Introduction
Human embryonic stem cells (hESCs) are a class of
pluripotent cells isolated from the inner cell mass of human
blastocysts. hESCs are hypothesized to be able to provide an
unlimited cell source for the treatment of numerous refractory
diseases in humans, including diabetes, leukemia, Parkinson's
disease and juvenile rheumatoid arthritis (1). A number of preliminary and clinical
studies have indicated that the repair of damaged tissues may be
achieved via the transplantation of embryonic stem cells or their
derived cells (2–4).
A key issue for the effective passage of hESCs is
the inhibition of spontaneous differentiation and the maintenance
of cell pluripotency. In previous studies, mouse embryonic
fibroblasts (MEFs) and human foreskin fibroblasts (hFFs) have been
used as feeder cells in a layer to support the growth of hESCs
(5–8). Different cells in the feeding layers
secrete various growth factors to support the pluripotency and
non-differentiation of hESCs. The ability of a culture medium to
support the growth of hESCs is evaluated by detecting the
expression of hESC markers. However, at present, it is not known if
the factors secreted by the feed layer have a role in maintaining
the non-differentiation status of hESCs.
Cytokines that maintain the pluripotency,
self-renewal and non-differentiation status of hESCs can be
directly added to the culture medium, secreted by the feeding
layers or activated by the feed layer cells. It has previously been
demonstrated that it is challenging for a culture system without a
feeding layer to maintain non-differentiation growth without the
addition of exogenous cytokines. Cytokines secreted by the feeding
layer are complicated and it is unknown which factors secreted by
the feeding layer are able to promote the proliferation and inhibit
the differentiation of hESCs. However, it is widely accepted that
the addition of basic fibroblast growth factor (bFGF) is beneficial
for the growth of hESCs; as such, bFGF has been widely applied in
the hESC culturing system, in the presence and absence of a feeding
layer (9,10). Saxena et al (11) reported that bFGF was able to support
the self-renewal of hESCs. In addition, self-renewal was achieved
by activation of the phosphoinositide 3-kinase (PI3K)/Akt/protein
kinase B (PKB) pathway and upregulation of integrin α6/β1.
In the present study the efficiencies of feeder
layers composed of various ratios of MEFs and hFFs were compared
with those of feeder layers composed of MEFs or hFFs alone. The aim
was to develop a novel approach to solve the problems present in
the regular culturing of hESCs.
Materials and methods
Materials
The present study was performed at the Reproductive
Medical Center of Nanning Second People's Hospital (Nanning,
China). Foreskin tissue samples were obtained from a 7-year-old boy
who had previously undergone circumcision at the Nanning Second
People's Hospital. Written informed consent was obtained froom the
parents of the participant. The present study was approved by the
ethics committee of Nanning Second People's Hospital. The hESC line
NS-1 was isolated from human blastocysts, according to the method
described in a previous study (12).
Clean-level mice (n=3) at 12.5–14.5 days of pregnancy were
purchased from Beijing Vital River Laboratory Animal Technology
Co., Ltd. (Beijing, China). Animal experiments were conducted in
accordance with animal ethics standards of Nanning Second People's
Hospital. Following anesthetization by abdominal injection with 40
mg/kg barbital (Guangdong Jiabo Pharmaceutical Co., Ltd.,
Guangzhou, China), the head, limbs and internal organs of the
pregnant mice were removed. Cell suspension was obtained by
conventional repeated trypsin (Sigma-Aldrich) digestion. Original
cells were frozen once the cells were confluent. A feeder layer
composed of MEFs was prepared by mitomycin C treatment, as
described previously (9), for 2–3 h
and inoculation onto a gelatin-coated dish (Sigma-Aldrich, St.
Louis, MO, USA) at the density of 1×108 cells/l.
Foreskin tissue was sterilized and cell suspension was obtained
using trypsin digestion. Original cells were frozen once the cells
were confluent. The feeder layers containing hFFs and mixed layers
containing hFFs + MEFs were prepared in a similar manner.
Cellular characterization
Cellular morphology was observed using
immunofluorescence staining, as described previously (10). Briefly, cells at generation 3–5 were
obtained by digestion with 0.2% dispase (Sigma-Aldrich) and placed
onto culturing dishes under coverslips. When 70–80% confluence was
achieved, the cells were washed 2–3 times with Dulbeccos
phosphate-buffered saline (DPBS; Sigma-Aldrich). The cells on the
coverslips were then fixed with cold methanol (75%) for 25 min.
After rinsing twice with DPBS, the cells were blocked with DPBS
containing 1% fetal bovine serum (FBS) for 40 min. After rinsing
twice with DPBS, the coverslips were incubated with primary
antibodies, including rabbit anti-human keratin polyclonal antibody
(1:100; E3260-1; Spring Bioscience Corporation, Pleasanton, CA,
USA) and rabbit anti-humna vimentin polyclonal antibody (1:100;
E4621-2; Spring Bioscience Corporation). Following incubation
overnight at 37°C in 5% CO2, the coverslips were rinsed
4 times with DPBS. Subsequently, the coverslips were incubated with
fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG
(1:100; E8921-0; Spring Bioscience Corporation) in the dark for
20–25 min. Following a further four rinses, propidium iodide was
added and the coverslips were incubated in the dark for 15 min.
Following a further three rinses, images were obtained immediately
using the DMIRE2 Fluorescent Microscope (Leica Microsystems GmbH,
Wetzlar, Germany). Fibroblasts exhibiting positive expression of
vimentin were stained green. Negative staining for keratin
indicated non-epithelial cells.
Detection of bFGF secreted by feeder
layers
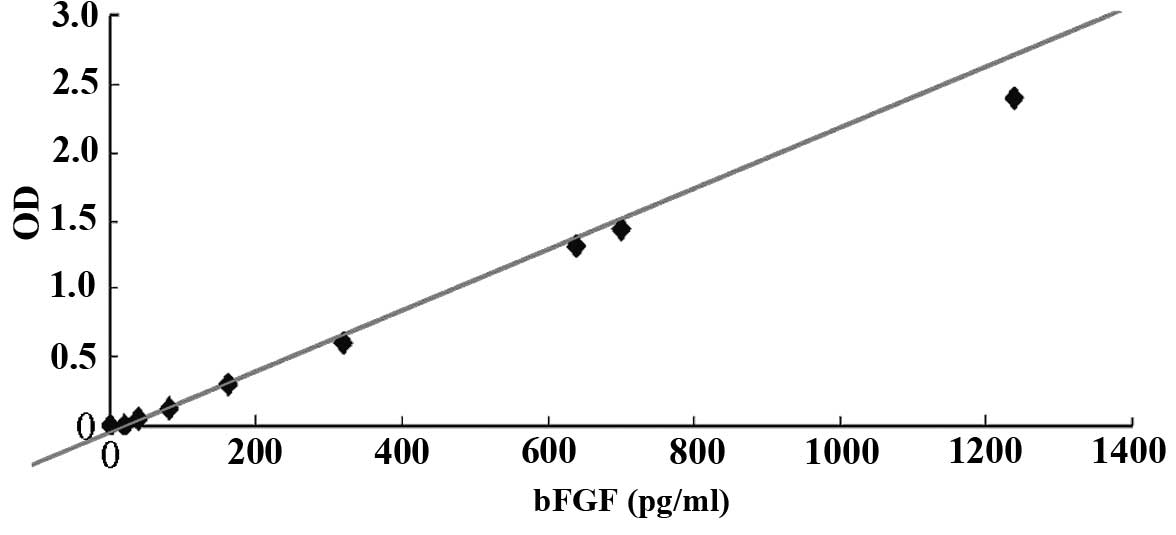
An enzyme-linked immunosorbent assay (ELISA; Fuzhou
Maixin Biotechnology Development Co., Ltd., Fuzhou, China) was used
to detect the concentration of bFGF secreted by the feeder layer
cells. Known concentrations of bFGF in Dulbeccos modified Eagles
medium (DMEM) and DMEM/F12 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) were used to generate a standard curve.
DMEM/F12 is designed for hESC culturing, and was supplemented with
20% KnockOut Serum Replacement formulation (SR; Thermo Fisher
Scientific, Inc.), which resulted in an osmotic pressure similar to
that of normal embryonic tissues. Following seeding of the hFFs or
MEFs for 24 h, the supernatant of the cells was isolated by
centrifugation (4°C, 256 × g), and the concentration of bFGF in the
supernatant was detected using the ELISA. Following treatment with
mitomycin C, the concentration of bFGF was determined in the
supernatant of the feeder layer cells with or without inoculation
of hESC (6, 24 and 72 h). In addition, 4 ng/ml exogenous bFGF
(Gibco; Thermo Fisher Scientific, Inc.) was added to the MEF feeder
layer, in order to sustain hESC self-renewal (13).
Preparation of mixed feeder
layers
The number of MEFs and hFFs were counted following
mitomycin C treatment using the Countess II FL Automated Cell
Counter (Thermo Fisher Scientific, Inc.). Subsequently, hFFs were
mixed with MEFs at ratios of 0:1, 1:2, 1:1, 2:1 and 1:0 and seeded
on gelatin-coated dishes at a density of 1×108
cells/l.
Passage of hESCs and morphological
observations
The hESC clones were mechanically cut into small
cell clumps (20–50 cells) using an attenuated Pasteur pipette
(Sanofi Pasteur MSD Ltd., Maidenhead, UK). The cell clumps were
then planted on the top of three feeder layers (MEFs, hFFs and hFFs
+ MEFs). The medium was changed every day and passage was made
every 5–6 days. The clone morphology was observed and recorded
using the CX41 phase contrast microscope (Olympus Corporation,
Tokyo, Japan).
Detection of non-differentiated hESCs
grown on the mixed feeder layers
The differentiation status was determined by
detecting the expression of alkaline phosphatase (AKP) and
octamer-binding transcription factor 4 (OCT-4). AKP was detected
using a commercial kit (Fuzhou Maixin Biotechnology Development
Co., Ltd.). Briefly, hESCs grown on the feeder layer for 5 days
were fixed with 90% ethanol. After washing, AKP labeling reagents
(Fuzhou Maixin Biotechnology Development Co., Ltd.) were added and
the cells were incubated for 15 min in the dark. When the color was
fully developed, the cells were visualized under the DVM6 Optical
Microscope (Leica Microsystems GmbH).
For immunohistochemical detection of OCT-4, hESCs
were washed with PBS and fixed with 40 g/l paraformaldehyde for 15
min. Subsequently, the cells were incubated for 40 min at room
temperature with rabbit anti-human OCT-4 polyclonal antibody (1:50;
ab19857; Abcam, Cambridge, MA, USA). Following washing with PBS,
the cells were incubated for 40 min at room temperature with
FITC-conjugated goat anti-rabbit IgG (1:200; ab21321, Abcam). OCT-4
expression was detected using the DMIRE2 Fluorescent Microscope.
hESC clones positive for OCT-4 exhibited a green color.
The mRNA expression levels of OCT-4 were determined
using a reverse transcription-polymerase chain reaction (RT-PCR),
according to a previous study (14).
Briefly, RNA was isolated from hESCs using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Reverse
transcription was performed using 1 µg RNA and the PrimeScript™ RT
Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China). PCR
was conducted in a C1000 Touch™ thermal cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), using the PCR Master Mix
(Promega Corporation, Madison, WI, USA) with 1 µl cDNA, 1 µl each
of sense and anti-sense primers, and 5 µl H2O. The PCR
cycling conditions were as follows: 95°C for 30 sec, followed by 32
cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec. The
primer sequences were as follows: OCT-4 sense,
5′-GACAACAATGAGAACCTTCAGGAGAA-3′ and anti-sense,
5′-TTCTGGCGCCGGTTACAGAACCA-3′; and GAPDH sense,
5′-GTCAGTGGTGGACCTGACCT-3′ and anti-sense,
5′-CACCACCCTGTTGCTGTAGCA-3′ (Sangon Biotech, Co., Ltd., Shanghai,
China). The fold-changes in gene expression were normalized to
GAPDH. PCR products were separated by 2% agarose gel
electrophoresis (Sigma-Aldrich) and visualized using the U-2900
UV–Vis Double Beam Spectrophotometer (Hitachi, Ltd., Tokyo, Japan).
The gel images were analyzed using ImageJ 2× software (National
Institutes of Health, Bethesda, MA, USA).
In vitro differentiation experiment. hESC
clones (1×106) were transferred onto culturing dishes
without feeder layers. The clones were cultured in embryonic stem
cell medium (Fuzhou Maixin Biotechnology Development Co., Ltd.)
without the basic components of fibroblast growth factors for 7–10
days. Morphology was observed under a microscope.
Statistical analysis
All statistical analyses were conducted using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. The differences between
two groups were compared using the Student's unpaired t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
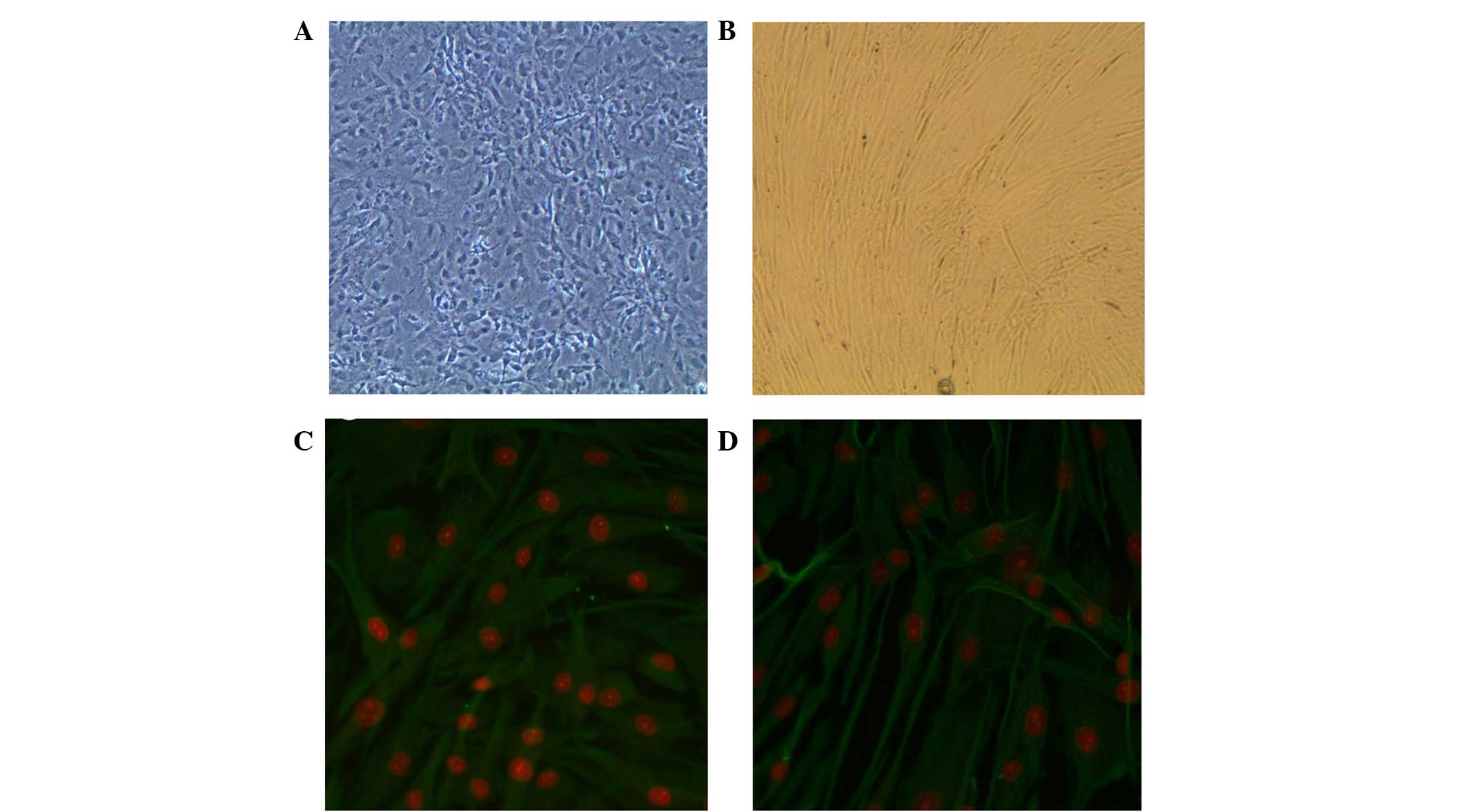
Characterization of MEFs and hFFs by
anti-vimentin fluorescence analysis
Anti-vimentin staining showed that MEFs and hFFs
obtained via standard procedure were positive for vimentin. Since
vimentin has been shown to be a marker of mesenchymal cells
(15), these results indicated the
presence of non-epithelial cells (Fig.
1).
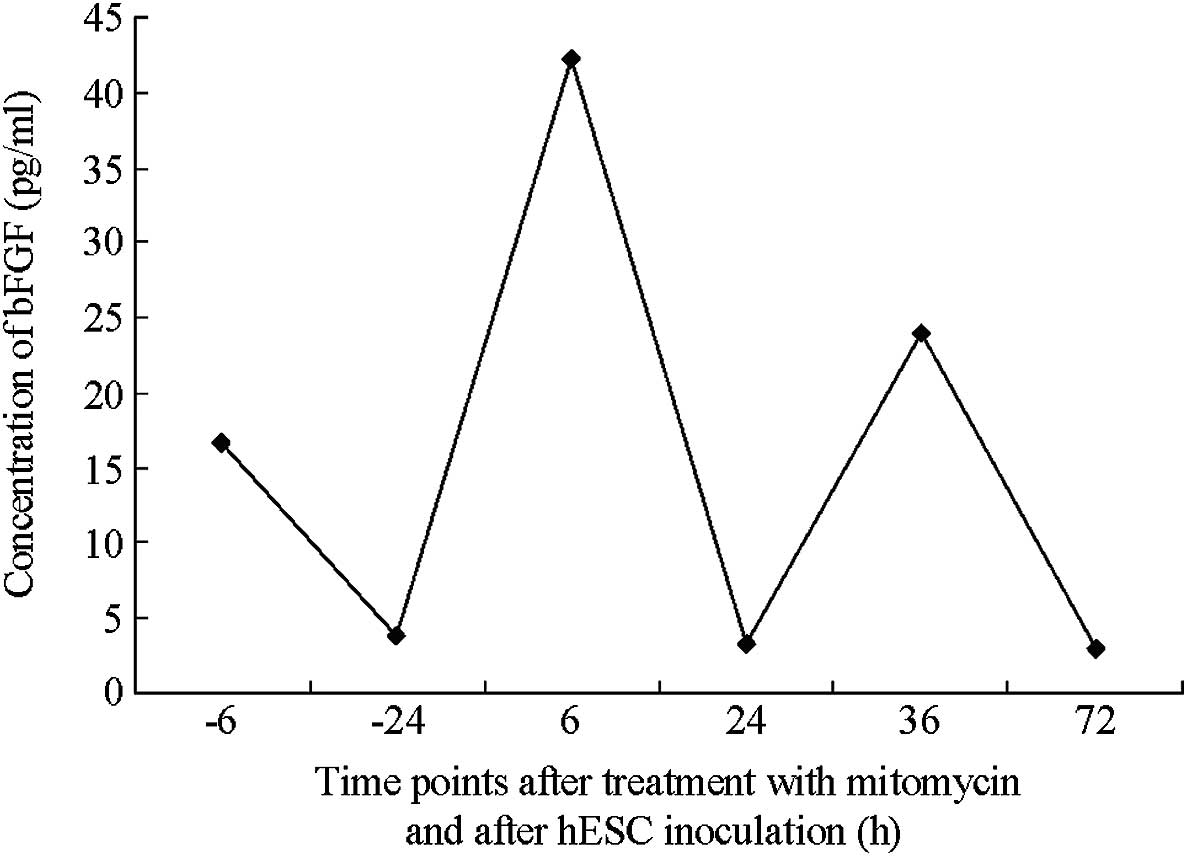
Secretion of bFGF by hFFs
An ELISA indicated that the content of bFGF in the
hFF feeder layer medium was not altered by the presence of
mitomycin C or by the replacement of FBS with SR. The absence of
bFGF in the FBS and SR indicated that the bFGF detected in the
medium had been secreted by the feeder layer. Furthermore,
mitomycin C treatment did not inhibit the autonomous secretion of
bFGF (Figs. 2 and 3). In addition, the maximal autonomous
secretion of bFGF was achieved in the first passage of feeder layer
cell inoculation. Following the adherent growth of hESCs, the
secretion of bFGF decreased significantly, suggesting that bFGF was
crucially involved in facilitating the adherent cell growth.
Furthermore, bFGF was mildly increased at the first passage of hESC
inoculation into the mixed feeder layer. The ratio of hFFs to MEFs
was 0:1, 1:2, 1:1, 2:1 and 1:0.
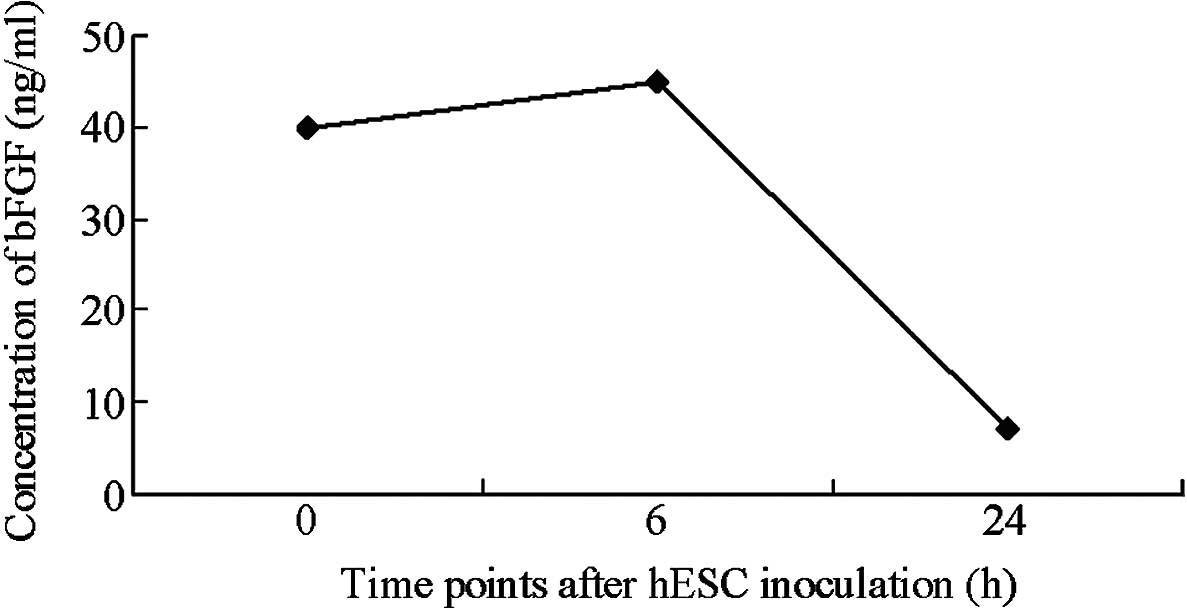
MEFs do not secrete detectable levels
of bFGF
bFGF content was rapidly reduced following the
adherent growth of hESCs. After 24 h of growth, bFGF content in the
medium was reduced to 7 pg/ml. Gradual reductions in bFGF and
significant increases in hESC proliferation indicated that bFGF was
utilized by the hESCs. bFGF is the most common additive in hESC
culture media and is able to markedly enhance the proliferation of
hESCs. Fibroblasts express multiple types of bFGF, while hESCs
express multiple types of bFGF receptor. The present results
suggest that bFGF is able to enhance cell adherence and clone
formation rate in the initial stages of hESC clone development and
support the proliferation of hESCs (Fig.
4). These results are consistent with the commonly accepted
hypothesis that bFGF is beneficial for the growth of hESCs.
Comparison of hESC growth between
different feeder layers
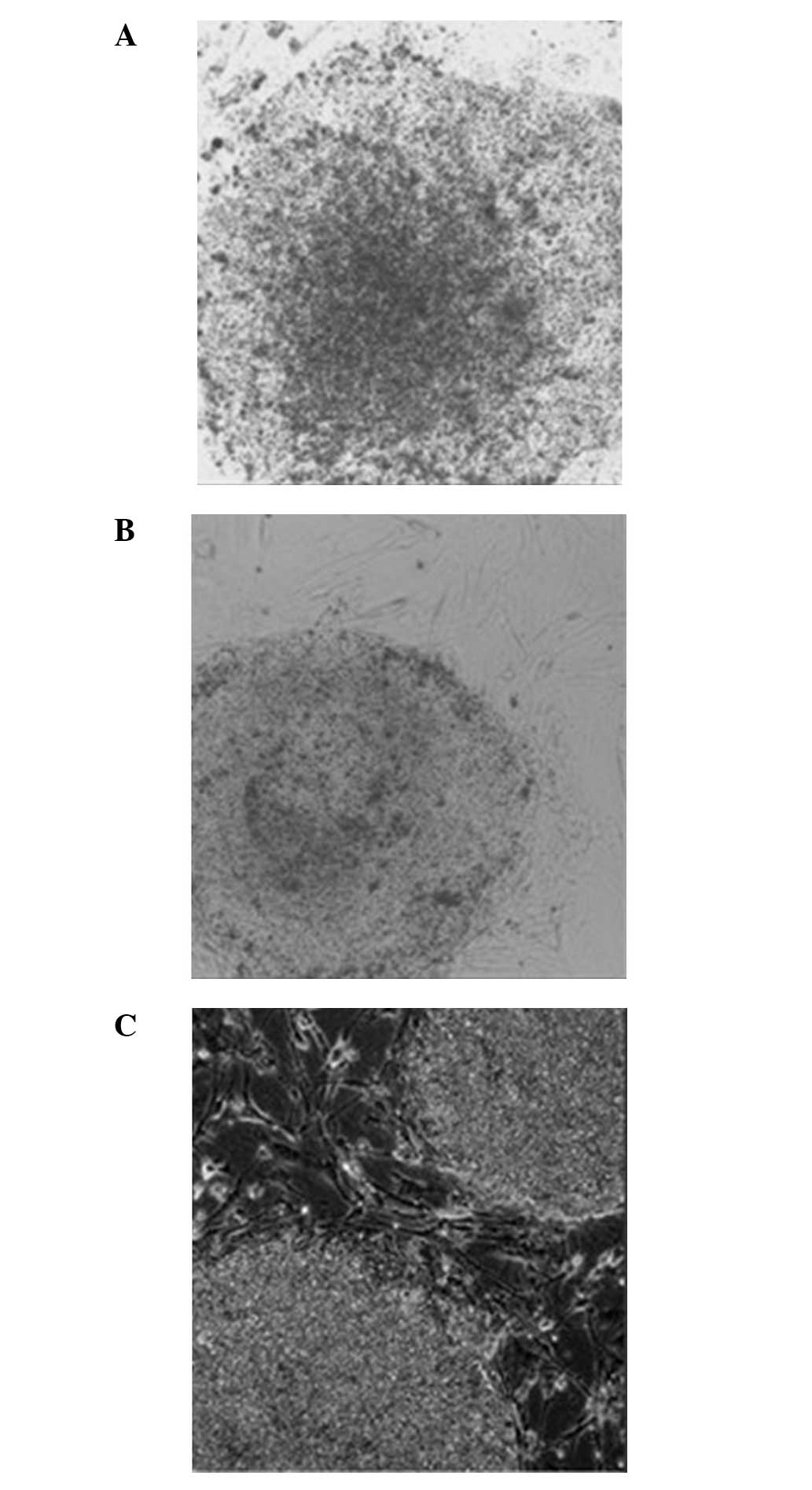
The hESCs grown on the MEF (0:1) feeder layer had a
clear clonal edge with distinct boundary with the surrounding
cells. However, the clones were flat without obvious upheaving
(Fig. 5A). These clones appeared
‘thin’ (Fig. 5A). The hESCs grown on
the hFF (1:0) feeder layer exhibited a clear boundary with the
surrounding cells. However, the clones were not plump and no
cellular accumulation was observed. In addition, the center of the
clonal mass was prone to differentiation. In addition, the clones
appeared ‘thin’ (Fig. 5B). The hESCs
grown on the mixed (1:1) feeder layer containing hFFs + MEFs had a
clear boundary, significant upheaving and exhibited accumulative
growth. Overall, the clones appeared ‘thick’. The morphology of
hESCs grown on the mixed (1:1) feeder layer was improved compared
with those grown on the MEF (0:1) or hFF (1:0) feeder layers
(Fig. 5C).
Growth of hESCs among the mixed feeder
layers with various ratios of MEF and hFF
In the feeder layer containing hFFs + MEFs at a
ratio of 1:0, hESCs exhibited a clear clonal boundary; however, the
clones were flat without obvious upheaving. Similar morphology was
observed in the feeder layer containing hFF + MEFs at a ratio of
1:2. In the feeder layer containing hFFs + MEFs at a ratio of 1:1,
the hESCs exhibited a clear clonal boundary and accumulative
growth. In addition, the clones had clear upheaving and were plump
and the cells had tight connections. Similar results were observed
when the ratio of MEF and hFF was 1:2. When the ratio of MEF and
hFF was 0:1, the edge of the hESC clones was evident. However, the
clones were not plump, with no obvious upheaving, and the center
was prone to differentiation.
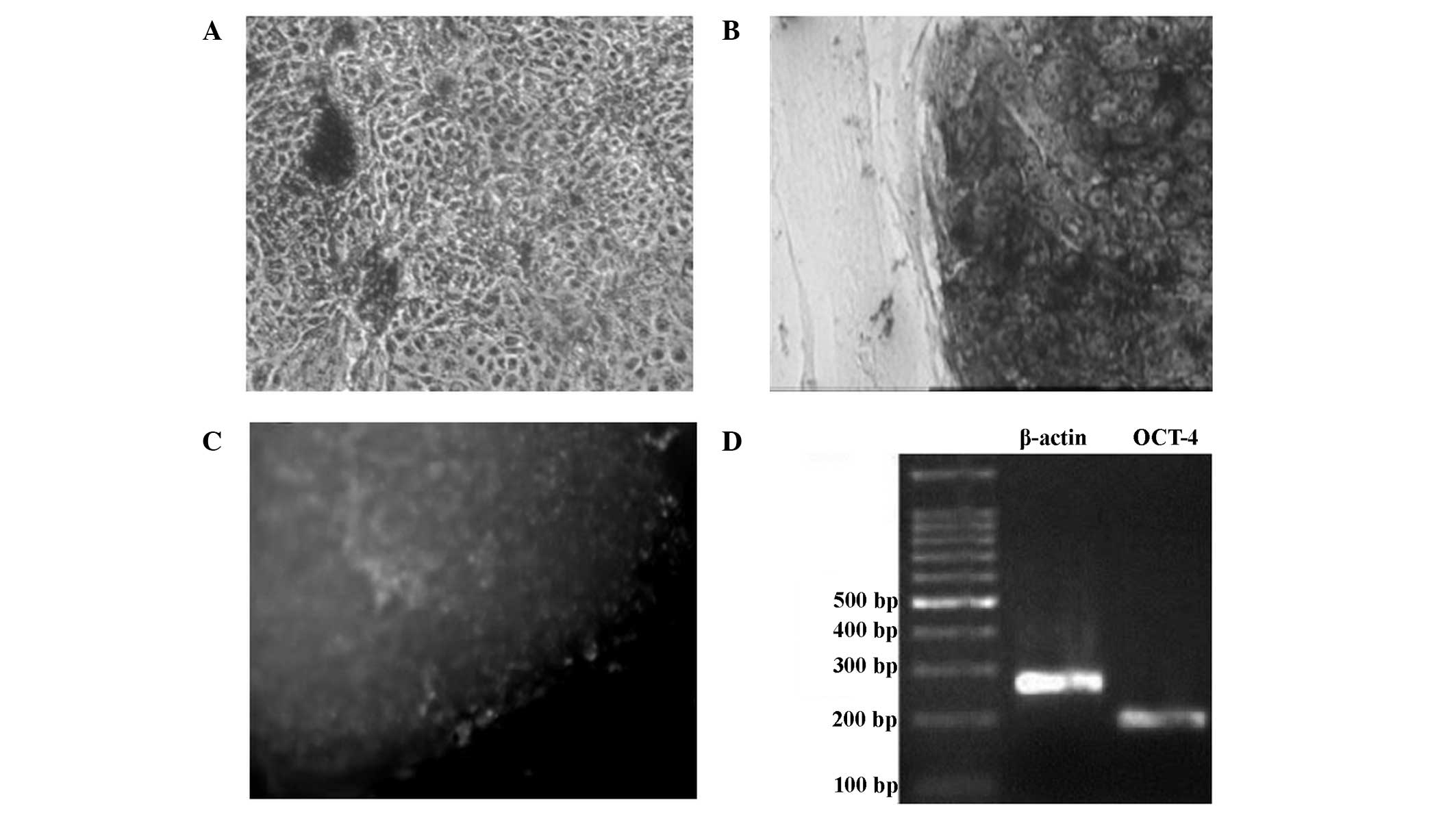
Detection of non-differentiation
status in the hESCs grown on the mixed feeder layers
The feeder layer cells and differentiated cells were
negative for AKP staining (Fig. 6A).
hESCs grown on the mixed feeder layers were stained dark purple by
the AKP staining, indicating a marked positive levels of AKP
expression, and that these cells were non-differentiated (Fig. 6B). Immunohistochemical analysis for
the detection of OCT-4 showed that non-differentiated hESCs
exhibited a green color, indicating that OCT-4 was markedly
expressed (Fig. 6C). The results of
the RT-PCR analysis showed that OCT-4 mRNA expression was present
in the non-differentiated hESCs (Fig.
6D).
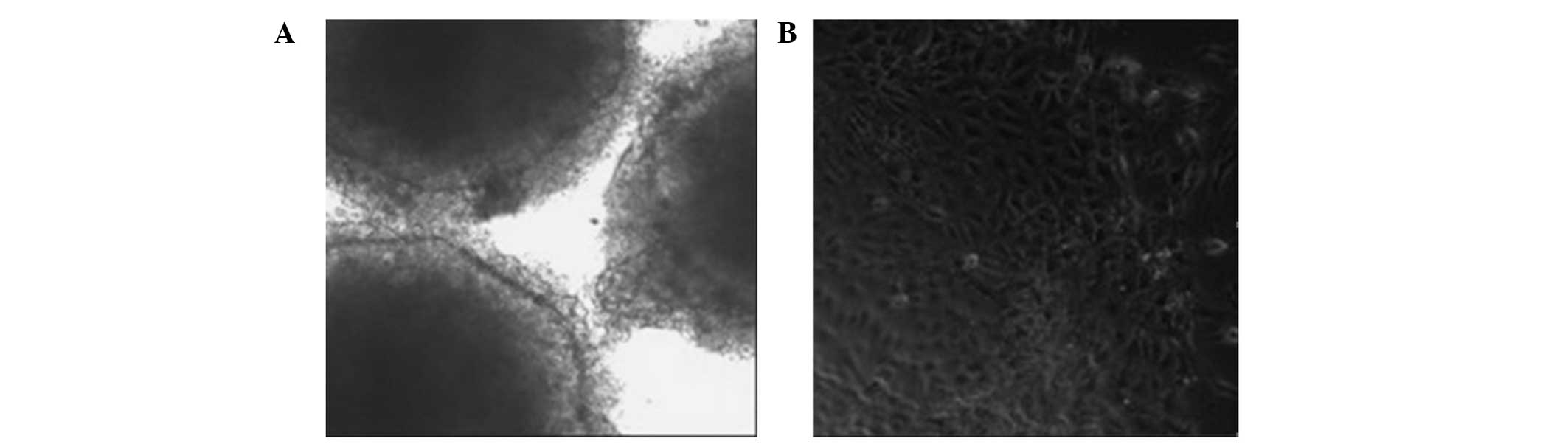
Formation of embryonic bodies
Following the removal of the feeder layer, the
remaining hESCs were able to form embryonic bodies in the
suspension cultures (Fig. 7A).
Following the achievement of adherence, the hESCs were able to
differentiate into cells with multiple morphologies. Normal hESCs
had large nuclei and a high nuclear-cytoplasmic ratio. When induced
to differentiate in vitro, they were able to form the
endoderm, desoderm and ectoderm layers (Fig. 7B).
Discussion
Different cell types in feeder layers are able to
secrete various growth factors to maintain the pluripotency and
non-differentiation of growth of hESCs. Currently, the ability of
the culture medium to support the growth of hESCs is evaluated by
detecting the expression of markers on the hESCs (16). However, it remained unclear whether
the factors secreted by the feeder layer were involved in the
maintenance of non-differentiation status of hESCs. Cytokines that
maintain the pluripotency, self-renewal and non-differentiation
status of hESCs may be directly added to the medium, or secreted by
the feeder layers, or activated by the feed layer cells. It is
difficult for the culture system alone, without a feeder layer, to
maintain non-differentiation growth if exogenous cytokines are not
added (17). The cytokine secretion
profiles of feeder layers are complicated, and it is unknown what
factors secreted by the feeder layer are involved in promoting the
proliferation and inhibition of hESC differentiation. It is
commonly accepted that the addition of bFGF is beneficial for the
growth of hESCs, and bFGF has been widely applied in hESC culturing
systems with or without a feeder layer (18). A previous study demonstrated that
bFGF promotes the proliferation of hESCs by binding to the bFGF
receptors expressed on hESCs, resulting in the upregulation of HLA
class I molecules, the downregulation of human leukocyte antigen-DR
and the activation of genes associated with the cell proliferation
(19). Saxena et al (11) showed that the ability of bFGF to
support the self-renewal of hESCs was necessary. In addition,
self-renewal was achieved by the activation of the PI3K/PKB pathway
and upregulation of integrin α6/β1.
The results of the present study show that the MEF
(0:1) feeder layer did not secrete detectable levels of bFGF. By
contrast, the hFF (1:0) feeder layer secreted bFGF. After 6 h of
inoculation, bFGF expression was detectable in the hFF tissues. The
quantity of bFGF secreted by the hFFs after 24 h of inoculation was
not significantly different compared with after 3 days of
inoculation, suggesting that an hFF feeder layer may be utilized
after 3 days of treatment with mitomycin C. These results are
consistent with a previous report (20), which demonstrated that an hFF feeder
layer is able to survive for an extended period following treatment
with mitomycin C. Thus, hFFs may be superior to MEFs for use in
feeder layers to support the growth of hESCs. In the present study,
exogenous bFGF (optimal concentration, 4 ng/ml) was added to the
MEF feeder layer, while bFGF was not added to the hFF feeder layer.
The results showed that hESCs exhibited marked adherent growth on
MEF and hFF feeder layers. However, the non-differentiation rate of
hESCs on the hFF feeder layer was reduced compared with that of
hESCs grown on the MEF feeder layer.
Previous studies have evaluated the impact of bFGF
on the non-differentiation growth of hESCs in an MEF culturing
system (21,22). Zhou et al (21) showed that bFGF induced MEF in a
concentration-dependent manner, and the optimal concentration of
bFGF to induce MEFs was 4 ng/ml. In addition, bFGF functions in
MEFs, but not hESCs. These results demonstrate that bFGF is
necessary for MEFs to support the non-differentiation growth of
hESCs. Xu et al (22) showed
that hESCs are able to maintain stable proliferation, pluripotency
and non-differentiation status if grown on an MEF feeder layer
supplemented with 160 or 250 ng/ml bFGF. Therefore, the
microenvironment of the different feeder layers varied. Currently,
the optimal concentration of bFGF required to support the growth of
hESCs is unknown. The function of bFGF may be affected by its
source (secreted or exogenous), purity, concentration and the
expression of bFGF receptor in hESCs.
It remains unclear whether the inhibition of mitosis
by mitomycin C (23) may promote the
secretion of new proteins or alter the secretion of growth factors
by feeder layer cells. In the present study, mitomycin C treatment
did not appear to inhibit the secretion of bFGF by hFFs. The peak
bFGF content was detected during the initial stage of hESC
inoculation in the hFF feeder layer. Following the adherent growth
of hESCs, the bFGF content was significantly reduced. The
concentration of bFGF in the MEF culturing system containing 4
ng/ml exogenous bFGF was reduced to 7 pg/ml after 24 h of hESC
growth. Thus, it was hypothesized that bFGF is able to enhance the
adherence and clone formation rate and support the proliferation of
hESCs at the initial stages of growth. In addition, bFGF content
was moderately increased during the initial period of hESC
inoculation in the MEF and hFF feeder layers, which is consistent
with a previous study (24),
indicating that hESCs are able to regulate their own self-renewal
and facilitate the secretion of bFGF.
In previous studies involving hESC lines and in
vitro culture, fibroblasts obtained from fetal mice at
12.5–14.5 days pregnancy were used as feeder cells to support the
growth of hESCs (25,26). Furthermore, a number of cell types
have been reported to be able to support hESC growth in
vitro, including hFFs (5–8), human
endometrial cells (27), human
placental fibroblasts (28), human
fetal skin cells (29) and
hESC-derived fibroblasts (30–32). In
the present study, MEFs and hFFs were shown to be able to support
hESC growth in vitro; however, the common problem remained
that the density of hESCs was low.
In conclusion, the present study combined MEFs and
hFFs at various ratios (1:1, 2:1 and 1:2) to produce a mixed feeder
layer in order to support the growth of hESCs in vitro. The
results indicated that the mixed feeder layer was able to promote
the growth of hESCs, with the hESCs remaining in an
undifferentiated state. Furthermore, the results suggested that a
hFFs + MEFs feeder layer at a ratio of 1:1 and 1:2 produced
comparable results, and were significantly more supportive of
cellular growth, as compared with non-mixed feeder layers. This may
be due to the weak adherence of MEFs, as compared with hFFs. Since
the MEFs after passage five were unable to support the
proliferation of hESCs, it may be that the factors required for
stem cell growth were predominantly derived from the hFFs. The
mixed feeder layer established in the present study may not only
support the growth of hESCs in vitro, but also replace the
conventional single cell feeder layer.
Acknowledgements
The present study was supported by the Scientific
and Technological Project of Guangxi Province (grant no.
0993003A-23) and the Major Science and Technology of Nanning (grant
no. 200801024C).
References
|
1
|
Whyte M, Hubbard R, Meliconi R, Whidborne
M, Eaton V, Bingle C, Timms J, Duff G, Facchini A, Pacilli A, et
al: Increased risk of fibrosing alveolitis associated with
interleukin-1 receptor antagonist and tumor necrosis factor-alpha
gene polymorphisms. Am J Respir Crit Care Med. 162:755–758. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bjorklund LM, Sánchez-Pernaute S, Chung S,
Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG,
Wahlestedt C, Kim KS and Isacson O: Embryonic stem cells develop
into functional dopaminergic neurons after transplantation in a
Parkinson rat model. Proc Natl Acad Sci USA. 99:2244–2349. 2002.
View Article : Google Scholar
|
|
3
|
Brüstle O, Jones KN, Learish RD, Karram K,
Choudhary K, Wiestler OD, Duncan ID and McKay RD: Embryonic stem
cell-derived glial precursors: A source of myelinating transplants.
Science. 285:754–756. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu S, Qu Y, Stewart TJ, Howard MJ,
Chakrabortty S, Holekamp TF and McDonald JW: Embryonic stem cells
differentiate into oligodendrocytes and myelinate in culture and
after spinal cord transplantation. Proc Natl Acad Sci USA.
97:6126–6131. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amit M, Margulets V, Segev H, Shariki K,
Laevsky I, Coleman R and Itskovitz-Eldor J: Human feeder layers for
human embryonic stem cells. Biol Reprod. 68:2150–2156. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hovatta O, Mikkola M, Gertow K, Strömberg
AM, Inzunza J, Hreinsson J, Rozell B, Blennow E, Andäng M and
Ahrlund-Richter L: A culture system using human foreskin
fibroblasts as feeder cells allows production of human embryonic
stem cells. Hum Reprod. 18:1404–1409. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inzunza J, Gertow K, Strömberg MA,
Matilainen E, Blennow E, Skottman H, Wolbank S, Ahrlund-Richter L
and Hovatta O: Derivation of human embryonic stem cell lines in
serum replacement medium using postnatal human fibroblasts as
feeder cells. Stem Cells. 23:544–549. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nieto A, Cabrera CM, Catalina P, Cobo F,
Barnie A, Cortés JL, del Barroso Jesus A, Montes R and Concha A:
Effect of mitomycin-C on human foreskin fibroblasts used as feeders
in human embryonic stem cells: Immunocytochemistry MIBI score and
DNA ploidy and apoptosis evaluated by flow cytometry. Cell Biol
Int. 31:269–278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thomson JA, Itskovitz-Eldor J, Shapiro SS,
Waknitz MA, Swiergiel JJ, Marshall VS and Jones JM: Embryonic stem
cell lines derived from human blastocytes. Science. 282:1145–1147.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Troy TC, Rahbar R, Diker B and Turksen K:
Immunolocalization in the epidermis. Methods Mol Biol. 289:113–120.
2005.PubMed/NCBI
|
|
11
|
Saxena S, Hanwate M, Deb K, Sharma V and
Totey S: FGF2 secreting human fibroblast feeder cells: A novel
culture system for human embryonic stem cells. Mol Reprod Dev.
75:1523–1532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gavrilov S, Marolt D, Douglas NC, Prosser
RW, Khalid I, Sauer MV, Landry DW, Vunjak-Novakovic G and
Papaioannou VE: Derivation of two new human embryonic stem cell
lines from nonviable human embryos. Stem Cells Int.
2011:7653782011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vaajasaari H, Ilmarinen T, Juuti-Uusitalo
K, Rajala K, Onnela N, Narkilahti S, Suuronen R, Hyttinen J,
Uusitalo H and Skottman H: Toward the defined and xeno-free
differentiation of functional human pluripotent stem cell-derived
retinal pigment epithelial cells. Mol Vis. 17:558–575.
2011.PubMed/NCBI
|
|
14
|
Ding Y, Yang H, Yu L, Xu CL, Zeng Y, Qiu Y
and Li DS: Feeder-free and xeno-free culture of human pluripotent
stem cells using UCBS matrix. Cell Biol Int. 39:1111–1119. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coulombe PA and Wong I: Cytoplasmic
intermediate filaments revealed as dynamic and multipurpose
scaffolds. Nat Cell Biol. 6:699–706. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu C, Inokuma MS, Denham J, Golds K, Kundu
P, Gold JD and Carpenter MK: Feeder-free growth of undifferentiated
human embryonic stem cells. Nat Biotechnol. 19:971–974. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirai H, Karian P and Kikyo N: Regulation
of embryonic stem cell self-renewal and pluripotency by leukaemia
inhibitory factor. Biochem J. 438:11–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salli U, Fox TE, Carkaci-Salli N, Sharma
A, Robertson GP, Kester M and Vrana KE: Propagation of
undifferentiated human embryonic stem cells with nano-liposomal
ceramide. Stem Cells Dev. 18:55–65. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yabut O and Bernstein HS: The promise of
human embryonic stem cells in aging-associated diseases. Aging
(Albany NY). 3:494–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang YC, Wang TW, Sun JS and Lin FH:
Investigation of mitomycin-C-treated fibroblasts in 3-D collagen
gel and conditioned medium for keratinocyte proliferation. Artif
Organs. 30:150–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou YP, Rochat A, Hatzfeld A, Peiffer I,
Barbet R, Hatzfeld J and Li ML: bFGF-stimulated MEF-conditioned
medium is capable of maintaining human embryonic stem cells. Fen Zi
Xi Bao Sheng Wu Xue Bao. 42:193–199. 2009.(In Chinese). PubMed/NCBI
|
|
22
|
Xu HF and Suming Z: Feeder-free growth of
human embryonic stem cells supported by basic fibroblast growth
factor. Zhong Guo Zu Zhi Gong Cheng Yan Jiu Yu Lin Chuang Kang Fu.
14:1111–1114. 2010.(In Chinese).
|
|
23
|
Chiba S, Lee YM, Zhou W and Freed CR:
Noggin enhances dopamine neuron production from human embryonic
stem cells and improves behavioral outcome after transplantation
into Parkinsonian rats. Stem Cells. 26:2810–2120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eiselleova L, Peterkova I, Neradil J,
Slaninova I, Hampl A and Dvorak P: Comparative study of mouse and
human feeder cells for human embryonic stem cells. Int J Dev Biol.
52:353–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li SS, Liu YH, Tseng CN, Chung TL, Lee TY
and Singh S: Characterization and gene expression profiling of five
new human embryonic stem cell lines derived in Taiwan. Stem Cells
Dev. 15:532–555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mandal A, Tipnis S, Pal R, Ravindran G,
Bose B, Patki A, Rao MS and Khanna A: Characterization and in vitro
differentiation potential of a new human embryonic stem cell line,
ReliCellhES1. Differentiation. 74:81–90. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JB, Lee JE, Park JH, Kim SJ, Kim MK,
Roh SI and Yoon HS: Establishment and maintenance of human
embryonic stem cell lines on human feeder cells derived from
uterine endometrium under serum-free condition. Biol Reprod.
72:42–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Genbacev O, Krtolica A, Zdravkovic T,
Brunette E, Powell S, Nath A, Caceres E, McMaster M, McDonagh S, Li
Y, et al: Serum-free derivation of human embryonic stem cell lines
on human placental fibroblast feeders. Fertil Steril. 83:1517–1529.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Richards M, Tan S, Fong CY, Biswas A, Chan
WK and Bongso A: Comparative evaluation of various human feeders
for prolonged undifferentiated growth of human embryonic stem
cells. Stem Cells. 21:546–556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu C, Jiang J, Sottile V, McWhir J,
Lebkowski J and Carpenter MK: Immortalized fibroblast-like cells
derived from human embryonic stem cells support undifferentiated
cell growth. Stem Cells. 22:972–980. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Q, Fang ZF, Jin F, Lu Y, Gai H and
Sheng HZ: Derivation and growing human embryonic stem cells on
feeders derived from themselves. Stem Cells. 23:1221–1227. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stojkovic P, Lako M, Stewart R, Przyborski
S, Armstrong L, Evans J, Murdoch A, Strachan T and Stojkovic M: An
autogeneic feeder cell system that efficiently supports growth of
undifferentiated human embryonic stem cells. Stem Cells.
23:306–314. 2005. View Article : Google Scholar : PubMed/NCBI
|