Introduction
Parathyroid hormone (1–34) [PTH
(1–34)] is currently the only anabolic agent
approved by the Food and Drug Administration (FDA) for the
treatment of osteoporosis in the USA (1). It is established that intermittent PTH
(1–34) administration increases mass, strength
and mineral density of bone, and improves bone microarchitecture
and fracture healing (2–4). Furthermore, previous in vitro
studies have indicated that intermittent delivery of PTH (1–34)
enhances the proliferation and differentiation of osteoprogenitor
cells in bone marrow, increases osteoblast activity and inhibits
osteoblast apoptosis (5–7). However, the precise molecular mechanism
underlying the effect of PTH (1–34) during
the osteogenic differentiation of bone mesenchymal stromal cells
(BMSCs) remains elusive.
A number of previous studies have reported that PTH
affects osteoblastic cells through activation of cyclic adenosine
monophosphate/protein kinase A (cAMP/PKA) (8,9),
Wnt/β-catenin (10–12) and mitogen-activated protein kinase
signaling pathways (13,14); however, the focus of previous studies
has particularly been on cAMP signaling. Several previous studies
have evaluated the role of PKA pathway in osteogenic
differentiation of hMSCs, and reported that pretreatment of human
MSCs with a cAMP analog or forskolin enhanced bone formation
(15–17). cAMP is a pivotal intracellular
signaling molecule, the main function of which is to activate the
cAMP-dependent PKA (18). Adenylate
cyclase is activated through dissociated G-proteins, causing the
conversion of adenosine triphosphate into cAMP (17). Subsequently, cAMP activates PKA, the
cAMP-responsive element binding (CREB) protein is phosphorylated,
and this translocates into the nucleus where it activates
transcription of target genes (17).
PTH signaling is mediated by a G protein-coupled
receptor, known as parathyroid receptor 1 (PTHR1) (19). Ligand binding to PTHR1 stimulates the
activation of adenylate cyclase mediated by the G protein subunit
Gαs, thereby stimulating cAMP production and the
subsequent activation of PKA. Wang et al (8) treated the rat osteoblast-like cell line
UMR 106 with PTH (1–34), revealing that PTH stimulates the
expression of the transcription factors runt related transcription
factor 2 (RUNX2) and osterix in vitro, and that induction of
RUNX2 mRNA expression is mediated through the activation of the
cAMP/PKA pathway. In another study, Nakao et al (9) evaluated cAMP and bone morphogenetic
protein (BMP) levels in MC3T3-E1 cells following the addition of
PTH, and found that PTH enhanced BMP activity by increasing cAMP
accumulation in MC3T3-E1 cells.
Although a number of previous studies have
demonstrated that the effects of PTH (1–34) are
associated with the cAMP/PKA pathway, it is unclear how
intermittent administration of PTH (1–34)
regulates osteogenic differentiation of BMSCs through the cAMP/PKA
pathway. The present study therefore aimed to investigate the
molecular mechanism of intermittent PTH (1–34)
application in the regulation of the proliferation and osteogenic
differentiation of BMSCs by the cAMP/PKA pathway.
Materials and methods
Isolation and culture of rat
BMSCs
A total of 20 male Sprague-Dawley rats (age, 5
weeks; weight, 100–120 g) were purchased from the Experimental
Animal Center of Sun Yat-Sen University (Guangzhou, China) for use
in the present study. The rats were acclimated to the housing
conditions for 7 days, during which the rats were maintained under
a 12-h light/dark cycle at 22°C with ad libitum access to
food and water. The current study was approved by the Animal Care
Committee of Sun Yat-Sen University and was in compliance with
guiding principles for the use of laboratory animals (20). Following sacrifice of the rats by
overdose with 10% chloral hydrate (intraperitoneal injection;
Guangzhou Chemical Reagent Factory, Guangzhou, China), the femora
and tibiae of the rats were removed. The ends of each bone were
dissected and the BMSCs were harvested by flushing out the bone
marrow with 5 ml Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) twice using a
syringe. The BMSCs were centrifuged at 1,000 × g for 5 min,
resuspended in DMEM containing 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) and incubated at 37°C with
5% CO2. The culture medium was changed every 2 days.
Upon reaching 80–90% confluence, the cells were subcultured. BMSCs
at passage 3 were used in all experiments.
PTH (1–34),
forskolin and H-89 administration
For all experiments, BMSCs were randomly divided
into four groups, as follows: No PTH treatment (control); PTH
(1–34) treatment (PTH; ProSpec-Tany TecnoGene
Ltd., Rehovot, Israel); PTH (1–34) plus
treatment with forskolin (FSK; Beyotime Institute of Biotechnology,
Haimen, China), an adenylyl cyclase activator; and PTH (1–34) plus
treatment with a PKA inhibitor (H-89; Beyotime Institute of
Biotechnology).
For the control group, 24 h after culture, the
medium was changed to fresh DMEM medium for 6 h, and then the cells
were cultured in PTH-free osteogenic medium containing 10 nM
dexamethasone, 10 mM β-glycerophosphate, and 50 µg/ml ascorbic
acid, (all Sigma-Aldrich, St. Louis, MO, USA) for the remaining 42
h of each 48-h cycle. In the PTH group, BMSCs were exposed to 10 nM
PTH (1–34) (7) in
DMEM medium for the first 6 h of each 48-h cycle and then washed
with phosphate-buffered saline (PBS) and cultured in osteogenic
medium for the subsequent 42 h. Similarly, BMSCs in the FSK and
H-89 groups were treated for 6 h with 10 nM PTH (1–34) plus
10 nM forskolin or 10 µM H-89 (21),
respectively, before being washed with PBS and cultured in
osteogenic medium for 42 h. In all groups, the medium was changed
every 48 h, and all groups were cultured for 14 days.
Cell proliferation assay
BMSCs were cultured in 96-well plates at a density
of 1×103 cells/well, and treated as described above.
Cell proliferation was assessed using a Cell Counting kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan) at 3,7,10
and 14 days. For this, the culture medium in all groups was removed
and changed to fresh culture medium. Subsequently, CCK-8 solution
was added (10 µl per well) and incubated for 2 h at 37°C with 5%
CO2. Finally, cell proliferation was assessed on the
basis of optical density values, measured at 450 nm using a Sunrise
microplate reader (Tecan Trading AG, Männedorf, Switzerland). Five
replicates of each sample at each time point were measured.
Alkaline phosphatase (ALP) activity
assessment
BMSCs were seeded in 6-well plates at a density of
5×104 cells/well in each group and treated as described
above. On days 7 and 14, ALP was extracted and detected using a
SensoLyte p-nitrophenyl phosphate (pNPP) ALP assay kit
(AnaSpec, Inc., Fremont, CA, USA) in accordance with the
manufacturer's protocol. Cells were initially lysed using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology), containing 10 mM Tris (pH 7.0), 1 mM EDTA, and 0.2%
Triton X-100, and the lysates were frozen at −80°C and then thawed.
Cell lysate was centrifuged for 15 min (10,000 × g, 4°C), after
which the supernatant was collected and combined with pNPP. ALP
activity was determined at 405 nm using the Sunrise microplate
reader. The values were normalized to total protein content,
measured using a NanoDrop 2000 spectrophotometer (NanoDrop; Thermo
Fisher Scientific, Inc., Wilmington, DE, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
On day 14, total RNA was extracted from the cultured
cells in each group using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.). Subsequent to RNA isolation, the concentration
and purity of RNA was assessed using a NanoDrop 2000
spectrophotometer. A total of 1,000 ng RNA per group was used for
cDNA synthesis using a SYBR-PrimeScript RT-PCR kit (Takara
Biotechnology Co., Ltd., Dalian, China). This included 0.5 µl
PrimeScript RT Enzyme mix I, 0.5 ml oligo dT primer, and 2 ml
PrimeScript Buffer to a final volume of 10 µl. A total of 1 µl cDNA
was then used for RT-qPCR in a 10-µl reaction volume containing 0.3
µl forward and 0.3 µl reverse primers (Table I), 5 µl SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd.) and 3.4 µl diethylpyrocarbonate-treated
water (Takara Biotechnology Co., Ltd.). qPCR was performed on each
cDNA sample in triplicate under the following cycling conditions:
Initial denaturation at 95°C for 5 min, followed by 40 cycles of
denaturation at 94°C for 30 sec, annealing at 60°C for 10 sec and
elongation at 72°C for 20 sec, and a final extension step at 72°C
for 10 min. Fluorescence data was analyzed using a CFX96 Real-Time
PCR Detection system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Gene expression levels were calculated using the
2−∆∆Cq method (22). Data
are presented as fold change relative to control samples.
 | Table I.Gene primers for quantitative reverse
transcription-polymerase chain reaction. |
Table I.
Gene primers for quantitative reverse
transcription-polymerase chain reaction.
| Gene | Primer | Sequence,
5′-3′ | Product size,
bp | GenBank accession
no. |
|---|
| RUNX2 | Forward |
GGGAACCAAGAAGGCACAGA | 171 |
NM_001278484.1 |
|
| Reverse |
GGTGGAATGGATGGATGGGG |
|
|
| Osterix | Forward |
AGGAGACGGGACAGCCAA | 113 |
NM_001037632.1 |
|
| Reverse |
AGGAAATGAGTGGGGAAAGGG |
|
|
| Collagen I | Forward |
GCGGAGGAGGCTATGACTTT | 162 |
NM_053356.1 |
|
| Reverse |
AGGCGAGATGGCTTATTCGT |
|
|
| Osteocalcin | Forward |
ACCTGTGGAGCAGAAATGGT | 187 |
NM_001033860.1 |
|
| Reverse |
GGCTGAAGTTGGTCGTTTGG |
|
|
| Osteopontin | Forward |
AGTGGTTTGCTTTTGCCTGT | 118 |
NM_012881.2 |
|
| Reverse |
AACTCGTGGCTCTGATGTTCC |
|
|
| β-actin | Forward |
GGAAATCGTGCGTGACATTA | 173 |
NM_031144.3 |
|
| Reverse |
CCACCCGTCCTCCAGTCC |
|
|
Alizarin Red S staining
To observe the osteogenic differentiation of BMSCs,
cells were subjected to Alizarin Red S staining. Cells were seeded
in 24-well plates at a density of 5×103 cells/well.
Following the 14-day culture, the osteogenic medium was removed
from the cells in each group. Cells were then washed twice with
ice-cold PBS and fixed with 70% ethanol for 30 min. Next, cells
were stained with 1% Alizarin Red S solution (pH 4.2;
Sigma-Aldrich) for 15 min and gently rinsed with dH2O.
The samples were imaged with an Axio Imager Z1 microscope (Zeiss
AG, Oberkochen, Germany).
Western blot analysis
Following the 14-day culture, cells in each group
were washed twice with ice-cold PBS and lysed in 80 µl Mammalian
Protein Extraction reagent and protease inhibitor cocktail tablets
(both Thermo Fisher Scientific, Inc.). The samples were centrifuged
for 15 min (12,000 × g, 4°C), the supernatant was extracted and
total protein concentration was determined using a NanoDrop 2000
spectrophotometer. The samples were denatured by heating for 5 min
at 95°C in loading buffer (Beyotime Institute of Biotechnology),
containing 0.5 M Tris·HCl, 0.5 M DTT, sodium dodecyl sulfate (SDS),
bromophenol blue and glycerol. Subsequently, equal volumes of the
samples (20 µl) were run on 12% SDS-polyacrylamide gels and
transferred to polyvinylidene fluoride membranes. The membranes
were blocked for 1 h with blocking reagent (Tris-buffered saline
containing 5% nonfat milk powder) and incubated overnight at 4°C
with primary antibodies, as follows: Rabbit anti-phosphorylated
CREB (p-CREB) monoclonal antibody (mAb; 1:1,000; cat. no. 9198),
rabbit anti-CREB mAb (1:1,000; cat. no. 9197), rabbit anti-RUNX2
mAb (1:1,000; cat. no. 12556), rabbit anti-osterix mAb (1:1,000;
cat. no. 13572) and rabbit anti-β-tubulin mAb (1:1,000; #15115; all
Cell Signaling Technology, Inc., Danvers, MA, USA). The membrane
was then incubated with alkaline phosphatase-conjugated goat
anti-IgG (1:2,000, cat. no. 7054; Cell Signaling Technology) for 1
h at room temperature, and the proteins were visualized using a
chemiluminescence kit (EMD Millipore, Billerica, MA, USA).
Densitometric analysis was performed using Photoshop CS5 (Adobe
Systems, Inc., San Jose, CA, USA).
Statistical analysis
All results are expressed as the mean ± standard
deviation. Comparative studies of means were performed by one-way
analysis of variance and Fisher's least significant difference
test, using SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA) and a
statistically significant difference was defined as P<0.05. All
experiments were repeated in triplicate.
Results
Intermittent PTH (1–34)
administration regulates proliferation of BMSCs via the cAMP/PKA
pathway
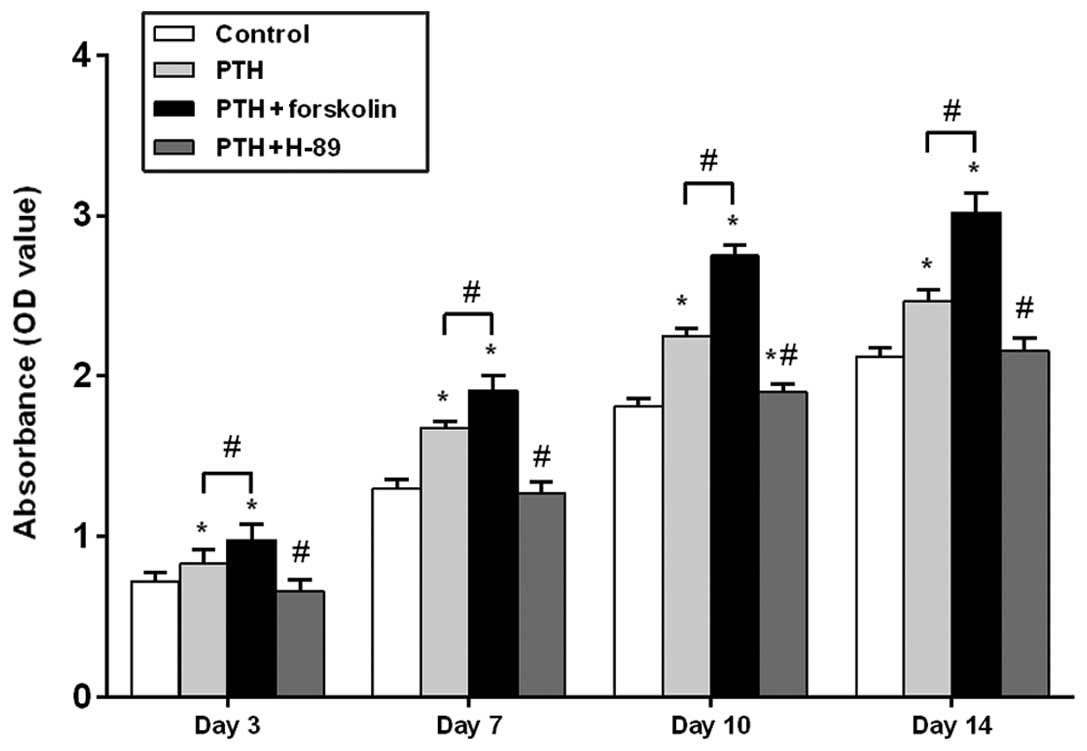
As shown in Fig. 1,
the cell number, as determined by the optical density using a cell
counting kit, gradually increased over time in all four groups.
After 3, 7, 10 and 14 days of intermittent PTH (1–34)
treatment, an increase in cell number was observed in the PTH group
compared with the control group, and there was a significant
difference between these groups at each time point (P<0.05). In
the PTH + FSK group, a significant increase in cell number was
observed on days 3, 7, 10 and 14 when compared with the control
group (P<0.05) and with the PTH group (P<0.05). Following
treatment of PTH (1–34) plus H-89, cell number was
significantly decreased compared with that in the PTH group at each
time point (P<0.05). No significant difference was observed
between the PTH + H-89 group and the control groups on days 3, 7
and 14 (P>0.05), but on day 10 an increase in cell number was
observed in the TH + H-89 group compared with the control group
(P<0.05).
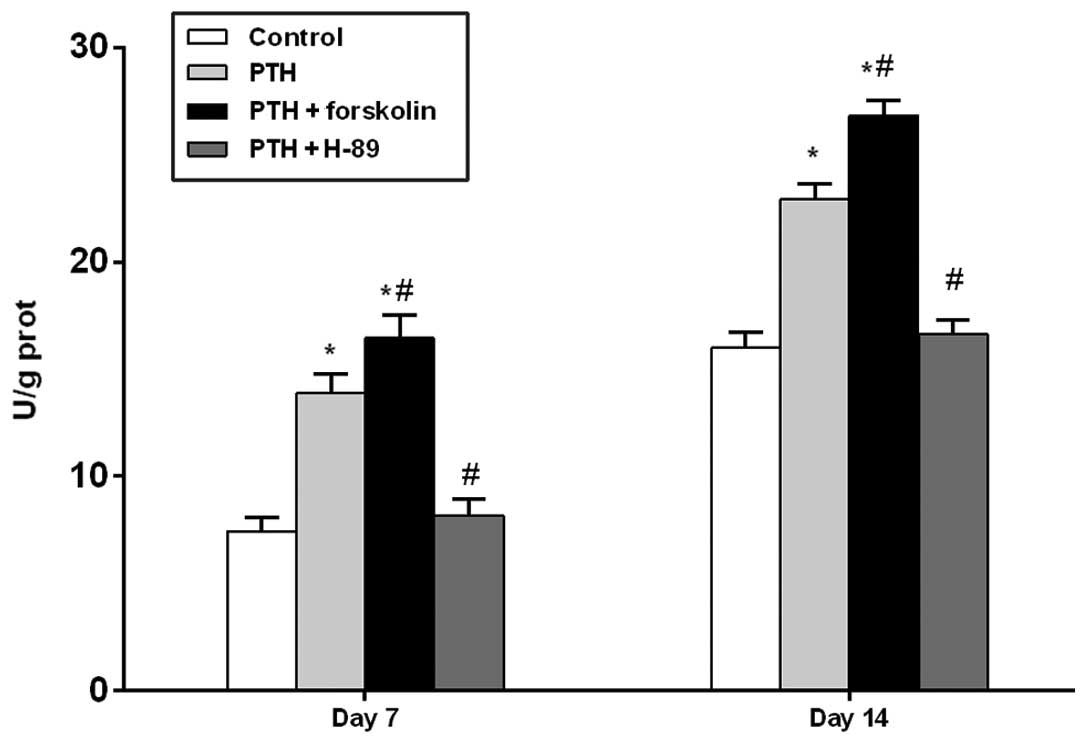
Assessment of ALP activity under
different treatment conditions
At days 7 and 14, the PTH and PTH + FSK groups
demonstrated significantly increased ALP activity compared with the
control group (P<0.05), and the PTH + FSK group also had
significantly increased ALP activity compared with the PTH group
(P<0.05). However, treatment with H-89 reduced ALP activity
compared with that in the PTH group (P<0.05; Fig. 2).
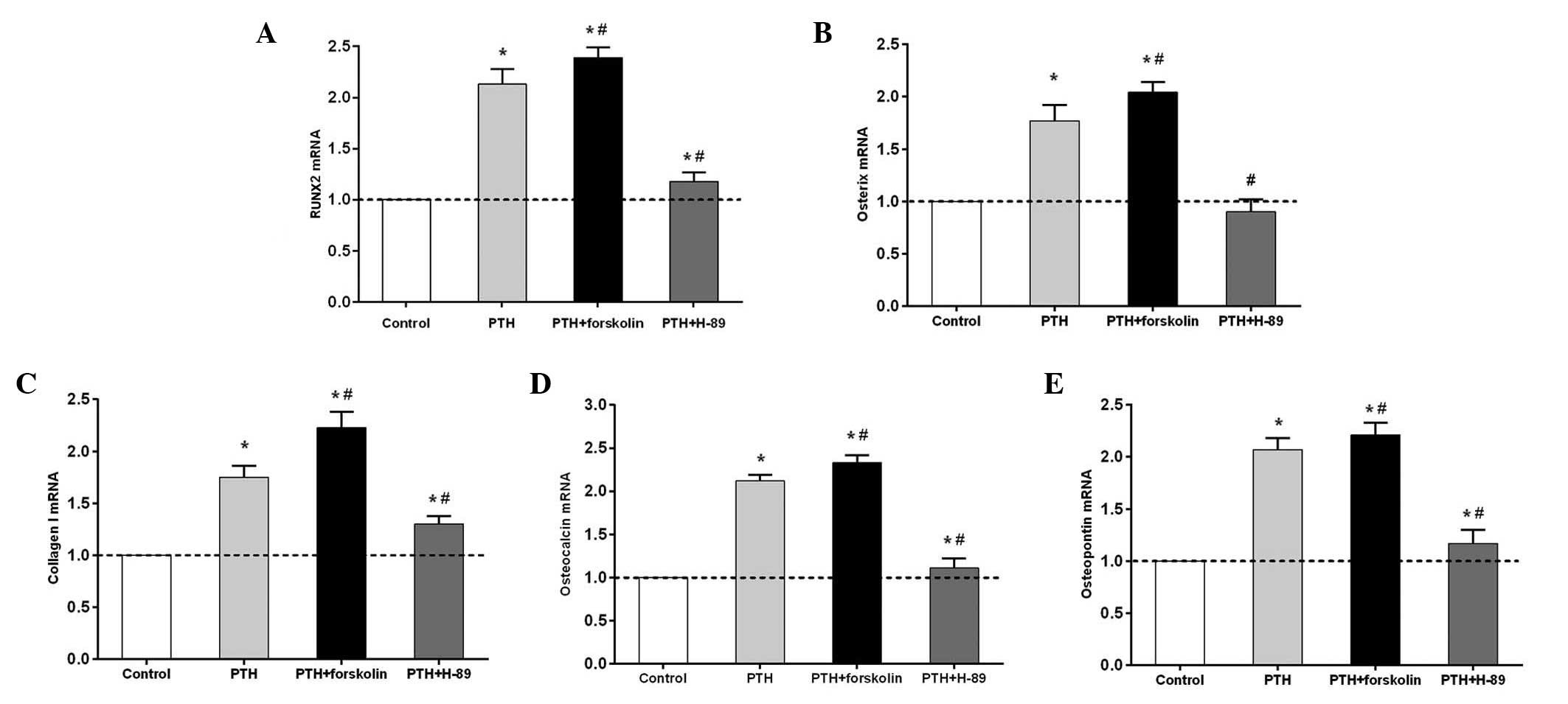
Intermittent PTH (1–34)
administration promotes osteogenic gene expression via the cAMP/PKA
signaling pathway
RUNX2, osterix, collagen I, osteocalcin and
osteopontin mRNA expression levels in the PTH and PTH + FSK groups
significantly increased compared with those in the control group
(P<0.05), but the levels of all genes examined were higher in
the PTH + FSK group than in the PTH group (P<0.05; Fig. 3). RUNX2, osterix, collagen I,
osteocalcin and osteopontin mRNA expression was significantly
downregulated in the PTH + H-89 group compared with the PTH group
(P<0.05), but the mRNA expression levels of these (with the
exception of osterix) were higher in the PTH + H-89 group than in
the control group (P<0.05). Notably, osterix mRNA expression
revealed a moderate but not statistically significant reduction in
the PTH + H-89 group compared with the control group.
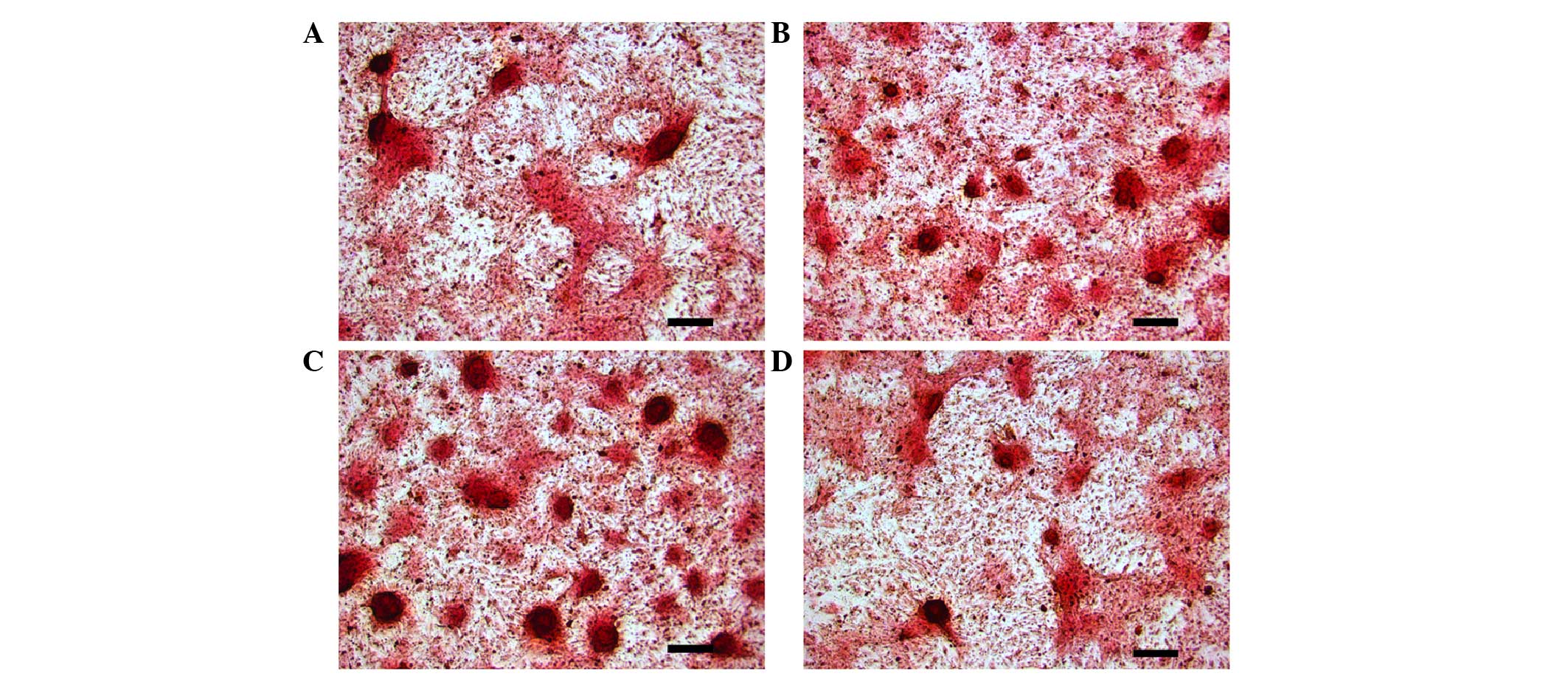
Effects of intermittent PTH (1–34)
application on mineralization of osteoblasts, induced from BMSCs
via the cAMP/PKA pathway
As indicated in Fig.
4, the PTH and PTH + FSK groups developed notably increased
mineralization compared with the control group (Fig. 4A–C). The mineralization effect of PTH
(1–34) plus forskolin was markedly increased
from that of PTH (1–34) treatment alone. However, treatment
with PTH (1–34) plus H-89 reduced mineralization
compared with that in the PTH group (Fig. 4B and D).
Intermittent PTH (1–34)
treatment increases osteogenic differentiation of BMSCs, mediated
by the cAMP/PKA signaling pathway
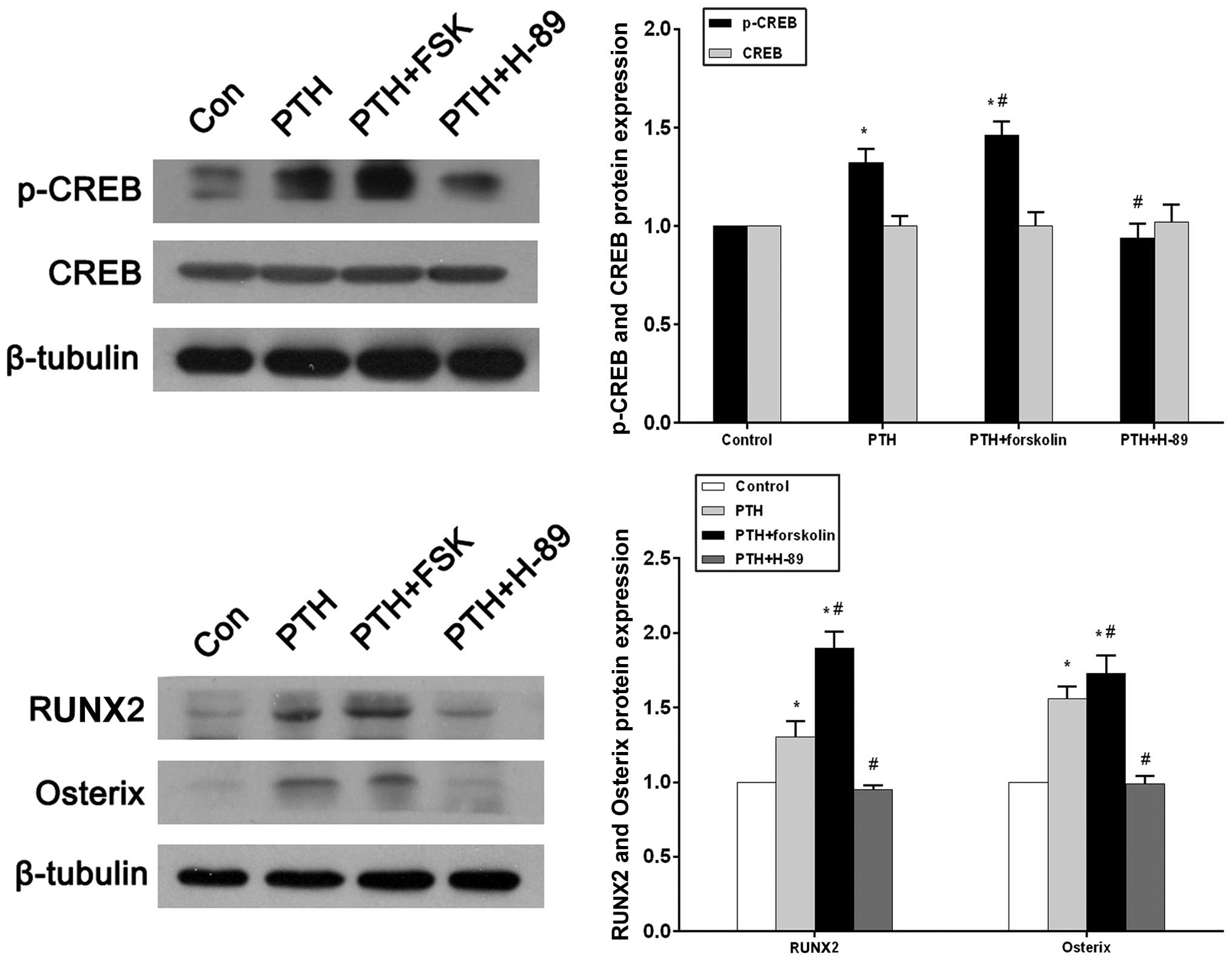
Western blotting and a densitometric analysis
revealed that p-CREB expression in the PTH group increased
significantly compared with that in the control group (P<0.05;
Fig. 5A). With the concurrent
administration of forskolin, p-CREB expression markedly increased
compared with that in the control group (P<0.05) and the PTH
group (P<0.05). In the PTH + H-89 group, p-CREB expression was
reduced compared with that in the PTH group (P<0.05), and did
not significantly differ from the p-CREB expression in the control
group (P>0.05; Fig. 5A).
Conversely, there were no statistical differences in CREB
expression between the four groups. (P>0.05; Fig. 5A). RUNX2 and osterix protein levels
were significantly increased in the PTH and PTH + FSK groups when
compared with the control group (P<0.05), and the FSK group also
exhibited increased expression of these proteins compared with the
PTH group (P<0.05). Significant reductions in RUNX2 and osterix
protein expression levels were observed in the PTH + H-89 group
compared with the PTH group (P<0.05), but a difference was not
observed compared with the control group (Fig. 5B).
Discussion
Previous studies have demonstrated the anabolic
effect of intermittent PTH (1–34) on
bone formation (23,24). Furthermore, a number of studies have
suggested that cAMP/PKA signaling is activated by PTH (1–34) during
osteogenic differentiation (8,9,21). However, precisely how the
intermittent administration of PTH (1–34)
regulates osteogenic differentiation of BMSCs via the cAMP/PKA
signaling pathway requires elucidation. In the present study, the
adenylyl cyclase activator forskolin and the specific PKA inhibitor
H-89 were used. A cell proliferation assay, osteogenic gene
testing, ALP activity detection, Alizarin Red S staining and
detection of p-CREB, RUNX2 and osterix protein expression were used
in the current study in order to determine the underlying molecular
mechanism by which intermittent PTH (1–34)
administration modulates the proliferation and osteogenic
differentiation of BMSCs via the cAMP/PKA signaling pathway. The
present findings indicated that intermittent PTH (1–34)
treatment regulates p-CREB to promote the proliferation and
osteogenic differentiation of BMSCs through the cAMP/PKA
pathway.
The results of the cell proliferation assay
demonstrated that PTH (1–34) stimulated BMSC proliferation,
forskolin enhanced PTH-stimulated BMSC proliferation and the
stimulating effect of PTH (1–34) was
blocked by H-89. The results indicated that intermittent PTH
(1–34) treatment promoted proliferation of
BMSCs through the cAMP/PKA pathway. Previously, Baron and Hesse
(25) had also suggested that PTH
stimulated the proliferation of MSCs. Nishida et al
(26) cultured bone marrow cells
isolated from the femora and tibiae of rats and treated them with
PTH, reporting that PTH induced apparent increases in the total
number of colony forming unit-fibroblasts. The findings of the
present study add to the current body of knowledge about the
effects of PTH (1–34) on BMSC proliferation via the cAMP/PKA
pathway.
RUNX2 and osterix are transcription factors used in
osteogenic lineage commitment expressed specifically at high levels
in osteoblasts. Thus, RUNX2 and osterix may be considered to be
osteoblast-specific transcription factors (27,28). In
support of the current results, Krishnan et al (29) revealed that PTH rapidly increases
RUNX2 mRNA and protein levels through activation of the cAMP/PKA
pathway and Wang et al (8)
demonstrated that PTH stimulates RUNX2 and osterix mRNA expression
via cAMP/PKA signaling. Furthermore, ALP is the most widely
recognized marker of osteoblast phenotypes and has an important
role during bone mineralization (30). A previous study reported that PTH
promotes osteogenic differentiation from MSCs by enhancing ALP
activity (7). Kao et al
(16) reported that treating BMSCs
with PTH increased the number of ALP-positive cells, and that
activation of cAMP signaling in BMSCs with forskolin markedly
enhanced ALP activity. It is also of note that collagen I is a
focal component of the extracellular matrix in the bone, and the
expression of collagen I typically occurs at the stage of matrix
synthesis during osteogenesis (31–33). In
addition, expression of osteocalcin and osteopontin mark the onset
of mineralization (34). Kao et
al (16) reported that direct
activation of cAMP signaling by forskolin in BMSCs elevated the
expression of osteoblast marker genes, such as RUNX2, osterix,
collagen I and osteocalcin, and that treatment of BMSCs with PTH
enhanced the ability of the subsequent differentiated osteoblasts
to mineralize. As with PTH, exposing BMSCs to forskolin also
increased mineralization. In the present study, and consistent with
these previous studies, the results of the ALP activity assay and
RT-qPCR revealed that ALP activity and RUNX2, osterix, collagen I,
osteocalcin and osteopontin expression were all upregulated by
intermittent PTH (1–34) application, and that the addition of
forskolin enhanced these effects to a greater degree than was
achieved by PTH (1–34) alone. Furthermore, the effects of PTH
(1–34) were inhibited by H-89. Alizarin Red S
staining revealed that intermittent PTH (1–34)
treatment significantly increased mineralization via the cAMP/PKA
pathway. Together, these data imply that intermittent PTH (1–34)
administration can affect osteogenesis by increasing the commitment
of BMSCs to the osteogenic lineage, via activation of the cAMP/PKA
pathway.
CREB is a cellular transcription factor (35) that is phosphorylated subsequent to
cAMP activation of PKA. CREB then translocates into the nucleus,
where it activates transcription of target genes (17). Siddappa et al (15) suggested that addition of
dibutyryladenosine-cAMP to human MSCs for 6 h resulted in increased
phosphorylation of the transcription factor CREB, which could be
inhibited by treatment with H-89. Furthermore, Tyson et al
(36) cultured UMR 106 cells and
treated them with PTH, finding that PKA is the enzyme responsible
for phosphorylating CREB in response to PTH. In the current study,
the western blot analysis demonstrated that intermittent PTH
(1–34) application upregulated the expression
of p-CREB, that administration of forskolin significantly increased
p-CREB expression in addition to that observed with PTH treatment
alone and that the effect of PTH on p-CREB expression could be
blocked by H-89. This trend was also observed in the protein
expression of RUNX2 and osterix.
In conclusion, the present in vitro study
demonstrated that intermittent PTH (1–34)
administration regulates the proteins downstream of the cAMP/PKA
signaling pathway, such as p-CREB, to enhance the proliferation,
osteogenic differentiation and mineralization of BMSCs.
Acknowledgements
This study was supported by the Guangdong Nature
Science Foundation in 2013 (grant no. S2013010015784).
References
|
1
|
Pettway GJ, Meganck JA, Koh AJ, Keller ET,
Goldstein SA and McCauley LK: Parathyroid hormone mediates bone
growth through the regulation of osteoblast proliferation and
differentiation. Bone. 42:806–818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Compston JE: Skeletal actions of
intermittent parathyroid hormone: Effects on bone remodelling and
structure. Bone. 40:1447–1452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neer RM, Arnaud CD, Zanchetta JR, Prince
R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S,
Genant HK, et al: Effect of parathyroid hormone (1–34) on fractures
and bone mineral density in postmenopausal women with osteoporosis.
N Engl J Med. 344:1434–1441. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang Y, Zhao J, Liao EY, Dai RC, Wu XP
and Genant HK: Application of micro-CT assessment of 3-D bone
microstructure in preclinical and clinical studies. J Bone Miner
Metab. 23(Suppl): S122–S131. 2005. View Article : Google Scholar
|
|
5
|
Jilka RL, Weinstein RS, Bellido T,
Roberson P, Parfitt AM and Manolagas SC: Increased bone formation
by prevention of osteoblast apoptosis with parathyroid hormone. J
Clin Invest. 104:439–446. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaback LA, do Soung Y, Naik A, Geneau G,
Schwarz EM, Rosier RN, O'Keefe RJ and Drissi H: Teriparatide (1–34
human PTH) regulation of osterix during fracture repair. J Cell
Biochem. 105:219–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang C, Frei H, Burt HM and Rossi F:
Effects of continuous and pulsatile PTH treatments on rat bone
marrow stromal cells. Biochem Biophys Res Commun. 380:791–796.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang BL, Dai CL, Quan JX, Zhu ZF, Zheng F,
Zhang HX, Guo SY, Guo G, Zhang JY and Qiu MC: Parathyroid hormone
regulates osterix and Runx2 mRNA expression predominantly through
protein kinase A signaling in osteoblast-like cells. J Endocrinol
Invest. 29:101–108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakao Y, Koike T, Ohta Y, Manaka T, Imai Y
and Takaoka K: Parathyroid hormone enhances bone morphogenetic
protein activity by increasing intracellular 3′,5′-cyclic adenosine
monophosphate accumulation in osteoblastic MC3T3-E1 cells. Bone.
44:872–877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kulkarni NH, Halladay DL, Miles RR,
Gilbert LM, Frolik CA, Galvin RJ, Martin TJ, Gillespie MT and Onyia
JE: Effects of parathyroid hormone on Wnt signaling pathway in
bone. J Cell Biochem. 95:1178–1190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Inoue Y, Canaff L, Hendy GN, Hisa I,
Sugimoto T, Chihara K and Kaji H: Role of Smad3, acting
independently of transforming growth factor-beta, in the early
induction of Wnt-beta-catenin signaling by parathyroid hormone in
mouse osteoblastic cells. J Cell Biochem. 108:285–294. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian Y, Xu Y, Fu Q and He M: Parathyroid
hormone regulates osteoblast differentiation in a
Wnt/β-catenin-dependent manner. Mol Cell Biochem. 355:211–216.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miao D, Tong XK, Chan GK, Panda D,
McPherson PS and Goltzman D: Parathyroid hormone-related peptide
stimulates osteogenic cell proliferation through protein kinase C
activation of the Ras/mitogen-activated protein kinase signaling
pathway. J Biol Chem. 276:32204–32213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rey A, Manen D, Rizzoli R, Ferrari SL and
Caverzasio J: Evidences for a role of p38 MAP kinase in the
stimulation of alkaline phosphatase and matrix mineralization
induced by parathyroid hormone in osteoblastic cells. Bone.
41:59–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siddappa R, Martens A, Doorn J, Leusink A,
Olivo C, Licht R, van Rijn L, Gaspar C, Fodde R, Janssen F, et al:
cAMP/PKA pathway activation in human mesenchymal stem cells in
vitro results in robust bone formation in vivo. Proc Natl Acad Sci
USA. 105:7281–7286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kao R, Lu W, Louie A and Nissenson R:
Cyclic AMP signaling in bone marrow stromal cells has reciprocal
effects on the ability of mesenchymal stem cells to differentiate
into mature osteoblasts versus mature adipocytes. Endocrine.
42:622–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doorn J, Siddappa R, van Blitterswijk CA
and de Boer J: Forskolin enhances in vivo bone formation by human
mesenchymal stromal cells. Tissue Eng Part A. 18:558–567. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang DC, Tsay HJ, Lin SY, Chiou SH, Li MJ,
Chang TJ and Hung SC: cAMP/PKA regulates osteogenesis, adipogenesis
and ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by
suppressing leptin. PLoS One. 3:e15402008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Potts JT: Parathyroid hormone: Past and
present. J Endocrinol. 187:311–325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawane T, Mimura J, Yanagawa T,
Fujii-Kuriyama Y and Horiuchi N: Parathyroid hormone (PTH)
down-regulates PTH/PTH-related protein receptor gene expression in
UMR-106 osteoblast-like cells via a 3′,5′-cyclic adenosine
monophosphate-dependent, protein kinase A-independent pathway. J
Endocrinol. 178:247–256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nozaka K, Miyakoshi N, Kasukawa Y, Maekawa
S, Noguchi H and Shimada Y: Intermittent administration of human
parathyroid hormone enhances bone formation and union at the site
of cancellous bone osteotomy in normal and ovariectomized rats.
Bone. 42:90–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rickard DJ, Wang FL, Rodriguez-Rojas AM,
Wu Z, Trice WJ, Hoffman SJ, Votta B, Stroup GB, Kumar S and Nuttall
ME: Intermittent treatment with parathyroid hormone (PTH) as well
as a non-peptide small molecule agonist of the PTH1 receptor
inhibits adipocyte differentiation in human bone marrow stromal
cells. Bone. 39:1361–1372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baron R and Hesse E: Update on bone
anabolics in osteoporosis treatment: Rationale, current status, and
perspectives. J Clin Endocrinol Metab. 97:311–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishida S, Yamaguchi A, Tanizawa T, Endo
N, Mashiba T, Uchiyama Y, Suda T, Yoshiki S and Takahashi HE:
Increased bone formation by intermittent parathyroid hormone
administration is due to the stimulation of proliferation and
differentiation of osteoprogenitor cells in bone marrow. Bone.
15:717–723. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakashima K and de Crombrugghe B:
Transcriptional mechanisms in osteoblast differentiation and bone
formation. Trends Genet. 19:458–466. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marie PJ: Transcription factors
controlling osteoblastogenesis. Arch Biochem Biophys. 473:98–105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krishnan V, Moore TL, Ma YL, Helvering LM,
Frolik CA, Valasek KM, Ducy P and Geiser AG: Parathyroid hormone
bone anabolic action requires Cbfa1/Runx2-dependent signaling. Mol
Endocrinol. 17:423–435. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stucki U, Schmid J, Hämmerle CF and Lang
NP: Temporal and local appearance of alkaline phosphatase activity
in early stages of guided bone regeneration. A descriptive
histochemical study in humans. Clin Oral Implants Res. 12:121–127.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Franceschi RT: The developmental control
of osteoblast-specific gene expression: Role of specific
transcription factors and the extracellular matrix environment.
Crit Rev Oral Biol Med. 10:40–57. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou Y, Guan X, Zhu Z, Gao S, Zhang C, Li
C, Zhou K, Hou W and Yu H: Osteogenic differentiation of bone
marrow-derived mesenchymal stromal cells on bone-derived scaffolds:
Effect of microvibration and role of ERK1/2 activation. Eur Cell
Mater. 22:12–25. 2011.PubMed/NCBI
|
|
33
|
Zhang C, Li J, Zhang L, Zhou Y, Hou W,
Quan H, Li X, Chen Y and Yu H: Effects of mechanical vibration on
proliferation and osteogenic differentiation of human periodontal
ligament stem cells. Arch Oral Biol. 57:1395–1407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Owen TA, Aronow M, Shalhoub V, Barone LM,
Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB and Stein
GS: Progressive development of the rat osteoblast phenotype in
vitro: Reciprocal relationships in expression of genes associated
with osteoblast proliferation and differentiation during formation
of the bone extracellular matrix. J Cell Physiol. 143:420–430.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bourtchuladze R, Frenguelli B, Blendy J,
Cioffi D, Schutz G and Silva AJ: Deficient long-term memory in mice
with a targeted mutation of the cAMP-responsive element-binding
protein. Cell. 79:59–68. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tyson DR, Swarthout JT and Partridge NC:
Increased osteoblastic c-fos expression by parathyroid hormone
requires protein kinase A phosphorylation of the cyclic adenosine
3′,5′-monophosphate response element-binding protein at serine 133.
Endocrinology. 140:1255–1261. 1999. View Article : Google Scholar : PubMed/NCBI
|