Introduction
Gliomas are a subtype of primary brain tumor that
are known to be aggressive and highly invasive (1). The most aggressive glioma manifestation
is glioblastoma; its invasive nature contributes towards the
ineffectiveness of current therapeutic strategies, such as
neurosurgery, radiation therapy and chemotherapy (2). Although efforts have been made to
advance cancer diagnosis and treatment, the impact of these
advances on the clinical outcome and prognosis for patients with
glioblastoma has not improved (3,4).
Therefore, it is of great importance to elucidate the molecular
mechanisms underlying glioblastoma in order to further explore
effective treatments for the disease.
The pituitary tumor transforming gene (PTTG) has
been demonstrated to serve a role in tumor initiation and
progression, including control of mitosis, cell transformation and
DNA repair (5). A whole-genome
expression profiling has revealed that PTTG1 and PTTG2 are
regulated in high-grade glioma, and may be involved in its
malignant progression (6). Moreover,
PTTG1 and PTTG2 have been identified in two glioblastoma-associated
stromal cell subtypes with different carcinogenic properties
present in the stroma of carcinomas (7). In addition, induced PTTG1 expression is
correlated with invasiveness and poor prognosis in glioma patients
(8), and increasing evidence
confirms a crucial role of PTTG1 in cell physiology and
tumorigenesis (9). However, the role
of PTTG2 expression in glioblastoma tumorigenesis remains
uncertain. Thus, there is a requirement for increased understanding
of the role of PTTG2 in glioblastoma progression in order to
determine novel target molecules.
In the present study, PTTG2 was overexpressed and
suppressed in the U251 human glioblastoma cell line following
transfection with pcDNA-PTTG2 and short interfering RNA
(siRNA)-PTTG2 plasmids, respectively. Following this, cell
proliferation, invasion and the apoptotic capacity were analyzed
in vitro. The current study aimed to investigate the
association between PTTG2 expression and glioblastoma
tumorigenesis, in order to provide further insight into the
mechanisms underlying glioblastoma disease and to determine a
theoretical foundation for therapeutically targeting PTTG2 in the
treatment of glioblastoma.
Materials and methods
Cell culture and plasmid
transfection
U251 human glioblastoma cell lines were obtained
from the Institute of Biochemistry and Cell Biology, Chinese
Academy of Sciences (Shanghai, China) and cultured in Dulbecco's
modified Eagle's medium (DMEM) containing 10% heat-inactivated
fetal bovine serum (Welgene Biotech, Co., Ltd., Taiwan). At 24 h
prior to transfection, 2xl05 U251 cells per well were
plated into 6-well plates and grown until they were 50–80%
confluent. Plasmids pcDNA-PTTG2 and siRNA-PTTG2 were purchased from
the American Type Culture Collection (Manassas, VA, USA) and were
transfected into U251 cells with Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for
24 h at room temperature, according to the manufacturer's
instructions. U251 cells transfected with a pcDNA vector were used
as the control (untreated) group. Cells from each group were
incubated at room temperature using normal DMEM media for 4 h and
were harvested after 48 h for further detection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from U251 cells from each
group using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) following treatment with TURBO DNase (Ambion; Thermo Fisher
Scientific, Inc.), and a spectrophotometer (NanoDrop 2000; Thermo
Fisher Scientific, Inc.) was used to measure the quality of RNA.
Next, 1 µl cDNA generated from 1 µg RNA was obtained by RT using
the PrimeScript RT Master Mix kit (RR036A; Takara Biotechnology,
Co., Ltd., Dalian, China). RT-qPCR was then performed using
Superscript III (Invitrogen; Thermo Fisher Scientific, Inc.) and
the expression level of PTTG2 was determined using the TaqMan Gene
Expression Assay (Hs00747713_sH; Applied Biosystems; Thermo Fisher
Scientific, Inc.). Reactions were conducted at 95°C for 10 min
followed by 40 cycles of 95°C for 15 sec, 60°C for 1 min and a
final dissociation stage of a gradient of 30–99°C using an ABI 7500
RT-PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Each sample was analyzed in triplicate. Finally, the
2−ΔΔCq method (10) was
used to calculate the expression level of PTTG2 according to the
expression of glyceraldehyde 3-phosphate dehydrogenase.
Western blot analysis
The expression of PTTG2 and caspase-3 at the protein
level were measured using western blot analysis. Cell lysates were
prepared using a radioimmunoprecipitation assay buffer (Roche
Diagnostics, Basel, Switzerland). Protein concentrations were
examined by a Bradford assay (Bio-Rad Laboratories, Inc., Madrid,
Spain). Protein bands were distinguished by sodium dodecyl
sulfate-polyacrylimide gel electrophoresis (Sigma-Aldrich, St.
Louis, MO, USA and Bio-Rad Laboratories, respectively) at 100 V for
2 h and subsequently transferred to nitrocellulose membranes (GE
Healthcare Life Sciences, Chalfont, UK). The membranes were blocked
with 5% nonfat dry milk for 1 h, then the membrane was probed using
specific rabbit primary antibodies against human caspase-3, PTTG2
and actin (all 1:1,000; Abgent, Inc., San Diego, CA, USA) overnight
at 4°C and incubated with horseradish peroxidase-conjugated
secondary antibody (1,5,000; all Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). The fold change in specific protein expression
was determined using β-actin expression as a loading control.
Densitometric measurements of the bands were analyzed using the
Kodak 2000R imaging system (Kodak, Rochester, NY, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
U251 cells (2xl04 cells/well) from each
group were seeded on 96-well plates. Next, 20 µl 0.5 mg/ml MTT
(Sigma-Aldrich) was added to each well after 0, 24, 48, 72 and 96 h
of transfection. Next, the plates were incubated for 4 h at 37°C,
followed by the addition of 200 µl dimethyl sulfoxide into each
well and further incubation for 30 min. Finally, the optical
density was read at a wavelength of 570 nm with a VERSAmax
microplate reader (Molecular Devices LLC, Sunnyvale, CA, USA). The
experiment was performed in triplicate. The cell
proliferation/viability was calculated using the obtained numerical
values using the following equation: Cell viability = [optical
density (OD) value of test group - OD value of blank group] / (OD
value of control group - OD value of blank group).
Matrigel Transwell assay
The invasion of glioblastoma cells in vitro
was measured using a Transwell chamber with a Matrigel-coated (0.78
mg/ml) upper membrane, which contained a 24-well insert with 8 mm
pore size (all BD Biosciences, Franklin Lanes, NJ, USA). The
transfected U251 cells were starved for 24 h and harvested. Next,
500 µl cells (1×105 cells/ml) from each group were added
to the upper insert of the chamber containing serum-free media
(Sigma-Aldrich). Meanwhile, the lower well of the chamber was
filled with DMEM with 10% bovine serum albumin (Sigma-Aldrich).
Following 12 h of incubation, 70% ice-cold ethanol was used to fix
the cells that had invaded the membrane and 0.1% crystal violet
(Sigma-Aldrich) was used to stain the cells for 15 min at room
temperature. Each determination was assayed in triplicate. Finally,
8 random high-power fields per chamber were counted under a Leica
DM2500 light microscope (magnification, ×100; Leica Microsystems
GmbH, Wetzlar, Germany) and the sum of the invasive cell number was
calculated to analyze the invasive ability.
Apoptotic analysis using Annexin
V/propidium iodide staining and flow cytometry
The apoptotic rate of U251 cells was detected using
flow cytometry. Analysis of Annexin V was examined using the
Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit
(Beijing Biosea Biotechnology, Co., Ltd., Beijing, China), in
accordance with the manufacturer's instructions. U251 cells
(5×106 cells/well) were resuspended and allowed to
attach overnight. Next, cells were isolated with trypsin
(Sigma-Aldrich), washed with ice-cold phosphate-buffered saline and
resuspended in 96 µl Annexin V binding buffer (BD Biosciences). A
total of 1 µl Annexin V-FITC and 5 µl propidium iodide solution
(both Sigma-Aldrich) was then added to cells, followed by
incubation away from light and on ice for 15 min. The cells were
analyzed at 488 nm by flow cytometry (BD FACSAria; BD Biosciences)
and the obtained numerical values were analyzed using CellQuest
version 3.0 software (BD Biosciences). A dual-color flow cytometric
method (11) was used to count the
Annexin V-positive cells as apoptotic cells.
Statistical analysis
The normal distribution of all collected data was
determined using the one-sample Kolmogorov-Smirnov test.
Enumeration data were analyzed using the χ2 or rank-sum
test. Measurement data was analyzed by Student's t-test (for two
groups) or one-way analysis of variance (for more than three
groups). A post-hoc Tukey's test was used to analyze comparisons
between groups. All variable data were analyzed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significantly difference.
Results
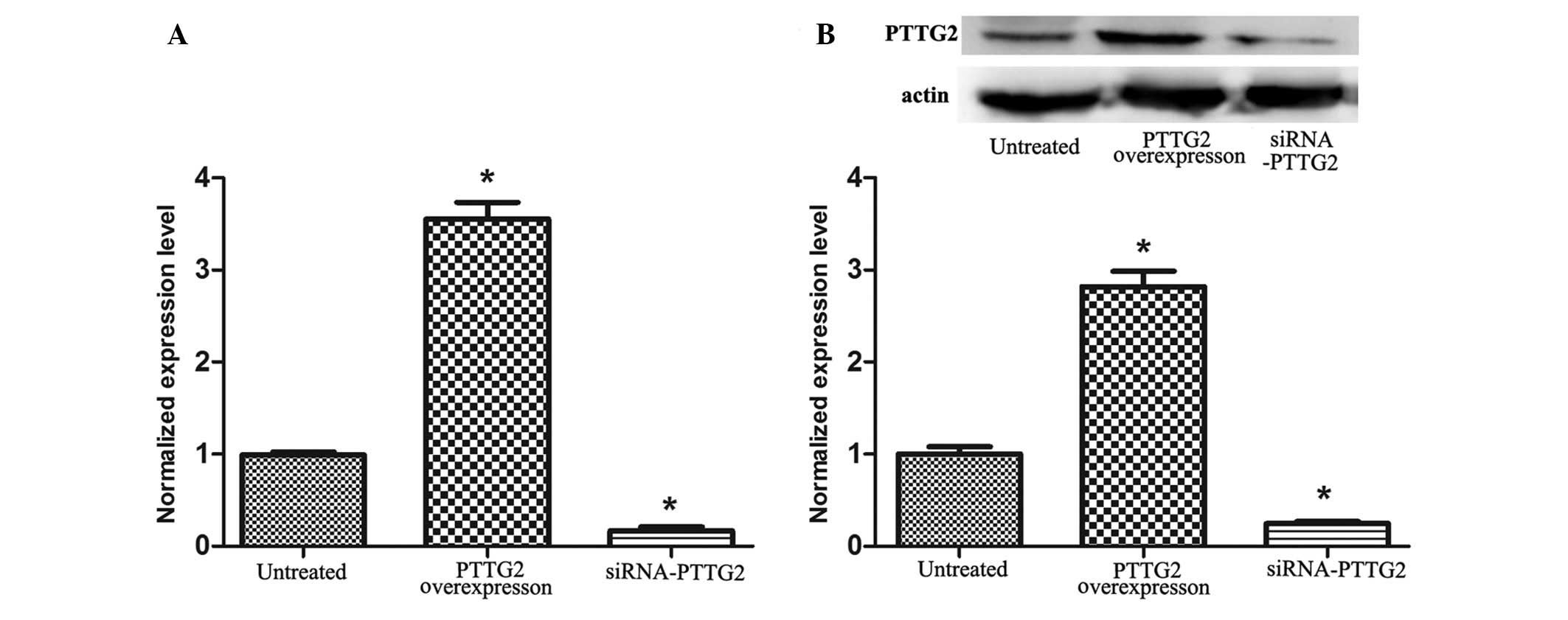
PTTG2 was successfully overexpressed
and knocked down in U251 cells
As presented in Fig.
1, the expression of PTTG2 mRNA and protein were determined.
The results demonstrated that the expression of PTTG2 mRNA and
protein (Fig. 1B) in U251 cells in
the PTTG2 overexpression group significantly increased in
comparison with the untreated group (P<0.05), whereas the
expression was significantly reduced in the siRNA-PTTG2
interference group in comparison with the untreated group
(P<0.05). This indicates that PTTG2 was successfully
overexpressed and suppressed in the study.
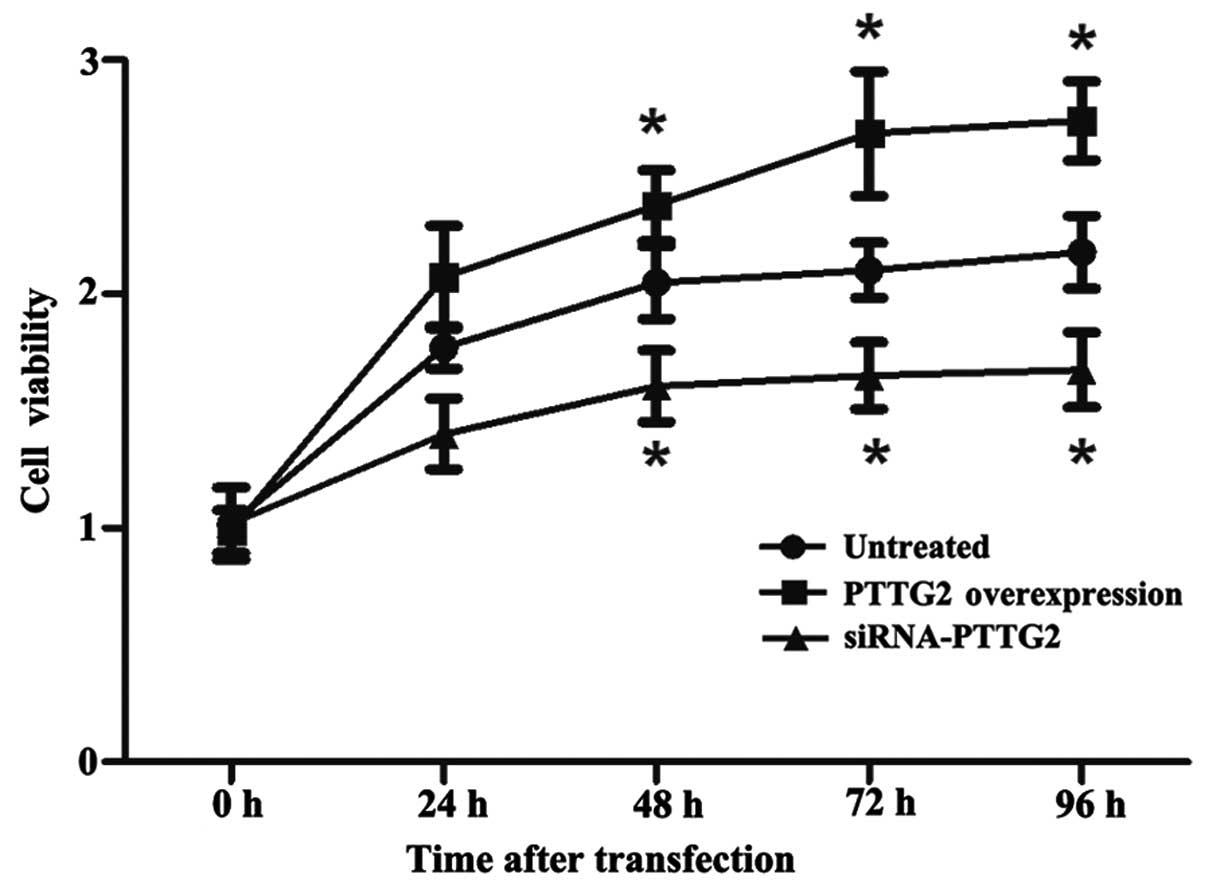
PTTG2 overexpression increases cell
viability
An MTT assay was performed to analyze the
proliferation of U251 cells in each group in an experimental period
of 96 h subsequent to transfection. As presented in Fig. 2, the cell proliferation/viability of
the PTTG2 overexpression group significantly increased over time in
comparison with the untreated group (P<0.05), whilst cell
proliferation/viability was reduced in the siRNA-PTTG2 interference
group in comparison with the untreated group (P<0.05).
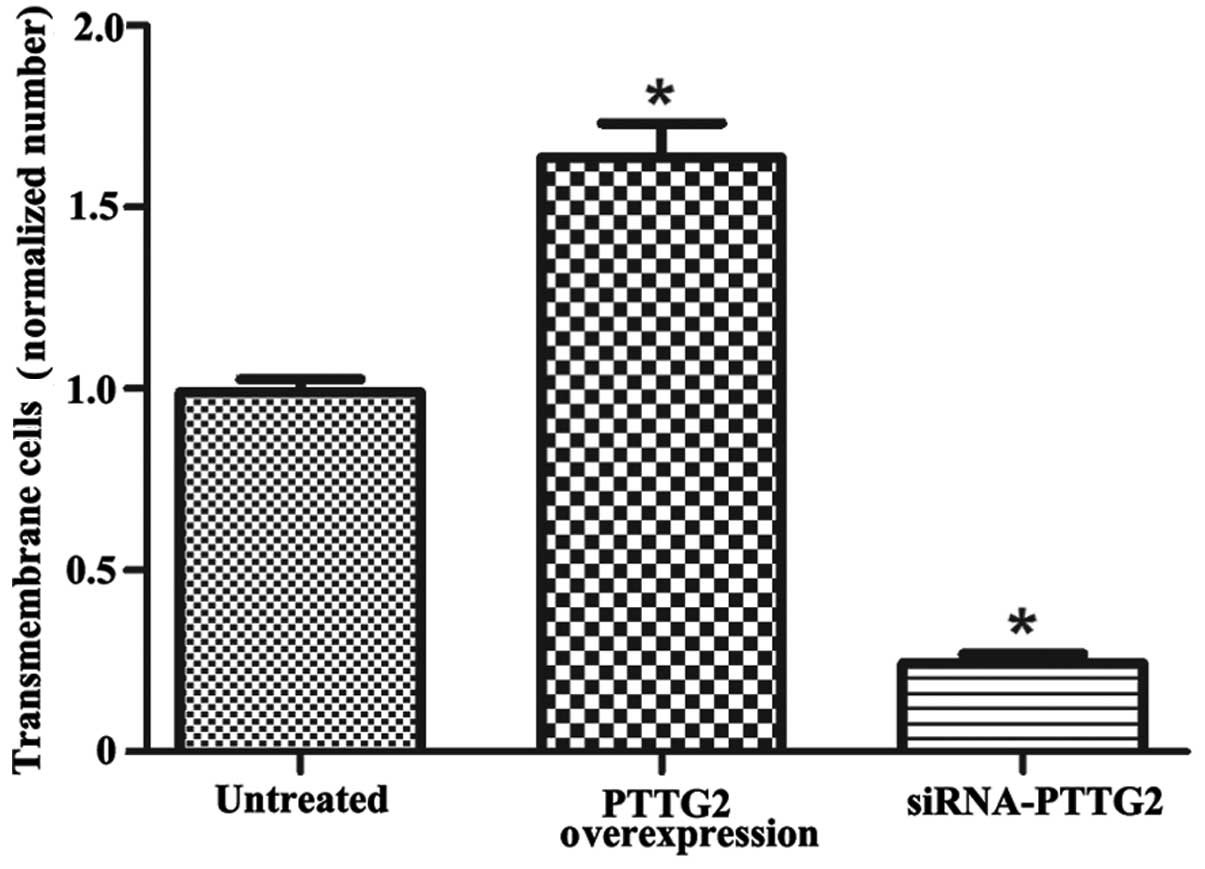
PTTG2 overexpression promotes cell
invasion
Fig. 3 displays the
cell invasion ability of each group. According to the sum of 8
random high-power fields from each group, the invasive cell number
in the PTTG2 overexpression group was significantly higher than
that of untreated group (1.63 times higher; P<0.05). Meanwhile,
the invasive cell number in the siRNA-PTTG2 interference group was
significantly reduced in comparison to the untreated group (28% of
untreated group; P<0.05).
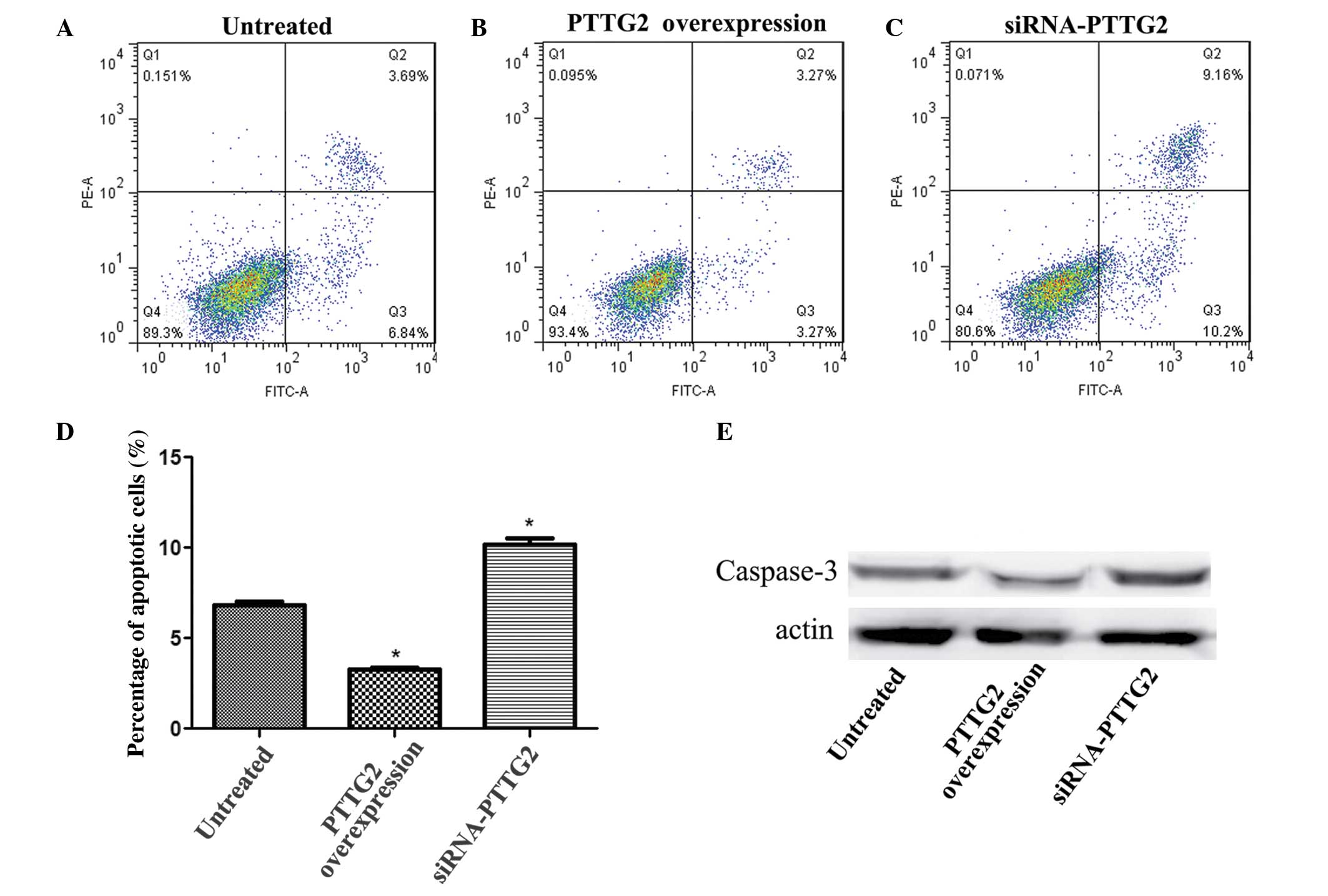
PTTG2 overexpression reduces cell
apoptosis
Flow cytometry was used to analyze the level of
apoptotic cells in each group. The results demonstrated that,
compared with the untreated group, the number of apoptotic cells in
the PTTG2 overexpression group was significantly reduced
(P<0.05; Fig. 4A and B) while the
number of apoptotic cells in the siRNA-PTTG2 interference group was
markedly increased (Fig. 4A and C).
In addition, the expression level of caspase-3 was determined to
verify the association between PTTG2 expression and cell apoptosis.
The expression level of caspase-3 was significantly reduced
(P<0.05) in the PTTG2 overexpression group and markedly reduced
in the siRNA-PTTG2 interference group, when compared with the
untreated group (Fig. 4D).
Discussion
Glioblastoma multiforme is an aggressive cancer that
has the highest rate of mortality among all malignant brain tumors
(12). Therefore, there is an urgent
requirement to identify novel target molecules to which more
effective therapeutic approaches can be developed. In the current
study, the results demonstrated that PTTG2 expression was strongly
associated with cell proliferation and invasion in the U251 human
glioblastoma cell line. Furthermore, PTTG2 was observed to induce
cell apoptosis in U251 cells. Together, these results indicate that
PTTG2 is likely to be associated with the progression of
glioblastoma.
PTTG2 belongs to PTTG family, which is highly
expressed in proliferating cells and serves an important role in
mediating tumorigenic functions in a number of tumors (13). A previous study used siRNA
interference to reveal that PTTG has key effects on cell
proliferation, cycle and apoptosis in U251 glioma cells (14). In addition, Ishikawa et al
(15) reported that PTTG could
induce angiogenesis by targeting basic fibroblast growth factor to
promote tumor progression. Angiogenesis is, therefore, considered
to be a key determinant and rate-limiting step in tumor growth and
metastatic spread (15).
PTTG2 is understood to share a high sequence
homology with PTTG1 (16).
Furthermore, overexpression of PTTG1 has been reported to induce
c-Myc expression and consequently result in increased levels of
cell proliferation (17). In the
present study, the cell proliferation/viability in the PTTG2
overexpression group significantly increased over time. As a
result, it is proposed that PTTG2 may serve a role in the
regulation of cell growth in glioblastoma.
Furthermore, invasiveness is a key molecular
mechanism that influences the malignant characteristics of gliomas
(2). Méndez-Vidal et al
(16) verified that PTTG2 serves an
important role in cell adhesion and may serve a potential role in
cell invasion. The study confirmed that knockdown of PTTG2 not only
results in concomitant downregulation of E-cadherin, but also leads
to the induction of epithelial-to-mesenchymal transition (EMT) and
elevates vimentin expression levels. In addition, Canel et
al (18) demonstrated that
E-cadherin mediates homophilic cell adhesion and that a loss of its
function may promote tumour invasion. Misregulated E-cadherin
expression is also observed as an aggressive brain tumor phenotype
in brain cancer, including glioblastoma (19). In addition, EMT has been demonstrated
to provide motility and invasive ability to cancer cells during the
progression of a number of types of human cancer (20), and vimentin intermediate filaments
have been recognized as an essential component involved in cell
invasion in lung cancer (21).
Zhou et al (22) suggested that vimentin expression
correlates with cell shape and motility in U-373 MG glioblastoma
cells. In the present study, the number of invasive cells in the
PTTG2 overexpression group was significantly increased compared
with that in the untreated group, and the number of invasive cells
in the siRNA-PTTG2 interference group was significantly reduced in
comparison with the untreated group. Therefore, the results suggest
that PTTG2 may promote cell invasion in U251 glioblastoma cells by
causing deregulation of E-cadherin or inhibiting EMT and reducing
vimentin expression levels.
In order to study the effect of PTTG2 on apoptosis,
the association between PTTG2 and caspase-3 was investigated. The
results demonstrated that the expression level of caspase-3 was
reduced as PTTG2 expression increased, which was also observed in a
study by Méndez-Vidal et al (16), indicating that PTTG2 may promote cell
apoptosis via the activation of caspase-3. In addition, caspase-3,
a widely accepted hallmark of programmed cell death, is able to
cleave a broad range of cellular substrates and promote activation
of DNA endonuclease (23); thus the
activation of caspases-3 is a key molecular stage in apoptotic cell
death in glioblastoma multiforme. Huang et al (24) demonstrated that curcuminoids can also
suppress cell growth and induce apoptosis in GBM 8401 cells through
affecting caspase-3-dependent signaling pathways. In the present
study, flow cytometry results demonstrated that the number
apoptotic cells in the PTTG2 overexpression group were
significantly reduced in comparison with the untreated group, while
the number of apoptotic cells in the siRNA-PTTG2 interference group
were reduced in comparison with the untreated group. Thus, it can
be hypothesized that PTTG2 induces cell apoptosis in U251
glioblastoma cells via caspase-3-dependent signaling pathways.
In conclusion, the current study indicates that
PTTG2 overexpression can promote cell proliferation and invasion
during the development of glioblastoma. Furthermore, PTTG2
overexpression may inhibit cell apoptosis in glioblastoma via
caspase-3-dependent signaling pathways. Together, these
observations provide an insight into the role of PTTG2 in
glioblastoma tumorigenesis and suggest that PTTG2 may serve as a
novel therapeutic target for the disease.
References
|
1
|
Maher EA, Furnari FB, Bachoo RM, Rowitch
DH, Louis DN, Cavenee WK and DePinho RA: Malignant glioma: Genetics
and biology of a grave matter. Genes Dev. 15:1311–1333. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rao JS: Molecular mechanisms of glioma
invasiveness: The role of proteases. Nat Rev Cancer. 3:489–501.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stewart LA: Chemotherapy in adult
high-grade glioma: A systematic review and meta-analysis of
individual patient data from 12 randomised trials. Lancet.
359:1011–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: European Organisation for Research and Treatment of
Cancer Brain Tumor and Radiotherapy Groups; National Cancer
Institute of Canada Clinical Trials Group: Radiotherapy plus
concomitant and adjuvant temozolomide for glioblastoma. N Engl J
Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boelaert K, McCabe CJ, Tannahill LA,
Gittoes NJ, Holder RL, Watkinson JC, Bradwell AR, Sheppard MC and
Franklyn JA: Pituitary tumor transforming gene and fibroblast
growth factor-2 expression: Potential prognostic indicators in
differentiated thyroid cancer. J Clin Endocrinol Metab.
88:2341–2347. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang P, Yan W, Zhang W, You G, Bao ZS and
Jiang T: Whole-genome messenger RNA profiling reveals genes
involved in malignant progression of glioma. Zhonghua Yi Xue Za
Zhi. 93:5–7. 2013.(In Chinese). PubMed/NCBI
|
|
7
|
Clavreul A, Etcheverry A, Tétaud C,
Rousseau A, Avril T, Henry C, Mosser J and Menei P: Identification
of two glioblastoma-associated stromal cell subtypes with different
carcinogenic properties in histologically normal surgical margins.
J Neurooncol. 122:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Genkai N, Homma J, Sano M, Tanaka R and
Yamanaka R: Increased expression of pituitary tumor-transforming
gene (PTTG)-1 is correlated with poor prognosis in glioma patients.
Oncol Rep. 15:1569–1574. 2006.PubMed/NCBI
|
|
9
|
Vlotides G, Eigler T and Melmed S:
Pituitary tumor-transforming gene: Physiology and implications for
tumorigenesis. Endocr Rev. 28:165–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Porra V, Bernaud J, Gueret P, Bricca P,
Rigal D, Follea G and Blanchard D: Identification and
quantification of fetal red blood cells in maternal blood by a
dual-color flow cytometric method: evaluation of the Fetal Cell
Count kit. Transfusion. 47:1281–1289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krex D, Klink B, Hartmann C, von Deimling
A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger
G, et al: Long-term survival with glioblastoma multiforme. Brain.
130:2596–2606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bradshaw C and Kakar SS: Pituitary tumor
transforming gene: An important gene in normal cellular functions
and tumorigenesis. Histol Histopathol. 22:219–226. 2007.PubMed/NCBI
|
|
14
|
Song-Bai X, Hong-Guang Z, Gang Z, Zuo X,
Hong-Quan Y and Shu-Hong K: The effect of PTTG gene silenced by
RNAi on inhibition of human glioblastoma U251 cells. Chin J
Gerontol. 7:645–647. 2008.
|
|
15
|
Ishikawa H, Heaney AP, Yu R, Horwitz GA
and Melmed S: Human pituitary tumor-transforming gene induces
angiogenesis. J Clin Endocrinol Metab. 86:867–874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Méndez-Vidal C, Gámez-Del Estal MM,
Moreno-Mateos MA, Espina-Zambrano ÁG, Torres B and Pintor-Toro JA:
PTTG2 silencing results in induction of epithelial-to-mesenchymal
transition and apoptosis. Cell Death Dis. 4:e5302013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pei L: Identification of c-myc as a
down-stream target for pituitary tumor-transforming gene. J Biol
Chem. 276:8484–8491. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Canel M, Serrels A, Frame MC and Brunton
VG: E-cadherin-integrin crosstalk in cancer invasion and
metastasis. J Cell Sci. 126:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lewis-Tuffin LJ, Rodriguez F, Giannini C,
Scheithauer B, Necela BM, Sarkaria JN and Anastasiadis PZ:
Misregulated E-cadherin expression associated with an aggressive
brain tumor phenotype. PLoS One. 5:e136652010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nieto MA: The ins and outs of the
epithelial to mesenchymal transition in health and disease. Annu
Rev Cell Dev Biol. 27:347–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kidd ME, Shumaker DK and Ridge KM: The
role of vimentin intermediate filaments in the progression of lung
cancer. Am J Respir Cell Mol Biol. 50:1–6. 2014.PubMed/NCBI
|
|
22
|
Zhou R and Skalli O: TGF-α differentially
regulates GFAP, vimentin, and nestin gene expression in U-373 MG
glioblastoma cells: Correlation with cell shape and motility. Exp
Cell Res. 254:269–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ray SK, Patel SJ, Welsh CT, Wilford GG,
Hogan EL and Banik NL: Molecular evidence of apoptotic death in
malignant brain tumors including glioblastoma multiforme:
Upregulation of calpain and caspase-3. J Neurosci Res. 69:197–206.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang TY, Tsai TH, Hsu CW and Hsu YC:
Curcuminoids suppress the growth and induce apoptosis through
caspase-3-dependent pathways in glioblastoma multiforme (GBM) 8401
cells. J Agric Food Chem. 58:10639–10645. 2010. View Article : Google Scholar : PubMed/NCBI
|