Introduction
Chronic obstructive pulmonary disease (COPD) is a
type of obstructive lung disease characterized by progressive
incompletely reversible poor airflow. COPD remains a major threat
to health, as it is the cause of >3 million cases of mortality
worldwide (1,2). Although COPD is incurable, its symptoms
may be alleviated and its progression could be slowed down. The
current available measures for reducing COPD-related mortality
primarily include smoking cessation and supplemental oxygen
(3). The limiting outcomes of these
measures highlight the need to for novel, potent and cost-effective
therapeutics for COPD (3). Although
it has been demonstrated that hypoxia-induced pulmonary
vasoconstriction (HPV) is a major initial factor of COPD, COPD is
often accompanied by hypoxia and hypercapnia. Furthermore, hypoxia
and hypercapnia-induced pulmonary vasoconstriction (HHPV) and
pulmonary hypertension (PH) are common complications of COPD
(4–6). Therefore, studies investigating the
alleviation of HHPV are of considerable significance in the
management of COPD.
Panax notoginseng saponins (PNS), are
extracted from P. notoginseng, a traditional Chinese
medicinal herb. The main active component of the plant is panax
notoginsenoside Rb1 (Rb1). Previous studies have suggested various
protective effects of PNS on various cardiovascular diseases
(7). For example, PNS could
ameliorate atherogenesis in rabbit models by decreasing blood
lipids and inhibiting inflammation (8). Furthermore, there is evidence that the
protective effect of PNS on atherosclerotic (AS) rats may be
mediated by increasing liver X receptor alpha expression, which is
related to inflammation and lipid metabolism (9). Furthermore, in vivo study has
indicated that total saponins of P. notoginseng may inhibit
vessel restenosis following vascular intimal injury by blocking the
proliferation of vascular smooth muscle cells and regulating
extracellular matrix protein accumulation in the endometrium
(10). However, there were are few
studies concerning the impact of PNS on HHPV.
Mitogen-activated protein kinases (MAPK) pathways
primarily include extracellular signal-regulated kinase (ERK1/2),
p38 mitogen-activated protein kinase (p38 MAPK) and JNK pathways.
It has been established that MAPK pathways are involved in smooth
muscle cell proliferation, migration and regulation of
intracellular calcium in pulmonary arterial smooth muscle cells
(PASMCs) (11,12). Furthermore, a recent study observed
that notoginsenoside R1 could inhibit hypoxia-hypercapnia-induced
vasoconstriction by inhibiting the ERK pathway activation in rat
models (13). Our own unpublished
data showed that PNS could mitigate hypoxia and hypercapnia-induced
PH via regulation of the MAPK pathway. Thus, the present study
aimed to investigate whether Rb1 could protect against HHPV, and
whether its possible underlying mechanism involved MAPK pathways.
PASMCs grown under the conditions of hypoxia and hypercapnia were
used to emulate HHPV in the study. Prior to the hypoxia and
hypercapnia exposure, PASMCs were exposed to various concentrations
of the ginsenoside Rb1. The expression and phosphorylation of ERK
and p38 at the protein and mRNA levels were detected.
Materials and methods
Experimental animals
Ten male specific pathogen-free Sprague-Dawley rats
(weight, 200–220 g) were provided by the Experimental Animal Center
of Wenzhou Medical College [Certificate No, SCXK (Zhe) 2008-0156;
Wenzhou, China]. Animals received food and water ad libitum,
and were housed in an environment at 18–20°C with 65–70% relative
humidity.
Rb1 (purity, >98%) was provided by the Natural
Medicine Research Center of Jilin University (Changchun, China).
Rb1 was dissolved in ultrapure water, prepared as 10 mg/ml stock
solution and stored in the dark at 4°C.
Primary pulmonary artery smooth muscle
cell (PASMC) isolation and culture
Rats were anesthetized via an intraperitoneal
injection of 5% chloral hydrate (0.7 ml/mg; Experimental Animal
Center of Wenzhou Medical University), then soaked in 75% alcohol
for 3 min. Pulmonary artery tissue segments (grade 2–4) were
obtained using a dissecting microscope (Leica Microsystems Holdings
GmbH, Wetzlar, Germany) in a clean platform. Endothelial cells were
removed with a sterile cotton swab. The tissue segments were then
cut into small pieces, and digested with 0.2% collagenase
(Sigma-Aldrich Chemie BV, Zwijndrecht, The Netherlands) for 2–4 h
at 37°C. After centrifugation, the supernatants were discarded and
the precipitates were rinsed with Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
plus 20% Gibco fetal bovine serum (FBS) in a dish (Corning Inc.,
Corning, NY, USA). Then, the dish with a large number of individual
muscle cells was cultured in a 5% CO2 incubator at 37°C.
After 7 days, cells were passaged.
PASMC morphology observation
Cellular morphology was observed under an inverted
phase contrast microscope (CK41; Olympus Corporation, Tokyo,
Japan).
Immunocytochemical detection of smooth
muscle-α-actin in PASMCs
Smooth muscle (SM)-α-actin in the PASMCs was
detected using immunocytochemistry staining, according to the
instructions of an immunohistochemistry kit (SA2008; Boster
Biotechnology Co., Ltd., Wuhan, China). Cells (1.0×104
cells/cm2) grown on glass coverslips were incubated with
mouse polyclonal antibody against SM-α-actin (1:200; sc-130616;
Takara Bio, Inc.). Finally, cells were stained with
3,3′-diaminobenzidine solution (Takara Bio, Inc.,) for
visualization. Hematoxylin (Sigma-Aldrich, St. Louis, MO, USA) was
added to counterstain nuclei. The stained cells were observed under
a light microscope (Olympus Corporation), with brown staining
indicating a positive result. Each slide was randomly observed in
three view fields. The percentage of positive cells in total cells
was calculated as cell purity. PASMCs with a cell purity >95%
were selected for further experiments (14).
Drug intervention and experimental
groups
PASMCs in the logarithmic phase from generations 2–5
were seeded in six-well plates at a density of 5×105
cells/ml, and cultured in high-glucose DMEM medium plus 10% FBS at
37°C in a 5% CO2 incubator. When the dish was ~80%
confluent (monolayer), the cells were divided into five groups:
Normal group (N); hypoxia and hypercapnia group (H); RbL
group, RbM group; and RbH group. N group was
cultured under 5% CO2 and 21% O2 for 24 h; H,
RbL, RbM and RbH groups were
cultured under 6% CO2 and 1% O2 for 24 h.
Prior to hypoxia and hypercapnia exposure, the RbL,
RbM and RbH groups were cultured in
serum-free medium for 24 h, and then exposed to 8, 40 and 100 mg/ml
Rb1 for 30 min, respectively. The H group was treated with the same
volume of ultrapure water (Rb1 vehicle) for 30 min.
Western blot and protein analysis
Cells were harvested, washed with ice-cold PBS three
times and lysed with lysis buffer (Cell Signaling Technology, Inc.,
Beverly, MA, USA) for 30 min on ice. Following centrifugation for 5
min at 4°C (300 × g), the supernatants were collected and protein
concentrations were evaluated using a Bicinchoninic Acid assay kit
(Pierce Biotechnology, Inc., Rockford, IL, USA). Western blot was
performed by adding equal quantities of protein (50 µg) for each
sample loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel
(Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently, the
proteins were transferred onto immobilon-P transfer membranes (EMD
Millipore, Shanghai, China). The membranes were immersed in
blocking solution (5% bovine serum albumin; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) for 2 h and incubated with
appropriate primary antibodies (1:1,000) overnight at 4°C. Then,
the membranes were incubated with appropriate anti-rabbit secondary
antibodies (1:4,000; Cell Signaling Technology, Inc., Danvers, MA,
USA) for 2 h at room temperature. Finally, the membranes were
developed using enhanced chemiluminescence (Super ECL Plus
detection reagent; P1010; Applygen Technologies, Inc., Beijing,
China). Eight duplications were made for each experiment. The
following antibodies used for western blot were purchased from Cell
Signaling Technology, Inc.: P-p38 rabbit monoclonal antibody
(1:1,000; cat. no. 9211); P-ERK rabbit monoclonal antibody
(1:1,000; cat. no. 9101), ERK rabbit monoclonal antibody (1:1,000;
cat. no. 9102), horseradish peroxidase-labeled goat anti-rabbit
secondary antibody (1:1,000; cat. no. 7074). Quantity One gel
software, version 4.4 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) was used to analyze the gray value of protein bands. The
ratios (P-p38 vs. T-p38, P-ERK vs. T-ERK) were defined as relative
value of P-p38 and P-ERK, which were used to express
phosphorylation of p38 and ERK, respectively.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Semi-quantitative RT-PCR was performed to detect
mRNA level of ERK1, ERK2, and p38 using a commercial PCR kit
(Takara Bio, Inc.). ERK1, ERK2 and p38 RNA were extracted from
PASMCs in different groups with TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) and then treated with DNase to remove
genomic DNA. First strand cDNA synthesis was carried out using the
cDNA Cycle kit (Invitrogen; Thermo Fisher Scientific, Inc.) and
used as template DNA. The reverse transcription mixture contained
2.0 µl template RNA, 4 µl 5X reaction buffer, 1 µl Moloney murine
leukemia virus reverse transcriptase (200 U/µl), 1 µl RNase
inhibitor (200 U/µl), 2 µl 10 mmol dNTPs mix and 1 µl
oligo(dT)18 primer.
The PCR mixture included 1 µl template DNA (cDNA),
12.5 µl PCR Master mix (2X), 1 µl forward primer, 1 µl reverse
primer and 9.5 µl nuclease-free water. Primers for ERK1 were:
5′-GCTGAATCACATCCTGGGTAT-3′ (upstream), and
5′-AGATCTGTATCCTGGCTGGAA-3′ (downstream). Amplification for ERK 1
was performed with the following conditions: Pre-denaturation at
94°C for 2 min, denaturation at 98°C for 10 sec, annealing at 54°C
for 15 sec, extension at 72°C for 1 min, and final extension at
72°C for 10 min. The size of the amplified fragment obtained by PCR
was 373 bp.
The primers for ERK2 were:
5′-GCAGGTGTTCGACGTGGGAAT-3′ (upstream), and
5′-GTGCAGAACATTAGGTGAATA-3′ (downstream). Amplification was
performed as follows: Pre-denaturation at 94°C for 2 min,
denaturation at 98°C for 10 sec, annealing at 56°C for 15 sec,
extension at 72°C for 30 sec and final extension at 72°C for 10
min. The size of the amplified fragment obtained by PCR was 394
bp.
Primers for p38 were: 5′-TCCAAGGGCTACACCAAATC-3′
(upstream), and 5′-TGTTCCAGGTAAGGGTGAGC-3′ (downstream).
Amplification was performed as following: Pre-denaturation at 94°C
for 2 min, denaturation at 98°C for 10 sec, annealing at 55°C for
15 sec, extension at 72°C for 1 min and final extension at 72°C for
10 min. The size of the amplified fragment obtained by PCR was 341
bp.
The primers for β-actin were:
5′-GAGACCTTCAACACCCCAGCC-3′ (upstream), and
5′-TCGGGGGATCGGAACCGCTCA-3′ (downstream). Amplification was
performed as following: Pre-denaturation at 94°C for 2 min,
denaturation at 98°C for 10 sec, annealing at 53°C for 15 sec,
extension at 72°C for 30 sec and final extension at 72°C for 10
min. The size of the amplified fragment obtained by PCR was 400
bp.
Amplification was conducted using a Px2 Thermal
cycler (Thermo Fisher Scientific, Inc.). PCR products were
separated by electrophoresis on 1.2% agarose gel and visualized by
staining with ethidium bromide (Sigma-Aldrich, St. Louis, MO, USA).
The absorbance value (A) of mRNA bands was measured by MUVB-20 gel
using a photographic analysis system (Ultra-Lum, Inc., Claremont,
CA, USA). Densitometric analysis for semi-quantification of the PCR
products was conducted using Quantity One software (Bio-Rad
Laboratories, Inc.). β-actin band was used as internal reference.
The ratios (ERK1 vs. β-actin, ERK2 vs. β-actin and p38 vs. β-actin)
were defined the relative A value of ERK1, ERK2 and p38,
respectively. Each experiment was repeated eight times.
Statistical analysis
SPSS software, version 17.0 (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis, and data were expressed
as the mean ± standard deviation. The significance of the
differences between the groups was determined using one-way
analysis of variance. Least significant difference and Dunnet's
t-test were used for homogeneity of variance and heterogeneity of
variance, respectively. The correlation between two variables was
analyzed using Pearson's bivariate correlation analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
PASMC morphology and
immunocytochemical observation
Fusiform PASMCs were observed under a microscope and
the cell morphology remained unchanged for several generations
(Fig. 1A and B). The
immunocytochemistry results showed that cytoplastic positive
staining of SM-α-actin was present in 98% of PASMCs. When observed
under a high-powered microscope (magnification, ×400),
brown-stained fiber filaments (SM-α-actin) were observed in
parallel to the long axis of the cell, with a pale blue oval
nucleus located in the center of the cell (Fig. 1C).
Effect of hypoxia/hypercapnia and Rb1
on the protein expression of phosphorylated ERK and p38
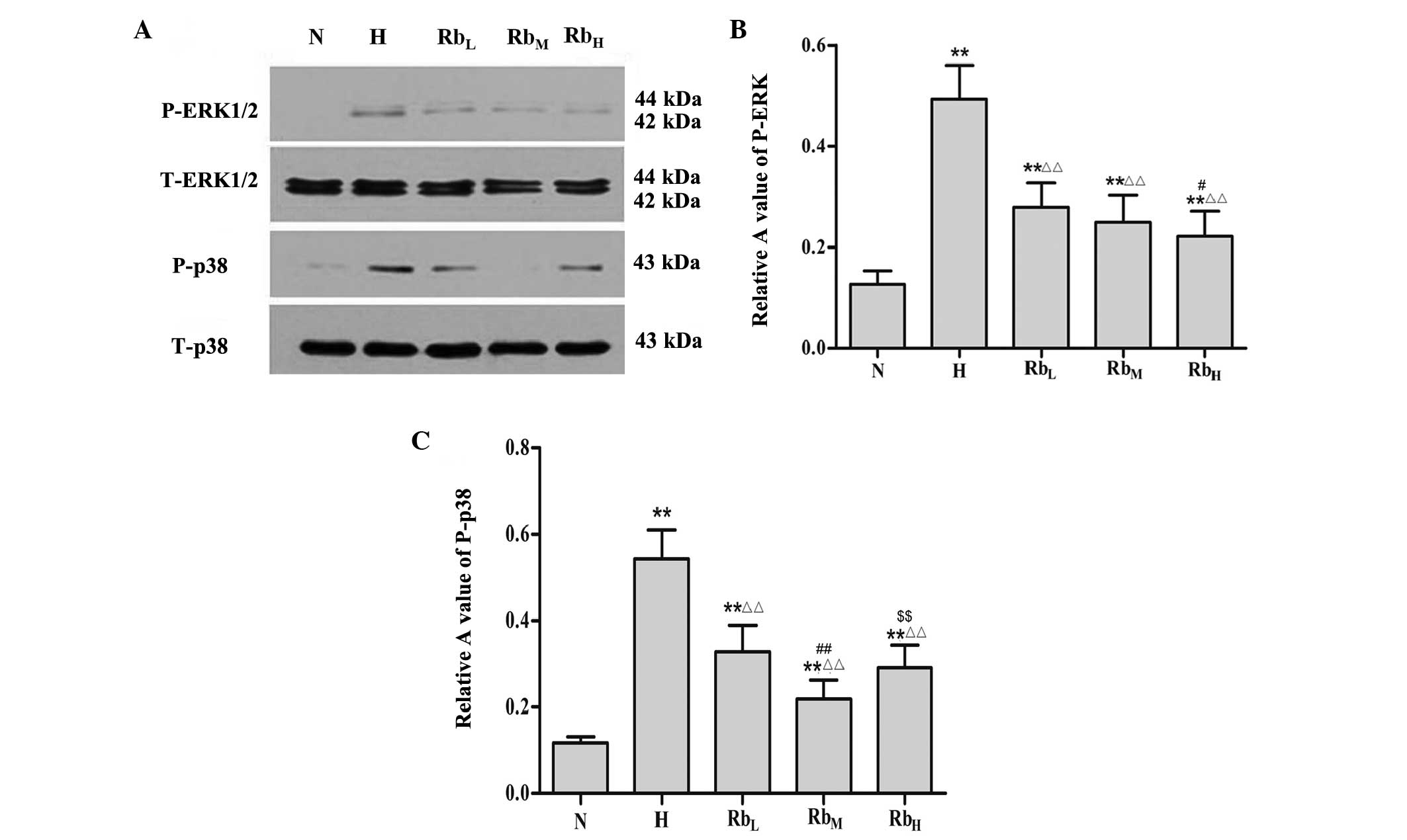
P-ERK expression was significantly higher in the H
group compared with the N group (P<0.01). Compared with the H
group, the RbL, RbM and RbH groups
exhibited decreased expression of P-ERK (P<0.01). Furthermore,
Rb1 suppressed the expression of P-ERK in a dose-dependent manner.
The P-ERK expression in the RbH group was significantly
lower compared with that in the RbL group (P<0.01).
However, no significant differences were detected between the
RbL and RbM groups, nor between the
RbM and RbH groups (P>0.05; Fig. 2A and B).
Similarly, the H group had elevated P-p38 expression
in comparison with the N group (P<0.01). Rb1 treatment led to
decreased P-p38 expression in the RbL, RbM
and RbH groups compared with the H group (P<0.01).
Among the three Rb1-treated groups, P-p38 expression was lowest in
the RbM group. P-p38 expression was significantly higher
in the RbL group compared with the RbM group
(P<0.01). Furthermore, there was no significant difference
between the RbL and RbH groups, and between
the RbM and RbH groups (P>0.05; Fig. 2A and C).
These results suggest that hypoxia and hypercapnia
resulted in the phosphorylation of ERK and p38, which was
subsequently inhibited by Rb1 treatment. The most effective
concentrations of Rb1 for inhibiting the protein expression of
P-ERK and P-p38 were 100 and 40 mg/ml, respectively.
Effect of hypoxia and hypercapnia, and
Rb1 on mRNA expression levels of ERK1, ERK2 and p38
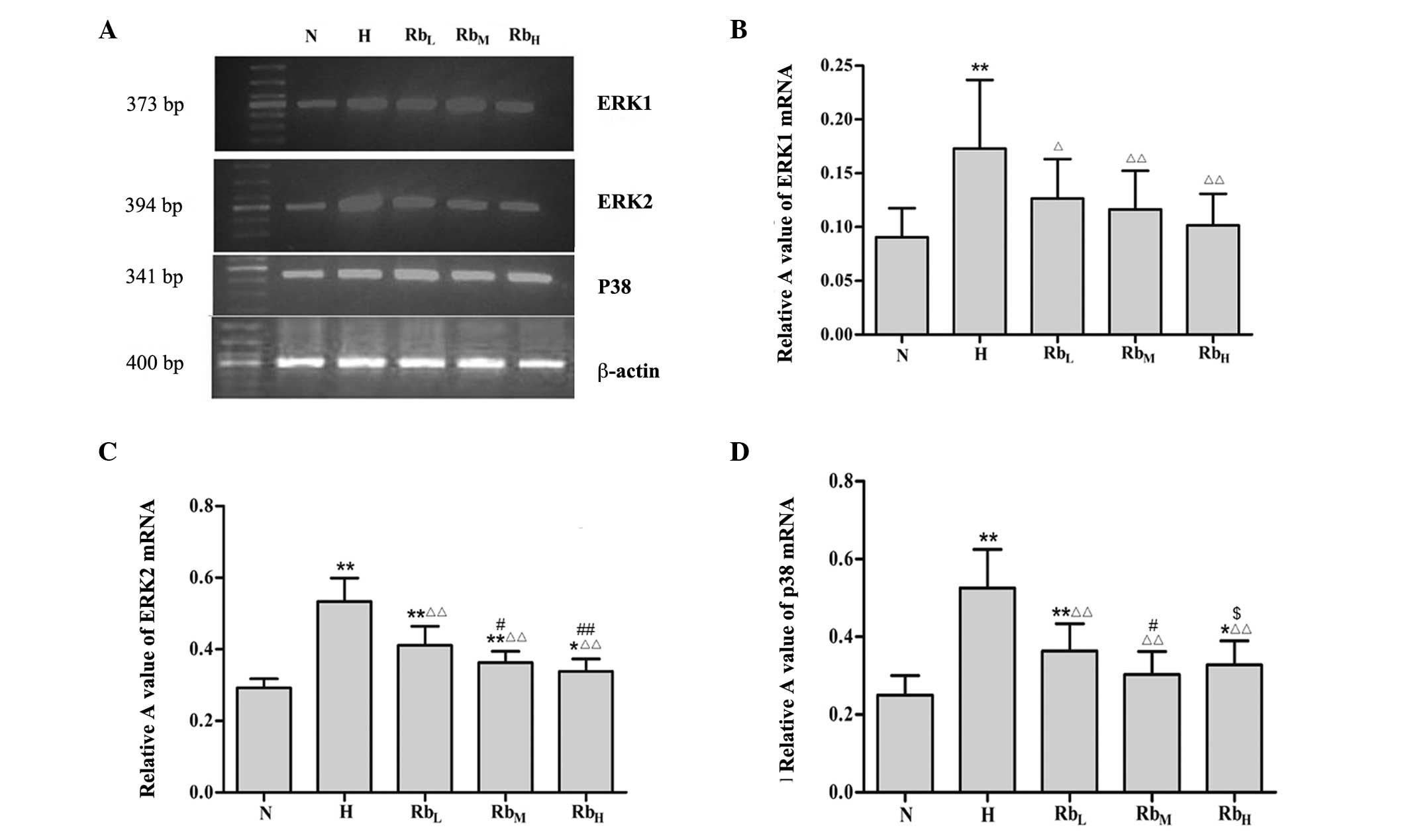
The results of the RT-PCR analysis showed that ERK1
mRNA levels were higher in the H group compared with the in N group
(P<0.01). Compared with the H group, the ERK1 levels were
inhibited in the RbL, RbM and RbH
groups (P<0.05), but remained higher compared with those in the
N group. Among the three Rb1-treated groups, ERK1 levels were
decreased in a dose-dependent manner; however, there were no
significant differences among the three groups (P>0.05; Fig. 3A and B).
As with ERK1, the expression of ERK2 was promoted in
the H group compared with the N group (P<0.01). This hypoxia and
hypercapnia-induced ERK2 promotion was mitigated in the
RbL, RbM and RbH groups
(P<0.05). The inhibition of ERK2 by Rb1 exhibited a
dose-dependent trend. There were significant differences between
the RbL and RbM groups (P<0.05), and
between the RbH and RbL groups (P<0.01)
(Fig. 3A and C).
Similarly, mRNA levels of p38 were increased in the
H group compared with the N group (P<0.01). By contrast, p38
levels were significantly downregulated in the RbL,
RbM and RbH groups in comparison with the H
group (P<0.01). Among the three groups, RbL had
significantly higher p38 levels compared with RbH,
whereas RbH had significantly higher p38 levels compared
with the RbM group (P<0.05; Fig. 3A and D).
These results indicate that the elevations in ERK1,
ERK2 and p38 mRNA levels induced by hypoxia and hypercapnia could
be inhibited by Rb1 treatment. The most effective concentration of
Rb1 for inhibiting ERK1/2 appeared to be 100 mg/ml, while 40 mg/ml
Rb1 was most effective for the inhibition of p38.
Analysis of correlation between the
expression of P-ERK1/2 protein and ERK1/2 mRNA, and between P-p38
protein and p38 mRNA
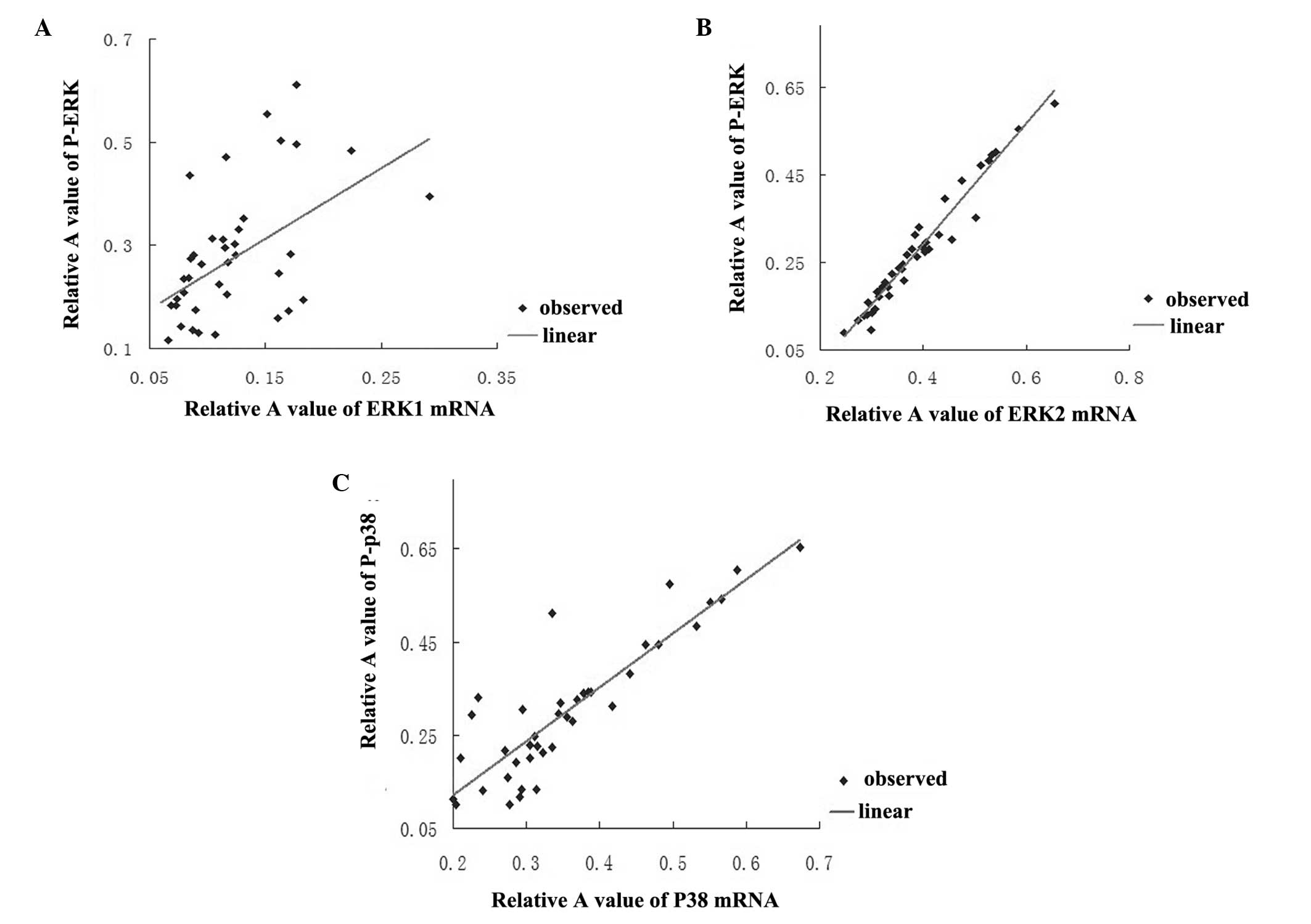
As shown in Fig. 4A and
B, in the Rb1-treated groups P-ERK protein expression was
positively correlated with ERK1 mRNA (r=0.5, P<0.01) and ERK2
(r=0.977, P<0.01) mRNA expression, respectively. Furthermore,
P-p38 protein expression was positively correlated with p38 mRNA
(r=0.884, P<0.01) in the Rb1-treated groups (Fig. 4C).
Discussion
COPD is a present health concern to a substantial
number of people, particularly elderly individuals. The
increasingly poor air quality appears to promote the incidence of
COPD (15). HHPV and PH are common
complications of COPD. The results of the present study showed that
exposing PASMCs to hypoxia and hypercapnia promoted ERK and p38
phosphorylation at the protein and mRNA level. The promoted ERK and
p38 phosphorylation was effectively inhibited by Rb1 treatment.
ERK and p38 pathways serve a variety of functions in
a number of biological activities, such as cell proliferation,
motility, differentiation and survival (16–18).
In vivo and in vitro studies have increasingly
indicated that hypoxia could lead to activation of ERK, JNK and p38
pathways, which is involved in pulmonary arteries remodeling
(19,20). Furthermore, hypercapnia could result
in ERK activation in alveolar epithelial cells in a time-dependent
manner (21). In support of these
previous studies, the present western blot and RT-PCR results
indicated that exposing cells to hypoxia and hypercapnia enhanced
the P-ERK protein expression, and ERK1 and ERK2 mRNA expression,
respectively. Consistent with ERK, P-p38 protein and p38 mRNA
expression were elevated in PASMCs grown under the conditions of
hypoxia and hypercapnia.
However, there has been uncertainty regarding the
effect of hypercapnia on ERK and p38 expression. For example, it
has been observed that ERK and p38 were not involved in the
mechanism underlying the attenuation of pulmonary epithelial wound
repair by hypercapnic acidosis (22). These contradictory results may be
attributed to the varying concentrations of CO2 used in
the different experimental models. Another potential explanation is
the varied sensitivity of different experimental models to the same
concentration of CO2 stimuli. In the present study, the
N group was cultured under 5% CO2 and 21% O2,
while the H group was grown under 6% CO2 and 1%
O2. Obviously, hypoxia stimulation produced a stronger
impact than hypercapnia on the PASMCs, indicating that the enhanced
phosphorylation of ERK and p38 may have predominantly resulted from
hypoxia rather than hypercapnia.
It has been established that PNS may function as a
free radical-scavenger and suppress ERK, JNK and p38 activation in
AS lesion rat models (23).
Similarly, Rg1, another active component of PNS, has been reported
to protect dopaminergic neurons against 6-hydroxydopamine (6-OHDA)
partly by compromising the 6-OHDA-induced ERK phosphorylation
(24). Furthermore, it has been
observed that ERK activation can be inhibited by R1 (active
component of PNS) treatment in human aortic smooth muscle cells
(25). In agreement with these
studies, Rb1 treatment appeared to suppress the increased P-ERK
protein expression, and ERK1/2 mRNA expression induced by hypoxia
and hypercapnia. Positive correlations were confirmed between the
P-ERK protein and ERK1 mRNA expression, and between the P-ERK
protein and ERK2 mRNA expression. Similarly, P-p38 protein and p38
mRNA were significantly reduced in response to Rb1. P-p38 protein
levels were positively correlated with p38 mRNA levels. These
results indicated that Rb1 may mitigate HHPV via the inhibition of
the ERK and p38 pathways. Furthermore, accumulating studies have
suggested that hypoxia may promote PASMC proliferation and induce
pulmonary vascular remodeling, and subsequently contribute to PH
(26–28). Thus, it is speculated that Rb1 may
affect the PASMCs proliferation and pulmonary vascular remodeling
via ERK and p38 pathways.
Notably, it was observed that the most effective
concentrations of Rb1 for ERK and p38 inhibition were 100 and 40
mg/ml, respectively. This indicated a discrepancy of sensitivity to
Rb1 treatment between ERK and p38 pathways. The complicated
mechanism governing the action of the two pathways has been
intensely investigated. Interactions between the two pathways have
been demonstrated in regulation of the chondrocyte development
(29). The two pathways also
function cooperatively in regulating RUNX2 transcription factor
phosphorylation and transcriptional activity (30). Furthermore, a variety of signaling
pathways such protein kinase A, protein kinase C and
phosphoinositide 3-kinase participate in regulating the downstream
ERK and p38 pathways (31–33). Further studies are required to
elucidate the relationship between the two pathways in response to
Rb1 treatment.
In conclusion, Rb1 appears to alleviate HHPV by
inhibiting the activation of ERK and p38 pathways. The present
study indicates the protective activity of Rb1 in the management of
COPD. Future studies are required to evaluate the effect of Rb1 on
PASMC proliferation and survival in vivo and in vitro
to clarify the underlying mechanism of Rb1 treatment against
HHPV.
Acknowledgements
This study was supported by Zhejiang Province
Traditional Chinese Medicine Science and Technology Key Project
(grant nos. 2008ZA017 and 2013ZZ011), and Zhejiang Province
Traditional Chinese Medicine Key Disciplines Construction Project
(grant no. 2012-XK-A28).
Glossary
Abbreviations
Abbreviations:
|
AS
|
atherosclerosis
|
|
COPD
|
chronic obstructive pulmonary
disease
|
|
ERK1/2
|
extracellular signal-regulated
kinase
|
|
HHPV
|
hypoxia and hypercapnia-induced
pulmonary vasoconstriction
|
|
HPV
|
hypoxia-induced pulmonary
vasoconstriction
|
|
MAPK
|
mitogen activated protein kinase
|
|
PASMCs
|
pulmonary arterial smooth muscle
cells
|
|
PH
|
pulmonary hypertension
|
|
PNS
|
Panax notoginseng saponins
|
|
p38 MAPK
|
p38 mitogen-activated protein
kinase
|
References
|
1
|
World Health Organization: The top 10
causes of death, 2000 and 2012. http://www.who.int/mediacentre/factsheets/fs310/en/Updated.
May;2014.
|
|
2
|
Burney P, Jithoo A, Kato B, Janson C,
Mannino D, Nizankowska-Mogilnicka E, Studnicka M, Tan W, Bateman E,
Koçabas A, et al: Chronic obstructive pulmonary disease mortality
and prevalence: The associations with smoking and poverty-a BOLD
analysis. Thorax. 69:465–473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pauwels RA, Buist AS, Calverley PM,
Jenkins CR and Hurd SS: GOLD Scientific Committee: Global strategy
for the diagnosis, management and prevention of chronic obstructive
pulmonary disease. NHLBI/WHO Global Initiative for Chronic
Obstructive Lung Disease (GOLD) Workshop summary. Am J Resp Crit
Care. 163:1256–1276. 2001. View Article : Google Scholar
|
|
4
|
Kennedy TP, Michael JR, Huang CK, Kallman
CH, Zahka K, Schlott W and Summer W: Nifedipine inhibits hypoxic
pulmonary vasoconstriction during rest and exercise in patients
with chronic obstructive pulmonary disease. A controlled
double-blind study. Am Rev Res Dis. 129:544–551. 1984.
|
|
5
|
Seemungal T, Harper-Owen R, Bhowmik A,
Moric I, Sanderson G, Message S, Maccallum P, Meade TW, Jeffries
DJ, Johnston SL and Wedzicha JA: Respiratory viruses, symptoms and
inflammatory markers in acute exacerbations and stable chronic
obstructive pulmonary disease. Am J Resp Crit Care Med.
164:1618–1623. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chaouat A, Naeije R and Weitzenblum E:
Pulmonary hypertension in COPD. Eur Respir J. 32:1371–1385. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Wang Y, Qiu L, Yu Y and Wang C:
Saponins of Panax notoginseng: Chemistry, cellular targets
and therapeutic opportunities in cardiovascular diseases. Expert
Opin Investig Drugs. 23:523–539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Zhang HG, Jia Y and Li XH: Panax
notoginseng saponins attenuate atherogenesis accelerated by
zymosan in rabbits. Biol Pharm Bull. 33:1324–1330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan JS, Liu DN, Huang G, Xu ZZ, Jia Y,
Zhang HG, Li XH and He FT: Panax notoginseng saponins
attenuate atherosclerosis via reciprocal regulation of lipid
metabolism and inflammation by inducing liver X receptor alpha
expression. J Ethnopharmacol. 142:732–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu L, Zhang W, Tang YH, Li H, Chen BY,
Zhang GM and Deng CQ: Effect of total saponins of ‘Panax
notoginseng root’ on aortic intimal hyperplasia and the
expressions of cell cycle protein and extracellular matrix in rats.
Phytomedicine. 17:233–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gan J, Li P, Wang Z, Chen J, Liang X, Liu
M, Xie W, Yin R and Huang F: Rosuvastatin suppresses
platelet-derived growth factor-BB-induced vascular smooth muscle
cell proliferation and migration via the MAPK signaling pathway.
Exp Ther Med. 6:899–903. 2013.PubMed/NCBI
|
|
12
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C
and Jensen LJ: STRING v9.1: Protein-protein interaction networks,
with increased coverage and integration. Nucleic Acids Res.
41:D808–D815, database issue. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Y, Lin L, Tang L, Zheng M, Ma Y, Huang
L, Meng W and Wang W: Notoginsenoside R1 attenuates hypoxia and
hypercapnia-induced vasoconstriction in isolated rat pulmonary
arterial rings by reducing the expression of ERK. Am J Chinese Med.
42:799–816. 2014. View Article : Google Scholar
|
|
14
|
Li X, Lu W, Fu X, Zhang Y, Yang K, Zhong
N, Ran P and Wang J: BMP4 increases canonical transient receptor
potential protein expression by activating p38 MAPK and ERK1/2
signaling pathways in pulmonary arterial smooth muscle cells. Am J
Resp Cell Mol Biol. 49:212–220. 2013. View Article : Google Scholar
|
|
15
|
Wen FQ and He B: Interpretation of Global
Strategy for the Diagnosis, Management and Prevention of Chronic
Obstructive Pulmonary Disease (GOLD) (revised 2011). Zhonghua Yi
Xue Za Zhi. 92:939–940. 2012.(In Chinese). PubMed/NCBI
|
|
16
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Waseem T, Duxbury M, Ashley SW and
Robinson MK: Ghrelin promotes intestinal epithelial cell
proliferation through PI3K/Akt pathway and EGFR trans-activation
both converging to ERK 1/2 phosphorylation. Peptides. 52:113–121.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SM, Lee CT, Kim YW, Han SK, Shim YS
and Yoo CG: Hypoxia confers protection against apoptosis via
PI3K/Akt and ERK pathways in lung cancer cells. Cancer Lett.
242:231–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin N, Hatton N, Swartz DR, Xia Xl,
Harrington MA, Larsen SH and Rhoades RA: Hypoxia activates
jun-N-terminal kinase, extracellular signal-regulated protein
kinase and p38 kinase in pulmonary arteries. Am J Respir Cell Mol
Biol. 23:593–601. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Welch LC, Lecuona E, Briva A, Trejo HE,
Dada LA and Sznajder JI: Extracellular signal-regulated kinase
(ERK) participates in the hypercapnia-induced Na, K-ATPase
downregulation. FEBS Lett. 584:3985–3989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O'Toole D, Hassett P, Contreras M, Higgins
BD, McKeown ST, McAuley DF, O'Brien T and Laffey JG: Hypercapnic
acidosis attenuates pulmonary epithelial wound repair by an
NF-kappaB dependent mechanism. Thorax. 64:976–982. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dou L, Lu Y, Shen T, Huang X, Man Y, Wang
S and Li J: Panax notogingseng saponins suppress RAGE/MAPK
signaling and NF-kappaB activation in apolipoprotein-E-deficient
atherosclerosis-prone mice. Cell Physiol Biochem. 29:875–882. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ge KL, Chen WF, Xie JX and Wong MS:
Ginsenoside Rg1 protects against 6-OHDA-induced toxicity in MES23.
5 cells via Akt and ERK signaling pathways. J Ethnopharmacol.
127:118–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang HS and Wang SQ: Notoginsenoside R1
inhibits TNF-alpha-induced fibronectin production in smooth muscle
cells via the ROS/ERK pathway. Free Radical Bio Med. 40:1664–1674.
2006. View Article : Google Scholar
|
|
26
|
Penumatsa KC, Toksoz D, Warburton RR,
Hilmer AJ, Liu T, Khosla C, Comhair SA and Fanburg BL: Role of
hypoxia-induced transglutaminase 2 in pulmonary artery smooth
muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol.
307:L576–L585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shan R, Chen L, Li X, Wu H, Liang Q and
Tang X: Hypoxia promotes rabbit pulmonary artery smooth muscle
cells proliferation through a 15-LOX-2 product
15(S)-hydroxyeicosatetraenoic acid. Prostaglandins Leukot Essent
Fatty Acids. 86:85–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei L, Yu X, Shi H, Zhang B, Lian M, Li J,
Shen T, Xing Y and Zhu D: 15-PGDH/15-KETE plays a role in
hypoxia-induced pulmonary vascular remodeling through
ERK1/2-dependent PAR-2 pathway. Cell Signal. 26:1476–1488. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hutchison MR: BDNF alters ERK/p38 MAPK
activity ratios to promote differentiation in growth plate
chondrocytes. Mol Endocrinol. 26:1406–1416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ge C, Yang Q, Zhao G, Yu H, Kirkwood KL
and Franceschi RT: Interactions between extracellular
signal-regulated kinase 1/2 and P38 Map kinase pathways in the
control of RUNX2 phosphorylation and transcriptional activity. J
Bone Miner Res. 27:538–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Araújo Herculano B, Vandresen-Filho S,
Martins WC, Boeck CR and Tasca CI: NMDA preconditioning protects
against quinolinic acid-induced seizures via PKA, PI3K and MAPK/ERK
signaling pathways. Behav Brain Res. 219:92–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hirota Y, Tsukazaki T, Yonekura A,
Miyazaki Y, Osaki M, Shindo H and Yamashita S: Activation of
specific MEK-ERK cascade is necessary for TGFβ signaling and
crosstalk with PKA and PKC pathways in cultured rat articular
chondrocytes. Osteoarthr Cartilage. 8:241–247. 2000. View Article : Google Scholar
|
|
33
|
Zhou D, Liu Y, Zhang X, Gu X, Wang H, Luo
X, Zhang J, Zou H and Guan M: Functional polymorphisms of the ABCG2
gene are associated with gout disease in the Chinese Han male
population. Int J Mol Sci. 15:9149–9159. 2014. View Article : Google Scholar : PubMed/NCBI
|