Introduction
Staphylococcus aureus (S. aureus), a
bacterium that grows in the human nose and skin and a major
pathogen for skin and soft-tissue infections, has previously been
treated with methicillin. S. aureus is a gram-positive
bacterium and a major nosocomial agent, responsible for several
hospital-acquired infections, including bacteremia, skin infections
and septic shock (1–3). Hospital-acquired infections comprise a
serious problem, particularly due to the increased prevalence of
methicillin-resistant S. aureus (MRSA) (4,5). This
pathogen is associated with various infectious diseases and has a
mortality rate of 36–50% (6,7). With increasing resistance to various
antibiotics, combination therapy appears to be a useful option,
particularly in developing countries where antibiotic availability
is limited (8–10). Furthermore, MRSA strains have been
found to be resistant not only to β-lactam antibiotics, but also to
fluoroquinolones and other types of antibiotics (8).
Luteolin (LUT), a polyphenolic flavonoid compound,
is present in numerous plant groups, including
Magnoliophyta, Pinophyta, Pteridophyta and
Bryophyta species. Dietary sources of LUT include carrots,
peppers, celery, olive oil, peppermint, thyme, rosemary and
oregano. LUT is considered to have diverse biological benefits,
including cardioprotective, antioxidant, anti-inflammatory and
anticancer activities (11–14). In addition, LUT has exhibited a
strong antiproliferative activity against various human cancer cell
lines, such as lung cancer cell lines, and is a widely used
ingredient in nutritional supplements (13,14).
However, the antimicrobial activity of LUT against S. aureus
has not yet been fully elucidated, but merits investigation.
The aim of the present study was to investigate the
potential of using LUT in combination with antibiotics as an
alternative therapeutic regime to overcome the drug-resistance of
MRSA strains, including reference strains and clinical
isolates.
Materials and methods
Reagents
Ampicillin (AM), oxacillin (OX), gentamicin (GT) and
luteolin (≥98.0%) were purchased from Sigma-Aldrich (St. Louis, MO,
USA). The structure of luteolin is shown in Fig. 1.
Bacterial strains and growth
conditions
Five clinical MRSA isolates (DPS-1 to −5) were
obtained from 5 different patients admitted to the Wonkwang
University Hospital (Iksan, South Korea), and two reference strains
were acquired from American Type Culture Collection (ATCC;
Manassas, VA, USA): S. aureus ATCC 33591 (MRSA) and S.
aureus ATCC 25923 (methicillin-sensitive S. aureus;
MSSA). Prior to each experiment, bacteria were stored in 30%
glycerol and frozen at −70°C. They were then cultured in
Mueller-Hinton broth (MHB) and Mueller-Hinton agar (MHA; Difco; BD
Biosciences, Sparks, MD, USA) by incubating at 37°C for 24 h.
Minimum inhibitory concentration
(MIC)
MICs were determined using the broth microdilution
method according to the guidelines of the Clinical and Laboratory
Standards Institute (15). Briefly,
a microorganism suspension was prepared by growing bacteria in
broth for 24 h, and adjusting to a 0.5 McFarland standard turbidity
(~1.5×108 CFU/ml). Final inoculums were adjusted to
1.5×106 CFU/ml. Serially diluted antibiotics and/or LUT
were then incubated with the inoculum at 37°C for 18 h. The MIC was
the lowest effective concentration of antimicrobial agent. At the
end of the incubation period, the wells were visually examined for
turbidity. Cloudiness indicated that bacterial growth had not been
inhibited at the concentration of antimicrobial agent in the
medium.
Checkerboard dilution test
Synergistic combinations were investigated using the
preliminary checkerboard method (16). The MIC was defined as the lowest drug
concentration, either alone or in combination, that inhibited
visible bacterial growth. In vitro interactions were
quantified by the fractional inhibitory concentration (FIC) index,
which was calculated as FIC = (MIC of drug A in combination/MIC of
drug A alone) + (MIC of drug B in combination/MIC of drug B alone).
FIC indices (FICI) were interpreted as follows: ≤0.5, synergy;
<0.5-≤0.75, partial synergy; >0.75-≤1.0, additive effect;
>1.0-≤4.0, indifference; and >4.0, antagonism. Finally, the
varying rates of synergy between two given agents were determined
(17). All experiments were
conducted in triplicate.
Methyl thiazolyl tetrazolium assay
(MTT) colorimetric assay
An MTT colorimetric assay for rapid detection of the
presence of bacteria was performed as previously described
(18–20). Briefly, a stock solution of 5 mg/ml
MTT (Sigma-Aldrich) was prepared in phosphate-buffered saline and
kept at −70°C. A final concentration of 1 mg/ml MTT was used in the
assay. Following incubation of the bacteria for 24 h at 37°C, 20 µl
yellow MTT was added to a 96-well microtiter plate and incubation
was continued for an additional 20 min. Blue color indicated the
presence of bacteria.
Time-kill curve assay
A time-kill curve assay was performed as previously
described (21) to study the effects
of antimicrobial agent concentration on bacterial growth over time.
A standard inoculum of ~106 CFU/ml was used. LUT (0.5
MIC) was used with antibiotics (0.5 MIC) in various combinations. A
test plate containing only MHB plus inoculum served as the control.
Viable strains were counted at different intervals up to 24 h at
37°C. The rate and extent of bacterial death were determined by
plotting viable colony counts (CFU/ml) against time in MHA. All
experiments were conducted in triplicate.
Statistical analysis
All experiments were performed at least in
triplicate. The data obtained from the experiments are presented as
the mean ± standard error. The statistical analysis was performed
using one-way analysis of variance followed by Dunnett's t-test
(SPSS software 19.0; IBM Corp., Armonk, NY, USA). P<0.01 was
considered to indicate a statistically significant difference.
Results
MICs and synergistic effect
Against all strains, the MIC of LUT was 62.5 µg/ml.
The synergistic effects of LUT with various antibiotics were
evaluated in MRSA strains using a checkerboard dilution assay. The
antibacterial effects of LUT alone, each antibiotic alone, and LUT
combined with one of the three antibiotics (AM, OX and GT) are
shown in Tables I–III. The presence of LUT reduced the MIC
of these antibiotics markedly against S. aureus strains. All
strains were found to be resistant to AM, OX and GT, with MIC
values ranging from 15.63 to 1,000 µg/ml. LUT + antibiotic
combinations exhibited markedly decreased MICs. In combination with
LUT, the MICs of AM, OX and GT underwent 2–16-, 0–16- and 4–16-fold
reductions, respectively. None of the aforementioned combinations
exhibited an antagonistic effect. These findings demonstrate the
promising potential of LUT plus antibiotic combination therapy in
the suppression of MRSA growth.
 | Table I.Interpreted FICI response for LUT + AM
combinations against MRSA and MSSA strains. |
Table I.
Interpreted FICI response for LUT + AM
combinations against MRSA and MSSA strains.
|
| MIC (µg/ml) |
|
|---|
|
|
|
|
|---|
|
| LUT | AM |
|
|---|
|
|
|
|
|
|---|
| Strain | Alone | +AM | Alone | +LUT | FICI |
|---|
| ATCC 33591 | 62.5 | 3.9 | 500 | 250 | 0.562 |
| ATCC 25923 | 62.5 | 3.9 | 15.63 | 0.975 | 0.125 |
| DPS-1 | 62.5 | 3.9 | 1,000 | 62.5 | 0.125 |
| DPS-2 | 62.5 | 3.9 | 1,000 | 62.5 | 0.125 |
| DPS-3 | 62.5 | 3.9 | 250 | 15.63 | 0.125 |
| DPS-4 | 62.5 | 3.9 | 250 | 15.63 | 0.125 |
| DPS-5 | 62.5 | 3.9 | 125 | 7.81 | 0.125 |
 | Table III.Interpreted FICI response for LUT +
GT combinations against MRSA and MSSA strains. |
Table III.
Interpreted FICI response for LUT +
GT combinations against MRSA and MSSA strains.
|
| MIC (µg/ml) |
|
|---|
|
|
|
|
|---|
|
| LUT | GT |
|
|---|
|
|
|
|
|
|---|
| Strain | Alone | +GT | Alone | +LUT | FICI |
|---|
| ATCC33591 | 62.5 | 3.9 | 62.5 | 3.9 | 0.125 |
| ATCC25923 | 62.5 | 3.9 | 62.5 | 3.9 | 0.125 |
| DPS-1 | 62.5 | 3.9 | 250 | 62.5 | 0.312 |
| DPS-2 | 62.5 | 31.25 | 500 | 31.25 | 0.562 |
| DPS-3 | 62.5 | 3.9 | 500 | 31.25 | 0.125 |
| DPS-4 | 62.5 | 3.9 | 500 | 31.25 | 0.125 |
| DPS-5 | 62.5 | 3.9 | 500 | 62.5 | 0.187 |
The combined use of LUT and AM, OX or GT antibiotics
against the ATCC 33591 MRSA strain resulted in a FICI of 0.125 or
0.562 (Tables I–III). None of the combinations exhibited
an antagonistic effect (FICI, >4.0). These results demonstrate
that combinations of LUT with antibiotics could be used for the
suppression of MRSA growth.
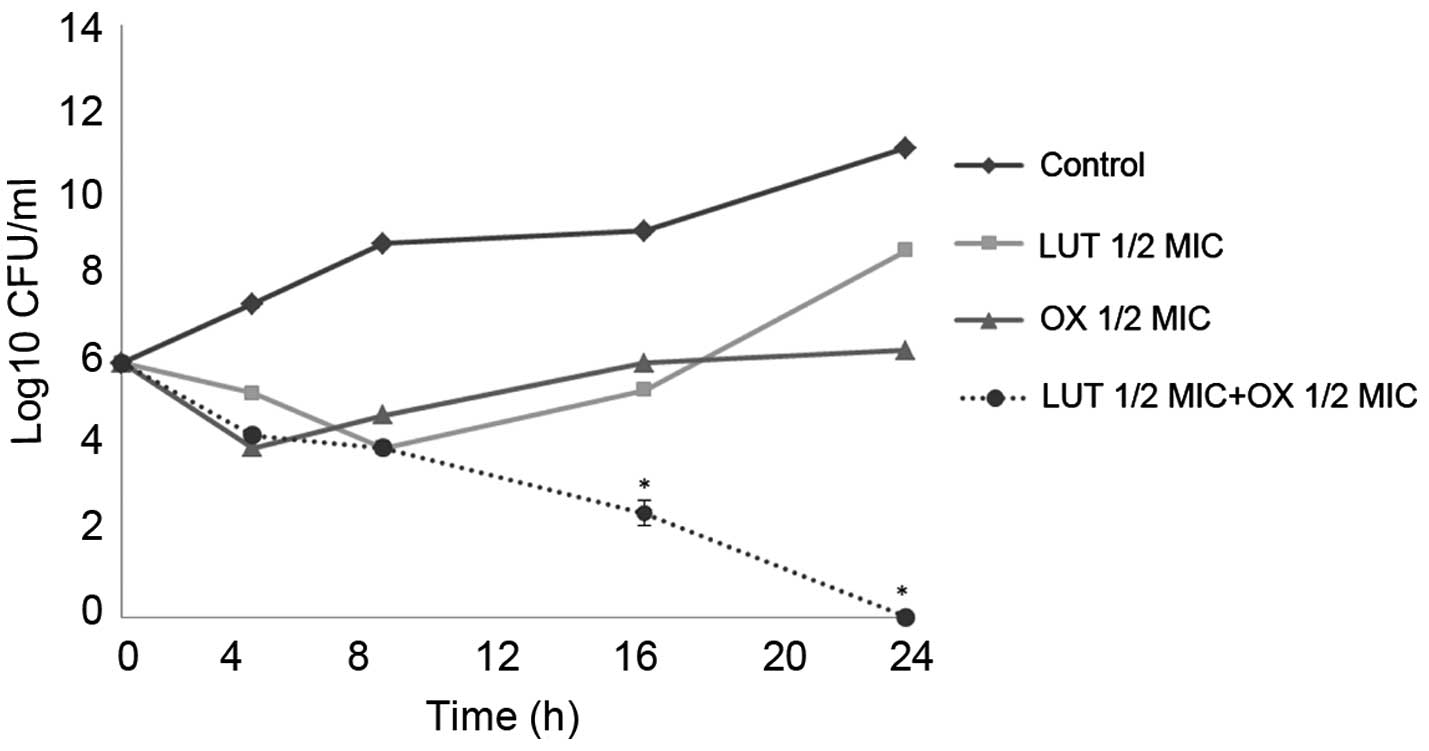
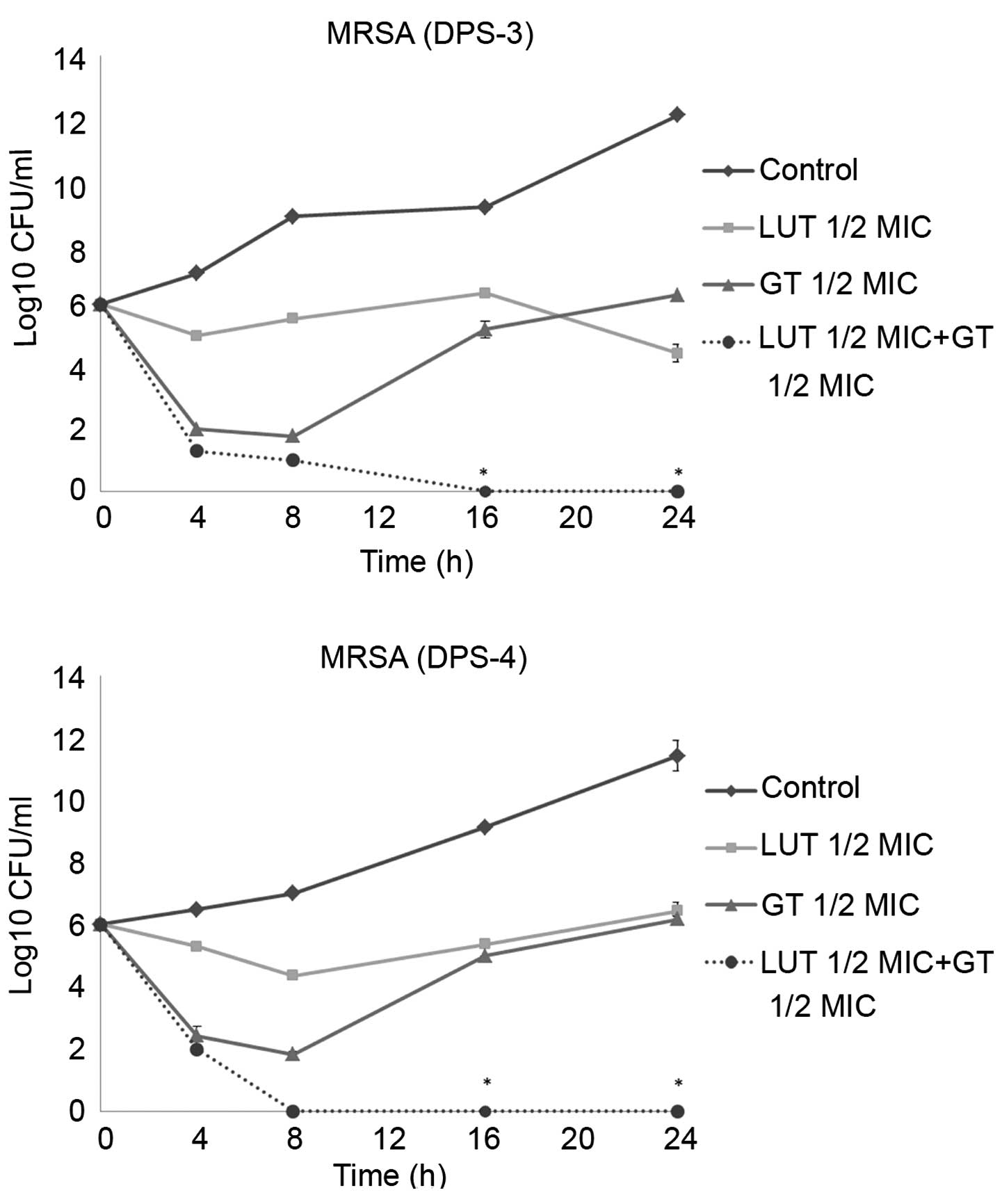
Time-kill curve assay
Time-kill tests were performed to examine the
synergistic effects of LUT and antibiotics over time. The control
did not exhibit a reduction in CFU counts, and LUT or antibiotic
alone did not induce cell death after 24 h. When used together, LUT
and antibiotics markedly reduced bacterial counts. As shown in
Figs. 2 and 3, the combination of ½MIC LUT + ½MIC
antibiotic completely Notably, the combination of ½MIC LUT + ½MIC
GT, completely inhibited the growth of S. aureus after 16
h.
Discussion
The resistance of S. aureus to drugs,
including synthetic penicillin and other conventional antibiotics,
is a major obstacle in the treatment MRSA infection, which is an
increasingly common type of infection, particularly among
hospitalized patients (22). While
vancomycin is often used as the only remaining effective antibiotic
against MRSA, even vancomycin-resistant species of S. aureus
have now been reported (22,23). In the absence of any effective
antibiotic treatment for multi-resistant infections, the
development of alternative methods for the prevention and treatment
of these diseases is required (22–24).
A consequence of the increase in the prevalence of
resistant bacteria is that novel antimicrobial drugs active against
infectious diseases are urgently required. Novel antimicrobial
agents with no associated toxic or side effects may be developed
from natural products. Combination therapy is the most commonly
recommended empirical treatment for bacterial infections in
intensive care units, since not all potential pathogens are
susceptible to monotherapy; in addition, it may aid the prevention
of antibacterial resistance (25).
The aim of the present study was to investigate combination therapy
with LUT and antibiotics against S. aureus, in order to find
a solution to the problem of multi-drug resistance.
To the best of our knowledge, this is the first
study investigating the potential of using antibiotics in
combination with LUT in the treatment of MRSA and MSSA. As an
indicator of the potency of LUT and antibiotic combinations against
resistant strains of S. aureus, MIC values were determined.
The antimicrobial activity of LUT was found to be moderate (MIC,
62.5 µg/ml). Checkerboard dilution tests were performed to
determine the action of LUT alone, as well as its synergistic
action with antibiotics against the 7 strains. Although LUT alone
had only a moderate inhibitory effect on MRSA growth, when a
non-growth inhibitory dose of LUT (62.5 µg/ml) or antibiotic was
used, the combination was shown to be highly effective, with a FICI
of 0.125–0.562. Similar effects were observed in the MSSA strain.
This experimental method clearly showed that LUT was a potent MRSA
growth inhibitor. The use of three different antibiotics, namely
AM, OX and GT, in combination with LUT clearly suppressed MRSA
growth, enabling the dose of the antibiotics to be reduced. The
time-kill curves confirmed the ability of LUT to increase the
antibacterial effects of the antibiotics, synergistically reducing
the bacterial counts below the lowest detectable limit after 8–24
h. The present study demonstrated the potential of LUT as an
effective therapeutic agent against MRSA, reinforcing the
possibility of substantially reducing the use of existing
antibiotics. The results obtained for the combinations tested in
the current study suggest that LUT can increase susceptibility to
antibacterial action, as well as reduce the inducible antibiotic
resistance of bacteria.
In conclusion, LUT may have potential as an
antibacterial drug candidate for clinical use against MRSA and
MSSA. The results of the present study are promising and may
support the use of drugs derived from natural products.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea,
the Ministry of Education, Science and Technology (grant nos.
2012-0004337 and 2008-0062484), and the Cooperative Research
Program for Agriculture Science & Technology Development
(project no. PJ00962202), Rural Development Administration,
Republic of Korea.
References
|
1
|
National Nosocomial Infections
Surveillance System: National Nosocomial Infections Surveillance
(NNIS) System Report, data summary from January 1992 to June 2002,
issued August 2002. Am J Infect Control. 30:458–475. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Selvey LA, Whitby M and Johnson B:
Nosocomial methicillin-resistant Staphylococcus aureus
bacteremia: Is it any worse than nosocomial methicillin-sensitive
Staphylococcus aureus bacteremia? Infect Control Hosp
Epidemiol. 21:645–648. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
López-Cortés LE, Gálvez-Acebal J, Del Toro
MD, Velasco C, de Cueto M, Caballero FJ, Muniain MA, Pascual A and
Rodríguez-Baño J: Effect of statin therapy in the outcome of
bloodstream infections due to Staphylococcus aureus: A
prospective cohort study. PLoS One. 8:e829582013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lowy FD: Staphylococcus aureus
infections. N Engl J Med. 339:520–532. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DeLeo FR, Otto M, Kreiswirth BN and
Chambers HF: Community-associated meticillin-resistant
Staphylococcus aureus. Lancet. 375:1557–1568. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baltch AL, Ritz WJ, Bopp LH, Michelsen PB
and Smith RP: Antimicrobial activities of daptomycin, vancomycin,
and oxacillin in human monocytes and of daptomycin in combination
with gentamicin and/or rifampin in human monocytes and in broth
against Staphylococcus aureus. Antimicrob Agents Chemother.
51:1559–1562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dancer SJ: The effect of antibiotics on
methicillin-resistant Staphylococcus aureus. J Antimicrob
Chemother. 61:246–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aqil F, Ahmad I and Owais M: Evaluation of
anti-methicillin-resistant Staphylococcus aureus (MRSA)
activity and synergy of some bioactive plant extracts. Biotechnol
J. 1:1093–1102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miranda-Novales G, Leaños-Miranda BE,
Vilchis-Pérez M and Solórzano-Santos F: In vitro activity effects
of combinations of cephalothin, dicloxacillin, imipenem, vancomycin
and amikacin against methicillin-resistant Staphylococcus
spp. strains. Ann Clin Microbiol Antimicrob. 5:252006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kastoris AC, Rafailidis PI, Vouloumanou
EK, Gkegkes ID and Falagas ME: Synergy of fosfomycin with other
antibiotics for Gram-positive and Gram-negative bacteria. Eur J
Clin Pharmacol. 66:359–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
López-Lázaro M: Distribution and
biological activities of the flavonoid luteolin. Mini Rev Med Chem.
9:31–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin Y, Shi R, Wang X and Shen HM:
Luteolin, a flavonoid with potential for cancer prevention and
therapy. Curr Cancer Drug Targets. 8:634–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seelinger G, Merfort I and Schempp CM:
Anti-oxidant, anti-inflammatory and anti-allergic activities of
luteolin. Planta Med. 74:1667–1677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seelinger G, Merfort I, Wölfle U and
Schempp CM: Anti-carcinogenic effects of the flavonoid luteolin.
Molecules. 13:2628–2651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clinical and Laboratory Standards
Institute (CLSI): Methods for dilution antimicrobial susceptibility
tests for bacteria that grow aerobically; approved standard. CLSI
Document M7-A7 (7th). (Wayne, PA, USA). CLSI. 2006.
|
|
16
|
Odds FC: Synergy, antagonism, and what the
chequerboard puts between them. J Antimicrob Chemother. 52:12003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mazumdar K, Dutta NK, Kumar KA and
Dastidar SG: In vitro and in vivo synergism between tetracycline
and the cardiovascular agent oxyfedrine HCl against common
bacterial strains. Biol Pharm Bull. 28:713–717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scheuber PH, Mossmann H, Beck G and Hammer
DK: Direct skin test in highly sensitized guinea pigs for rapid and
sensitive determination of staphylococcal enterotoxin B. Appl
Environ Microbiol. 46:1351–1356. 1983.PubMed/NCBI
|
|
19
|
Abate G, Mshana RN and Miörner H:
Evaluation of a colorimetric assay based on
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
for rapid detection of rifampicin resistance in Mycobacterium
tuberculosis. Int J Tuberc Lung Dis. 2:1011–1016.
1998.PubMed/NCBI
|
|
20
|
Shi YJ, Chen J and Xu M: A new method for
antimicrobial susceptibility testing of in vitro-cultured bacteria
by means of resonance light scattering technique. J Microbiol
Biotechnol. 18:118–123. 2008.PubMed/NCBI
|
|
21
|
Chang SC, Chen YC, Luh KT and Hsieh WC: In
vitro activities of antimicrobial agents, alone and in combination,
against Acinetobacter baumannii isolated from blood. Diagn
Microbiol Infect Dis. 23:105–110. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roth DM, Senna JP and Machado DC:
Evaluation of the humoral immune response in BALB/c mice immunized
with a naked DNA vaccine anti-methicillin-resistant
Staphylococcus aureus. Genet Mol Res. 5:503–512.
2006.PubMed/NCBI
|
|
23
|
Pearson H: ‘Superbug’ hurdles key drug
barrier. Nature. 418:4692002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohwada A, Sekiya M, Hanaki H, Arai KK,
Nagaoka I, Hori S, Tominaga S, Hiramatsu K and Fukuchi Y: DNA
vaccination by mecA sequence evokes an antibacterial immune
response against methicillin-resistant Staphylococcus
aureus. J Antimicrob Chemother. 44:767–774. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drago L, De Vecchi E, Nicola L and
Gismondo MR: In vitro evaluation of antibiotics' combinations for
empirical therapy of suspected methicillin resistant
Staphylococcus aureus severe respiratory infections. BMC
Infect Dis. 7:1112007. View Article : Google Scholar : PubMed/NCBI
|