Introduction
Bone grafting is performed on millions of patients
suffering from bone trauma and bone defects caused by wars, traffic
accidents, sports injuries, diseases and natural disasters. Bone
grafting is the second most frequently performed type of tissue
transplant following blood transfusion (1). In 1995, Crane et al (2) proposed the concept of using bone tissue
engineering for the repair of bone defects. Since then, this
technology has achieved marked progress, in fundamental research
and clinical application in repairing bone defects (3,4) and in
total joint replacement (5,6). There are three types of scaffold
materials used in bone tissue engineering: Natural, artificially
synthesized inorganic, and artificially synthesized polymers.
Generally, a single type of scaffold material does not satisfy the
current requirements for an extracellular scaffold, which are as
follows: Enhanced mechanical strength, improved degradation time
and increased biological activity. Therefore, numerous studies have
investigated the use of two or more materials in the composition of
scaffold materials (7–9). By selecting appropriate components and
adjusting the proportion of each, composite materials with
adjustable degradation characteristics and mechanical properties
may be produced.
It has been widely recognized that, following
autogenous bone, allograft bone is the best substitute material for
bone grafting. Demineralized bone matrix (DBM), prepared for
allograft cortical bone through grinding, washing and
decalcification, is a natural sustained-release carrier of bone
morphogenetic proteins with favorable bone induction, bone
conduction and biocompatibility properties (10,11).
However, DBM has poor mechanical properties and is difficult to
model (12). The most widely applied
artificially synthesized polymer scaffold material is polylactic
acid (PLA), which has useful mechanical properties and is easy to
model, but has no biological activity and poor cellular affinity
(13). Therefore, a composite of DBM
and PLA together may exhibit advantages compared with either
material alone.
With effective utilization of antibiotics,
concurrent infection of bone defects are well controlled. However,
the postoperative infection rate of bone defects caused by open
injuries remains as high as 20%, even when debridement is conducted
early in the procedure (14). The
prevention and treatment of infected bone defects is more difficult
compared with that of a simple bone defect (15). When a bone defect is infected, bone
grafting in the first stage does not facilitate the union of the
defect, but rather exacerbates the infection (16). Furthermore, single-dose
administration of antibiotics at the site of bone grafting may not
be effective, as systemic antibiotic administration cannot provide
an effective concentration of antibiotics at the bone grafting site
and can also have potential adverse side effects (17). One solution to this problem is to
combine antibiotics with a scaffold material to prepare an
anti-infective tissue-engineered bone material that is able to
maintain a high local concentration of antibiotics at the bone
grafting position, and a low concentration in the circulation. This
may reduce the infection rate by increasing antibacterial activity
locally at the bone grafting site (18).
With these considerations, the present study
investigated the effect of a novel gentamicin sulfate (GS)/DBM/PLA
porous composite for the treatment of an infected femoral condyle
defect in a rat model.
Materials and methods
Preparation of DBM and PLA
The ethics committee of Shanxi Medical University
(Taiyuan, China) approved the present study. Human cortical bones
from numerous healthy and certified donors were ground and sifted
to prepare bone powder with a diameter of 200–300 µm. The bone
powder was frozen, ultrasonically washed with distilled water for 3
days, decalcified in 0.6 N HCl at 4°C for 48 h and lyophilized. A
1-g sample of 0.5×105, 1×105 or 5
×105 Da PLA (Daigang Biomaterial Co., Ltd., Jinan,
China) was mixed with 2 g NaCl (diameter, 200–300 µm, determined by
molecular sieve), at a PLA-to-NaCl ratio of 1:2. The mixed
composition was put into a 10-ml supercritical cylinder reactor
vessel linked to supercritical (SC)-CO2 reaction
apparatus (SFE-2; Applied Separations, Inc., Allentown, PA, USA).
The pressure of the container was gradually raised to 20 MPa and
the temperature was increased to 37°C, and was maintained at
37±1°C, with the pressure variation kept within 0.1 MPa of this.
After maintaining the temperature and pressure for 30 min, they
were decreased slowly over 15 min to room temperature and pressure.
PLA samples were then removed from the vessel and leached with
distilled water for 48 h to completely remove the NaCl. Following
lyophilization, the samples were packed in a three-layer aseptic
package (Fuhua Medical Packing Co., Ltd., Nantong, China) and
irradiated with 20 kGy 60Co (China Nuclear Power
Engineering Co., Ltd, Beijing, China) for sterilization.
Preparation of porous GS/DBM/PLA
composite biomaterial
To produce the biomaterial, 0.6 g DBM (particle
size, 200–300 µm; Shanxi Provincial Tissue Bank, Taiyuan, China)
and 0.4 g PLA (molecular weight, 1×105; Daigan
Biological Materials, Jinan, China) were weighed at a DBM-to-PLA
ratio of 3:2, in addition to 0.5 g GS powder (BBI Life Sciences
Corporation, Shanghai, China) and 2 g NaCl (diameter, 200–300 µm).
These materials were all white powders and were mixed thoroughly
prior to being placed into the 10-ml supercritical reactor vessel.
The vessel was then linked to the supercritical CO2
reaction apparatus and the preparation proceeded as described for
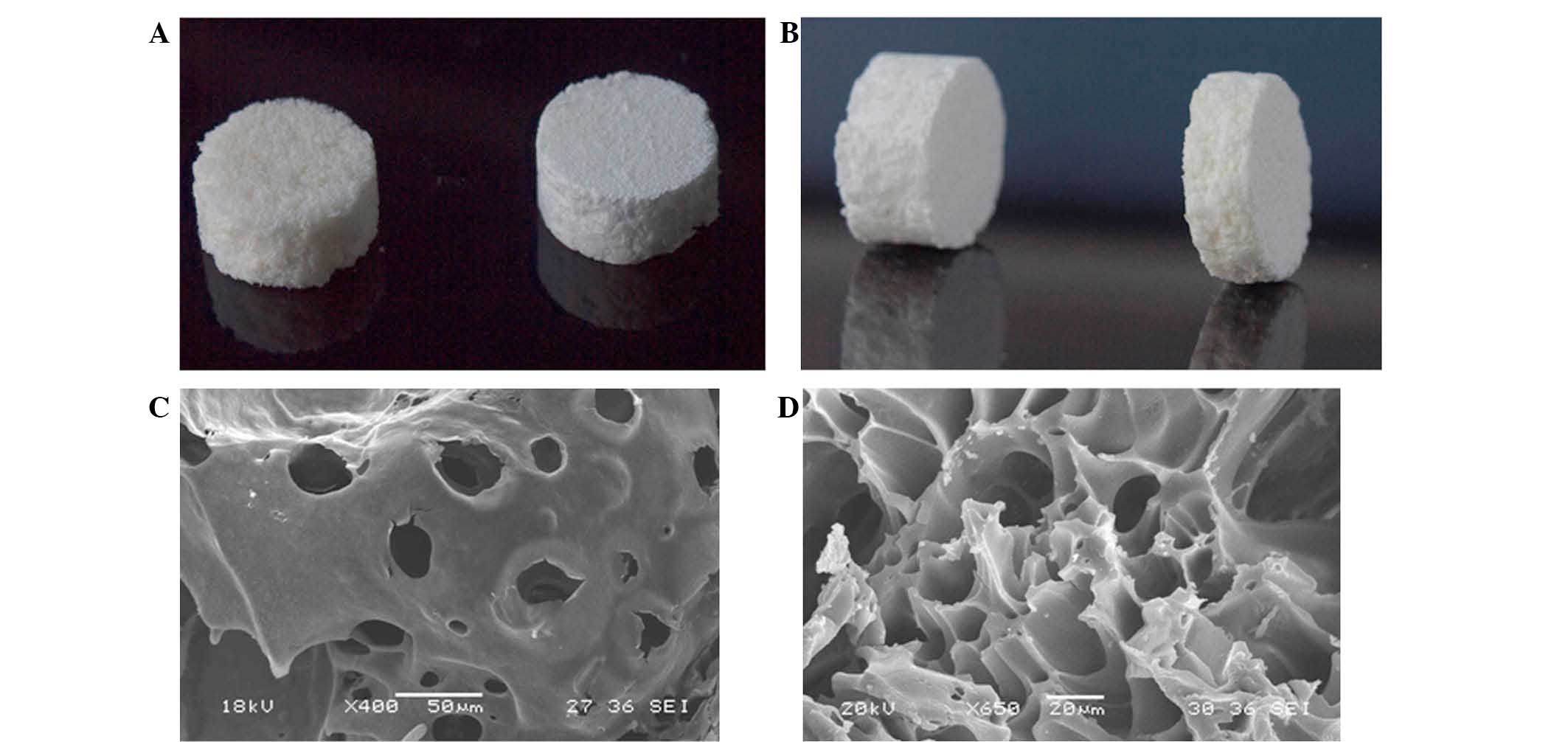
the DBM and PLA. Optical and scanning electron microscope
(JSM-6360LVV; JEOL, Ltd., Tokyo, Japan) images of the composite
biomaterial are presented in Fig. 1.
GS/DBM/PLA was prepared to a ratio of 5:3:2.
Characteristics of the porous
GS/DBM/PLA composite biomaterial
For the evaluation of the biomechanical properties
of the composite biomaterials, specific gravity was measured as an
indication of porosity, and compressive strength and elastic
modulus were determined using an RGT-20A microcomputer-controlled
electronic universal testing machine (Shenzhen Reger Instrument Co.
Ltd., Shenzhen, China). Cytotoxicity was detected in the mouse
fibroblast cell line L-929 (Shanxi Provincial Tissue Bank) using an
MTT assay. Briefly, mouse fibroblast cells at passage 3 were
cultured together with extracts of GS/DBM/PLA and DBM/PLA in
96-well culture plates, with DMEM medium serving as a control.
After culturing for 1, 2, 3, 4, 5, 6 and 7 days, MTT solution
(Sigma-Aldrich, St. Louis, MO, USA) was added to each well and the
cells were cultured for an additional 4 h. Subsequent to this, 150
µl DMSO was added to each well and the optical density was examined
using a Model 680 microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at a wavelength of 570 nm to determine cell
viability in the presence of these compounds. Next, the prepared
GS/DBM/PLA composite was trimmed to uniform size (10 × 5 mm).
Fifteen test tubes were prepared and 10 ml normal saline was added
to each under sterile conditions. The prepared GS/DBM/PLA materials
were put into test tube 1, soaked for 4 h at 37°C and the next day
removed to test tube 2. Then, the process was repeated from tube 2
to tube 3, successively up to tube 15 (day 15). During this time,
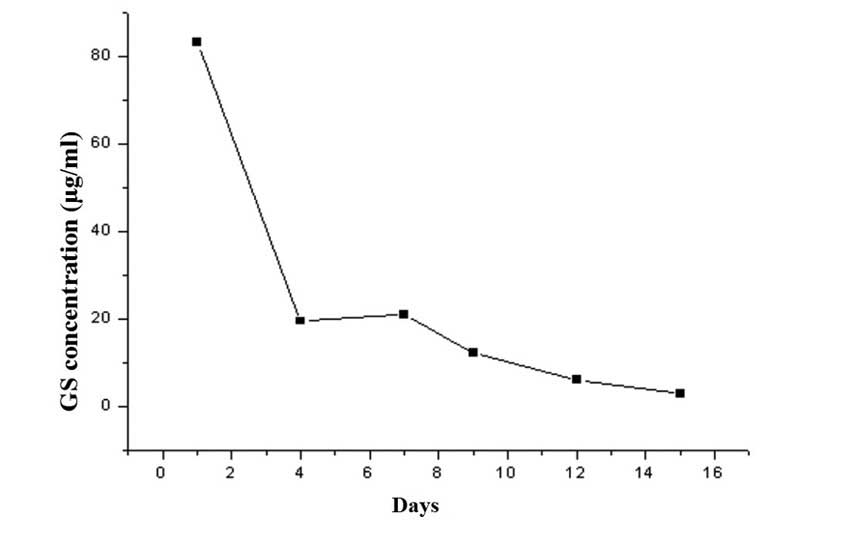
the gentamicin in the GS/DBM/PLA composite gradually leached into
the normal saline. A microbial inhibitory concentration test was
then conducted on the saline in each of the tubes to determine the
antibacterial concentration at different times and to produce an
in vitro release curve of gentamicin (Fig. 2). Subsequent to this, prepared
GS/DBM/PLA composite was trimmed to uniform size (5 × 5 mm), and
placed into the muscle of rats (described below). At postoperative
days 1, 4, 7, 9, 12 and 15, 3 mm muscular tissue around the
implanted materials was removed and 2 mm blood was collected from
the abdominal aorta [using heparin anticoagulant (Sigma-Aldrich)].
The biological samples were stored in a cryogenic refrigerator
(DW-25L92; Haier Group, Qindao, China) at −20°C. Then, under
sterile conditions, the preserved muscle tissue and blood were
homogenized in phosphate-buffered saline solution, centrifuged for
10 min at 37°C, 2,300 × g, and the supernatant was collected.
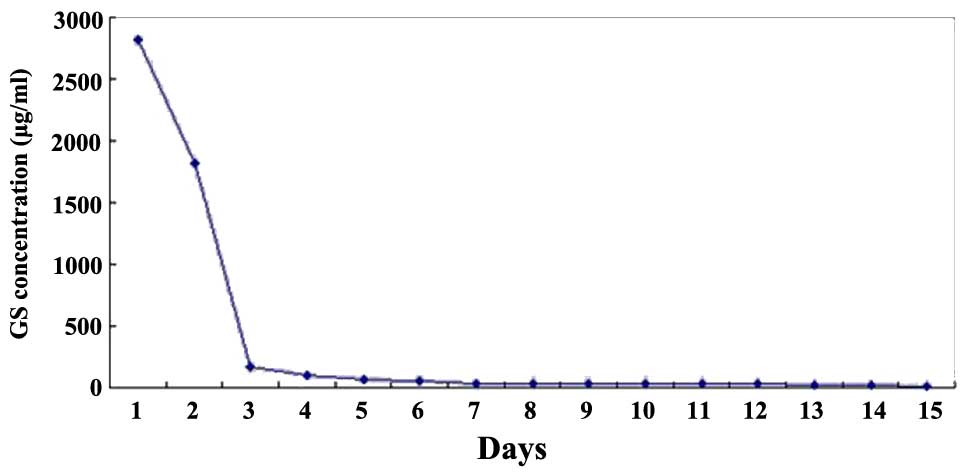
Microbial inhibitory concentration was detected in this saline by a
golden Staphylococcus microbiological test, based on flat
colony counting methods (Shanxi Medical University), and an in
vivo release curve was mapped (Fig.
3).
Preparation of the animal model
A total of 84 Wistar rats were used (Shanxi Medical
University Medical Laboratory Animal Center). These were kept at
26°C, in pathogen-free conditions with a 12–12 h light-dark cycle.
The body weight of rats was ~300 g, and the sample included male
and female rats. The requisite approval was obtained from the local
ethics committee. The rats were divided into four groups, as
follows: The control group, in which no experiment was conducted;
the GS/DBM/PLA plus Staphylococcus aureus (SA) group; the
DBM/PLA plus SA group; and the DBM/PLA without SA group, with 21
rats per group. The rats were anesthetized with 0.6% pentobarbital
sodium (30 mg/100 g; Merck Millipore, Darmstadt, Germany) and fixed
prostrate to the operating platform. Following depilation and
sterilization around the femoral condyle, with drapes covering the
skin, subcutaneous tissue and deep fascia were incised by layer.
The muscles were bluntly dissected to prepare for a bilateral
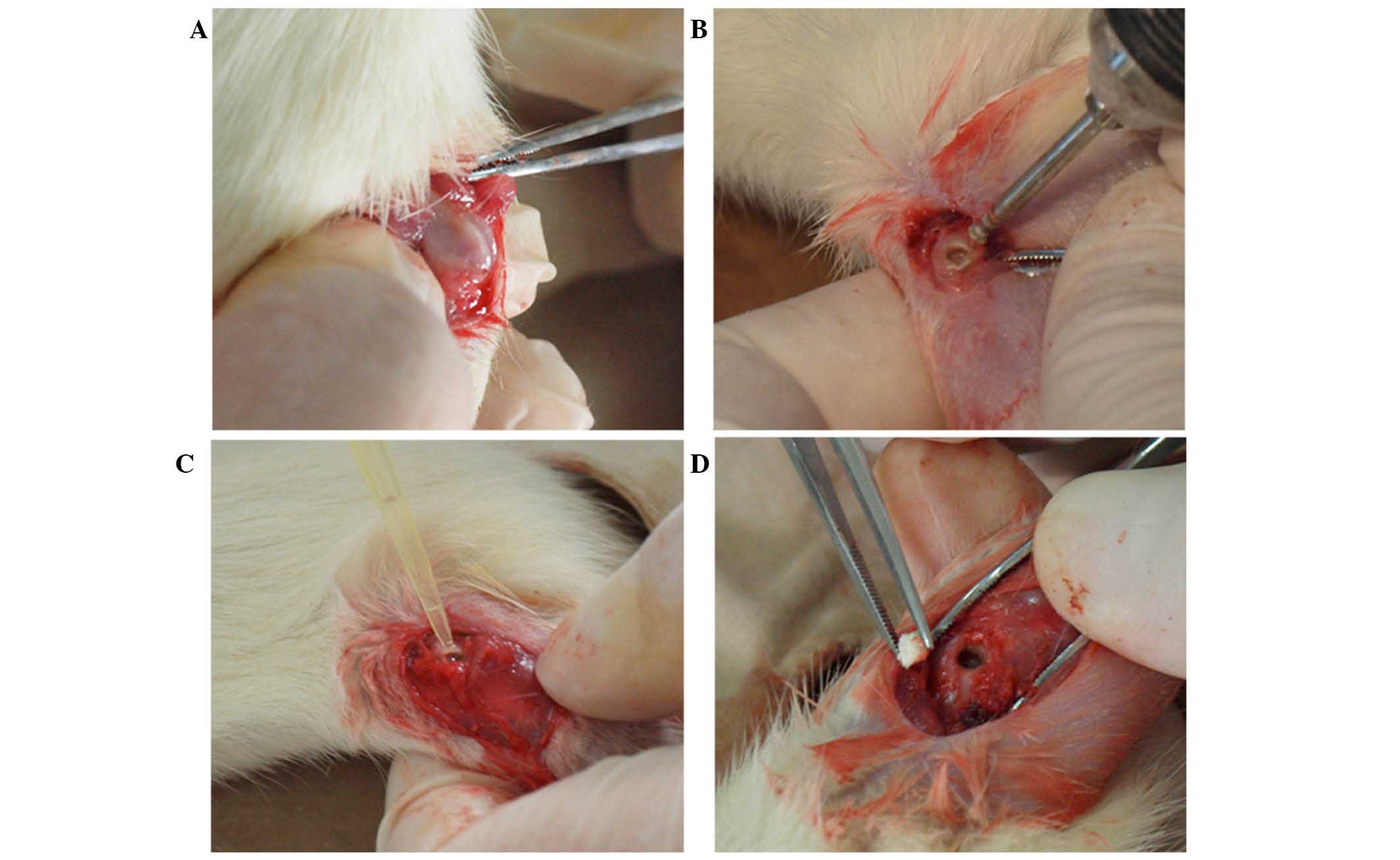
femoral condyle defect. A 1-cm incision on the lateral femoral
condyle was made to expose the lower end of the femur. The incision
did not penetrate into the articular cavity in order to protect the
articular cartilage (Fig. 4A). A
dental ball drill (diameter, 1.6 mm) was applied to drill in from
the lateral side of the femoral condyle along the bone shaft axis
(Fig. 4B), reaching the bone cortex
on the opposite side but not damaging it. Then, the defect in the
condyle was enlarged until it was 60–70% of the volume of the
femoral condyle (Fig. 4C). The
defect was washed with sterile saline and dried with a sterile
gauze. Then, 10 µl SA standard solution (1×1011 CFU/ml;
Shanxi Medical University) was injected into the defect by pipette
(Fig. 4D). After 30 min, ~80 mg
GS/DBM/PLA or DBM/PLA was implanted into the defect. Following
suturing, the incision was tightly covered, but the operated limb
was not fastened. The rats were fed conventionally following the
operation. Rats were sacrificed prior to the following experiments
using an injected overdose of pentobarbital sodium (40–60 mg/100
g), at 4, 6 or 8 weeks after the first operation.
Radiographic observation and bone
density analysis
Three rats (six sites of femoral condyle defects) in
each group were selected and anesthetized with 0.6% pentobarbital
sodium (30 mg/100 g) at 4, 6 and 8 weeks after the operation
described above. To investigate new bone growth and reconstruction
of bone, radiographs were captured using an SMX-1000 Plus X-ray
inspection system (Shimadzu, Kyoto, Japan). The grayscale value per
unit area of defect was measured using image analysis software
(Image-Pro Plus version 5.0; Media Cybernetics, Inc., Rockville,
MD, USA) to evaluate the bone repair condition instead of bone
mineral density.
Biomechanical compression tests
Four rats from each group were selected and the left
femoral condyles were inspected at 4, 6, and 8 weeks after the
operation. For comparison, the left femoral condyles of the Wistar
rats in the control group with normal age and weight were inspected
at the same position. The femoral condyles were fixed onto the
biomechanics machine (WD-P4204; Jinan Test Machine Co., Ltd.,
Jinan, China) table and compressed at a rate of 5 mm/min. During
the process, the femoral condyle displacement/time and
displacement/load were determined. A displacement of 2 mm (femoral
condyle thickness, ~5 mm) was defined as structural damage and this
pressure (the breaking load) was used to evaluate the carrying
capacity (compression area, ~24 mm2).
Histological observation
Four rats from each group were selected and the
right femoral condyles were inspected at 4, 6 and 8 weeks after the
operation. The bone at the bilateral femoral condyle (weight, ~2 g)
was excised until sterile conditions. The samples were then fixed
in 10% neutral buffered formalin for 24 h, decalcified in 50%
formic acid, dehydrated in an 80–100% ethyl alcohol series, a
95–100% hyaline xylene series and prepared for paraffin embedding.
The samples were sectioned longitudinally at 5 µm and stained with
hematoxylin and eosin (Sigma-Aldrich). The bone union in the
grafted area was then observed under an optical microscope (IX73;
Olympus Corporation, Tokyo, Japan).
Statistical analysis
Data analysis was performed using SPSS version 18.0
statistical software (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. The porosity was
analyzed by rank correlation, and intergroup comparisons were
carried out by one-way analysis of variance using the multi-sample
average. If the homogeneity of variance was satisfied, the
intergroup multiple comparisons were conducted with the method of
least significance difference, and for heterogeneity, Tamhanes T2
post hoc test was applied to the intergroup multiple comparisons.
Comparisons between two group were performed using the Students
t-test or t-test (for heterogeneity of variance) using the
independent sample average. The optical density value measured by
the cytotoxicity test was analyzed through factorial design
variance analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Physical and chemical properties of
GS/DBM/PLA and DBM/PLA
The porosity of the composite biomaterials
GS/DBM/PLA (5:3:2) and DBM/PLA (3:2) were evaluated using a t-test.
The porosity of GS/DBM/PLA (77.08%) was significantly reduced
compared with DBM/PLA (79.71%) (t=2.877; P=0.021; Table I). The compressive strength and
elastic modulus of the GS/DBM/PLA and DBM/PLA materials were
evaluated using a t-test (Table
II). The t-values were 2.056 and 2.028, and the P-values were
0.074 and 0.077, respectively. Thus, there was no significant
difference between the two groups with regards to compressive
strength or elastic modulus, although the compressive strength and
elastic modulus of GS/DBM/PLA was slightly inferior to that of
DBM/PLA. Table III presents the
data demonstrating the effects of GS/DBM/PLA and DBM/PLA composite
gentamicin leachate on cellular proliferation and Table IV presents the cytotoxicity rating
of GS/DBM/PLA and DBM/PLA composites. There are significant
differences in the cell optical density values at different time
points (F=1376.194; P<0.001), and the difference is also
significant between GS/DBM/PLA and DBM/PLA (F=2.983; P<0.001).
From days 3 to 7, there is no statistical difference among the
control, DBM/PLA and GS/DBM/PLA groups. The relative rate of
cellular proliferation from days 3 to 7 was >90% following
treatment with GS/DBM/PLA composite leachate and the cell toxicity
rating was 0 or 1, indicating that the GS/DBM/PLA composite was
almost non-cytotoxic relative to the DBM/PLA composite
material.
 | Table I.Porosity of GS/DBM/PLA and DBM/PLA
composite biomaterials (n=5). |
Table I.
Porosity of GS/DBM/PLA and DBM/PLA
composite biomaterials (n=5).
| Composite
material | Porosity (%) |
|---|
| GS/DBM/PLA |
77.08±1.63a,b |
| DBM/PLA |
79.71±1.23a,b |
 | Table II.Compressive strength and elastic
modulus of GS/DBM/PLA and DBM/PLA composite biomaterials (n=5). |
Table II.
Compressive strength and elastic
modulus of GS/DBM/PLA and DBM/PLA composite biomaterials (n=5).
| Composite
material | Compressive
strength | Elastic
modulus |
|---|
| GS/DBM/PLA |
100.99±7.28a,b |
13.82±2.00c,d |
| DBM/PLA |
108.71±4.19a,b |
11.82±0.94c,d |
 | Table III.OD values for the proliferation of
mouse fibroblast L929 cells in the presence of composite
biomaterials (n=5). |
Table III.
OD values for the proliferation of
mouse fibroblast L929 cells in the presence of composite
biomaterials (n=5).
|
| Composite
biomaterial |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Day | Control | DBM/PLA | GS/DBM/PLA | Mean OD | F-value | P-value |
|---|
| 1 | 0.392±0.040 | 0.515±0.022 | 0.395±0.051 | 0.434±0.070 | 15.618 | <0.001 |
| 2 | 0.474±0.060 | 0.577±0.027 | 0.457±0.044 | 0.503±0.069 | 10.152 | 0.003 |
| 3 | 0.582±0.019 | 0.546±0.044 | 0.584±0.019 | 0.570±0.033 | 2.616 | 0.114 |
| 4 | 0.716±0.081 | 0.744±0.047 | 0.702±0.040 | 0.721±0.057 | 0.651 | 0.539 |
| 5 | 1.306±0.053 | 1.272±0.043 | 1.282±0.066 | 1.287±0.053 | 0.504 | 0.616 |
| 6 | 1.388±0.045 | 1.346±0.029 | 1.392±0.037 | 1.375±0.041 | 2.328 | 0.140 |
| 7 | 1.325±0.027 | 1.304±0.036 | 1.316±0.035 | 1.315±0.032 | 0.478 | 0.632 |
| Mean |
0.883±0.415a,b |
0.901±0.366a,b |
0.876±0.413a,b | 0.886±0.394 | 4.318 | <0.001 |
| F | 380.161 | 562.808 | 498.743 | 1376.194 | – | – |
 | Table IV.Cell toxicity of GS/DBM/PLA and
DBM/PLA leachates. |
Table IV.
Cell toxicity of GS/DBM/PLA and
DBM/PLA leachates.
|
| Day |
|---|
|
|
|
|---|
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|
| GS/DBM/PLA | 0 | 1 | 0 | 1 | 1 | 0 | 1 |
|
DBM/PLAa | 0 | 0 | 1 | 0 | 1 | 1 | 1 |
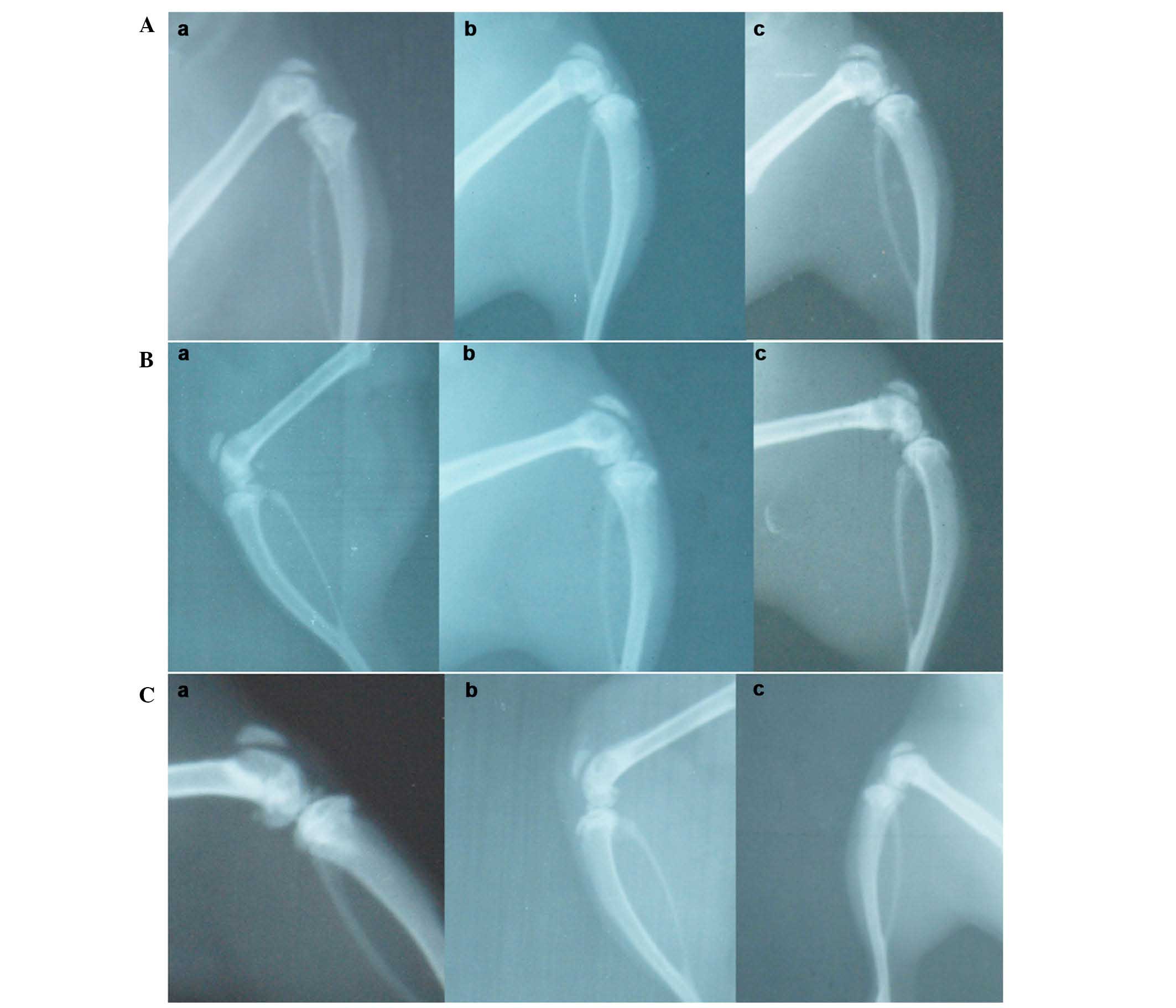
X-ray observation
Representative X-rays of the three different groups
are presented in Fig. 5. In the
GS/DBM/PLA group containing SA, at 4 weeks following implantation,
the defect repair area appeared cloudy, and the density of this was
highest at the center of the repair area (Fig. 5A). At 6 weeks after implantation, the
flocculent resistance projection grew denser (Fig. 5A). At 8 weeks following implantation,
the resistance projection at the defect region was nearly as dense
as normal bone (Fig. 5A). In the
DBM/PLA group containing SA, at 4 weeks following implantation
there was clear and uniform flocculent resistance projection in the
defect repair area and the defect center was radiolucent (Fig. 5B). At 6 weeks following implantation,
the flocculent resistance projection grew denser and more uniform,
but there remained a large area of transparent zones that could be
observed in the defect area (Fig.
5B). At 8 weeks following implantation, the flocculent
resistance projection was more dense and the transparent zones in
the center of the defect were smaller and irregular (Fig. 5C). In the DBM/PLA group without SA,
at 4 weeks following implantation, there was flocculent resistance
projection in the defect repair area and the transparent zones in
the center of defect were not obvious (Fig. 5A). At 6 weeks following implantation,
the flocculent resistance projection was uniform and dense
(Fig. 5B). At 8 weeks following
implantation, the defect region was nearly as dense as normal bone
(Fig. 5C).
Analysis of bone density
measurement
Variance analysis was conducted to evaluate the gray
values (bone mineral density) of materials collected at 4, 6 and 8
weeks post-operation, and there was significant difference recorded
at each point (Table V). At 4 weeks,
F=56.248, P<0.001; at 6 weeks, F=57.704, P<0.001; and at 8
weeks, F=25.793, P<0.001. Further intergroup comparisons
demonstrated statistical differences between each pair of groups
(P<0.001). The grey value of the DBM/PLA group without SA was
highest, and that of the DBM/PLA group containing SA was the
lowest. In addition, the grey value of the GS/DBM/PLA group
containing SA was significantly higher compared with the DBM/PLA
group containing SA. These results suggest that the DBM/PLA
material could promote the repair of bone defects and that
GS/DBM/PLA was beneficial to bone defect repair as a result of its
ability to reduce local infection.
 | Table V.Bone mineral density (pixels; gray
value) of bone repair in the area surrounding the composite
material (n=6). |
Table V.
Bone mineral density (pixels; gray
value) of bone repair in the area surrounding the composite
material (n=6).
|
| Gray value
(pixels) |
|---|
|
|
|
|---|
| Composite
material | Week 4a | Week 6b | Week 8c |
|---|
| Non-operated
control | 2.223±0.328 | 2.505±0.574 | 3.993±0.597 |
| GS/DBM/PLA |
1.230±0.265d |
1.693±0.296d |
2.850±0.555d |
| DBM/PLA |
3.333±0.420d,e |
4.520±0.494d,e |
5.968±1.035d,e |
Biomechanical compression tests
Variance analysis was conducted to evaluate the
breaking load and breaking strength of composite materials at 6 and
8 weeks following operation, and there was significant difference
between these values (Table VI). At
6 weeks, F=50.381, P<0.001, and at 8 weeks, F=93.291,
P<0.001. Further intergroup comparison demonstrated that, except
for the GS/DBM/PLA group containing SA and the DBM/PLA group
without SA at 6 weeks (P=0.097), there was statistical difference
among the other groups (P<0.05). The analysis indicated that the
breaking load and breaking strength were highest in the normal
control group, followed by the DBM/PLA group without SA, and that
the breaking load and breaking strength were lowest in the DBM/PLA
group containing SA, which was lower than those in the GS/DBM/PLA
group containing SA. These differences were statistically
significant and suggest that the DBM/PLA material can promote the
repair of bone defects and that GS/DBM/PLA supported bone defect
repair applying its anti-infection properties.
 | Table VI.Anti-compression biomechanical
evaluation of the femoral condyle in the presence of a composite
material with, or without, SA (n=4). |
Table VI.
Anti-compression biomechanical
evaluation of the femoral condyle in the presence of a composite
material with, or without, SA (n=4).
|
| Week 6a | Week 8b |
|---|
|
|
|
|
|---|
| Composite
material | Load (N) | Strength (MPa) | Load (N) | Strength (MPa) |
|---|
| GS/DBM/PLA (with
SA) | 288.284±31.255 | 12.012±1.302 | 363.029±23.828 | 15.126±0.993 |
| DBM/PLA (with
SA) |
192.376±15.811c |
8.01±0.659c |
248.687±21.465c |
10.362±0.894c |
| DBM/PLA (without
SA) |
322.530±32.802d |
13.439±1.367d |
412.902±11.748a,d |
17.204±0.490c,d |
| Normal control
group |
88.696±22.174c,d,e |
17.651±1.012c,d,e |
527.878±33.655c,d,e |
21.995±1.402c,d,e |
Histological observation
In the GS/DBM/PLA group containing SA, after 4
weeks, a small area of inflammatory tissue was observed among the
implanted biomaterial (Fig. 6).
After 6 weeks, mesenchymal cells grew into the implanted bone, and
a number of inflammatory cells and polykaryocytes were also
observed. Osteoblasts could be seen around the implanted material.
After 8 weeks, the implanted area was completely substituted by new
bone tissue and the condition of bone union was similar to that of
the DBM/PLA group without SA at 8 weeks. In the DBM/PLA group
containing SA after 4 weeks, the implanted material was covered by
inflammatory fibrous tissue in which a large amount of inflammatory
cells were observed. After 8 weeks, the implanted material was
almost absorbed by the inflammatory fibrous tissue, despite some
DBM fragments, and the implanted area was saturated with
inflammatory fibrous tissue.
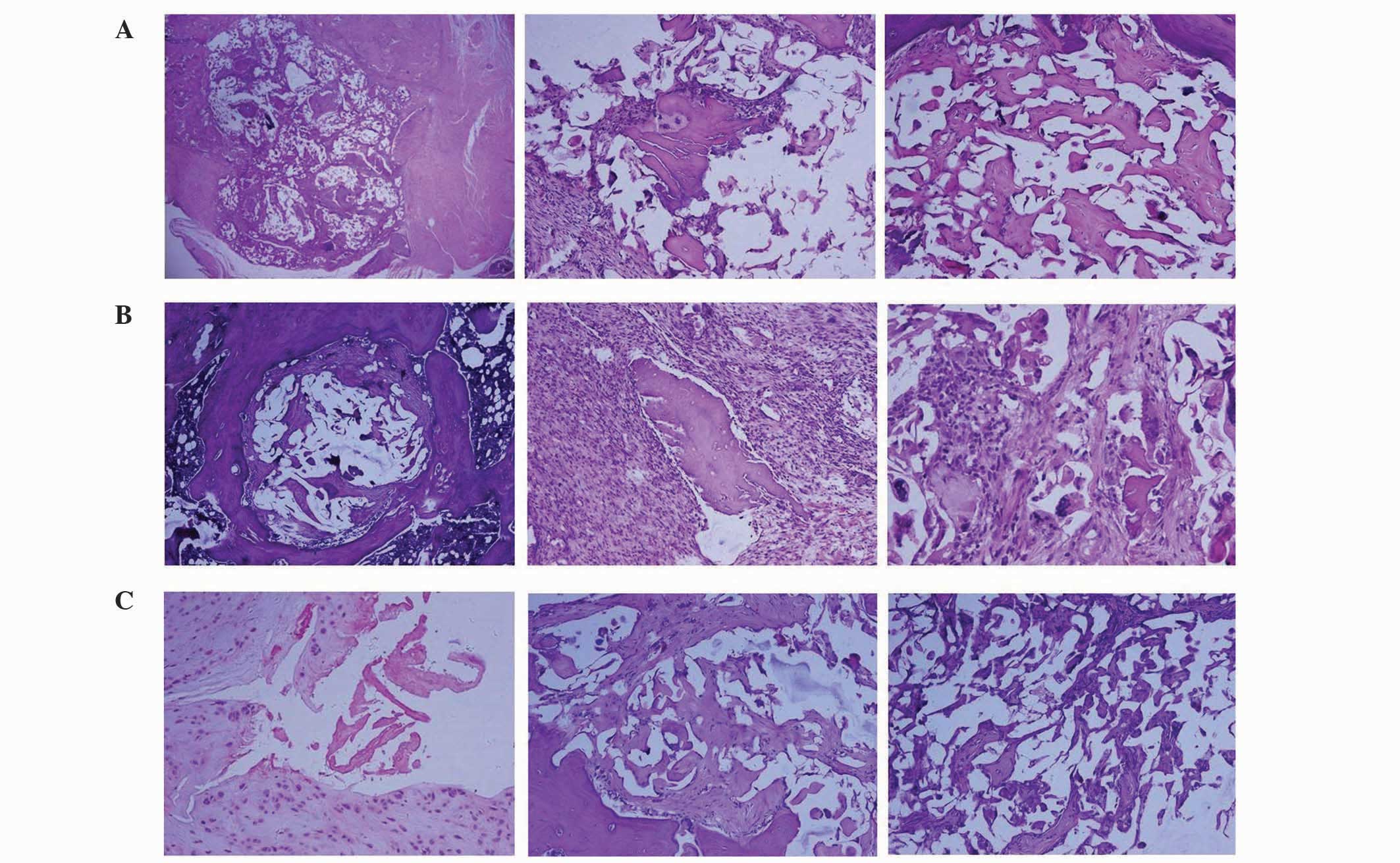 | Figure 6.Histological images, stained with
hematoxylin and eosin, of biomaterial implanting (A) after 4 weeks,
displaying a small area of inflammatory tissue among the implanted
biomaterial, (B) after 6 weeks, displaying mesenchymal cells
growing into the implanted bone with a number of inflammatory
cells, multinucleated cells around the demineralized bone matrix
and osteoblasts in the lacuna, and (C) after 8 weeks, when the
implanting area was fully covered with new-borne bone tissue,
showing complete dissipation of inflammation (magnification,
×100). |
Discussion
In the present study, the GS/DBM/PLA porous
composite was prepared using a SC-CO2 fluid technique,
and DBM/PLA was the sustained-release carrier for GS. It was
observed that the porous GS/DBM/PLA composite material accelerated
repair of bone defects while controlling infection in the rat
model.
Poly(methyl methacrylate) (PMMA) was the earliest
sustained-release drug material applied in clinical practice, and
it achieves evident curative effect in the treatment of bone
infections and bone defect repairs. However, PMMA has a number of
limitations, including poor histocompatibility, heat release during
polymerization, toxicity of its monomer, a second operation
required to remove it and inadequate drug release (19). These disadvantages of PMMA impose
many restrictions on its clinical application (20,21).
A number of studies have been conducted on the
materials that can provide sustained-release of antibiotics. For
anti-infective bone defect repair, studies have investigated
composites to contain antibiotics including allogeneic bone
(22,23), alginates (24), hydroxyapatites (25) and beta tricalcium phosphate (26). However, appropriate biological
activity, degradability and mechanical strength cannot be satisfied
by a single material, and therefore creating an anti-infective
material that contains two different methods of producing
sustained-release of antibiotic with desirable properties is a
vital area of research. The DBM/PLA material prepared in the
present study was demonstrated to have favorable biocompatibility,
bone inductive activity and mechanical properties. When composited
with GS, it exhibited good anti-infective ability, effectively
inhibiting the growth of SA and promoting the union of bone
defects.
After grafting, degradable material may fill the
cavity of the bone defect and release a high concentration of
antibiotics. Compared with systemic administration, this method
avoids adverse antibiotic side effects as well as bacterial drug
resistance (27). In addition,
degradable material does not require a second operation to remove
it; space for bone growth is provided as the material degrades
(28). The degradable
sustained-release materials PLA, polyglycolic acid and their
copolymer poly(glycolide-co-lactide) have numerous advantages,
including high local drug concentration, long maintenance time,
lack of toxic and allergic reactions and acceptable compatibility
with most antibiotics (29,30). In addition, these materials can
usually be metabolized in the human body and their decomposition
products, carbon dioxide and water, have no toxic or other adverse
side effects in humans (31).
The GS/DBM/PLA porous composite prepared in the
current study combined the advantages of its three components: The
easy modelling and good biomechanical properties of PLA, the
biocompatibility and bone induction properties of DBM and the
broad-spectrum antibacterial activity of GS. As a result, the
composite has the favorable properties required to treat infected
bone defects. As previously reported, when the porosity of a
biomaterial is >60%, new bone tissue can grow from the surface
inwards along the intercommunicated pores, forming a network
structure (32). The porosity of the
GS/DBM/PLA porous composite in the present study was 77.1%, and its
compressive strength and elasticity modulus were 101.0±7.3 and
13.8±2.0 MPa, respectively. Cell compatibility is another important
indicator affecting bone tissue engineering material. The
cytotoxicity grade of the GS/DBM/PLA porous composite in this study
was rated as 0 or 1, which is almost nontoxic. This satisfies the
essential requirements for bone tissue engineering scaffold
material.
In the present study, the materials were processed
at <37°C to ensure bioactivity during compositing. During the
SC-CO2 reaction process, low pressure may lead to
oversized and uneven pores, while excessive pressure may cause
complete swelling of the material and collapse of the pores
(33). The reaction pressure in the
present study was 20 MPa and ideal porosity was obtained; the
porosity of the DBM/PLA composite was >65% and increased with
the proportion of DBM, reaching 79.7% at the optimal composite
proportion of DBM/PLA (3:2). High porosity enlarges the contact
area between the scaffold material and cells, thus promoting the
cell adhesive growth and facilitating the entrance of oxygen and
nutrients, and the excretion of metabolic products.
Response time is another crucial factor that
influences the size of pores in PLA material. The porosity of the
material formed from pure PLA after 30 min of SC-CO2
reaction is relatively high and the pore size is ~300 µm; a
reaction that is much shorter or longer is not beneficial to the
formation of pores on the PLA surface (34). If the reaction time is too short the
segmental motion will be incomplete, and if the reaction time is
too long the chain segments will rearrange and there will be fewer
pores. Adequate infiltration time ensures that carbon dioxide will
infiltrate the sample sufficiently, and after pressure reduction
and foaming, a porous scaffold material with high porosity will be
obtained (35,36). Therefore, in the present study the
reaction time was set to 30 min.
The pressure reduction time also has a marked
influence on the porosity of the composite material. If the
pressure reduction time is too short, the PLA will rapidly separate
from the supercritical system and the curing speed will be high.
Furthermore, the speed of CO2 overflowing out of the PLA
will be high, leading to a low expansion rate (37). If the pressure reduction time is
prolonged to 15 min, the curing speed of the PLA and the overflow
speed of the carbon dioxide will reduce, leaving sufficient time
for a uniform foam to form and solidify, resulting in a microporous
structure of relatively large diameter (38). On this basis, the current study
adopted 15 min as the pressure reduction time.
Bucholz (39)
proposed that the pore diameter of bone substitute material should
not be <100 µm, in order to avoid stunting the growth of bone.
However, if the pore diameter is >500 µm, the strength and cell
adhesion rate of the material would be adversely affected. Porter
et al (40) considered that a
pore diameter of >200 µm is essential for bone conduction, and a
pore diameter between 200 and 400 µm is ideal for bone growth.
Therefore, the pore diameter of the DBM/PLA porous composite
prepared in the present study was between 200 and 400 µm. Thus, the
material was able to promote the growth of bone tissue into the
material and facilitate cell adhesion and nutrient diffusion. In
addition, there were small pores (diameter, 20–30 µm) on the pore
wall of the material which may assist the growth of bone tissue and
blood vessels.
In conclusion, the GS/DBM/PLA porous composite
prepared by the SC-CO2 fluid technique has favorable
porosity, mechanical properties, bone induction/conduction ability,
local antibacterial ability and bone defect repair efficacy.
Therefore, the GS/DBM/PLA porous composite is a potentially viable
bone tissue engineering material for the repair of infective bone
defects.
Acknowledgments
The present study was supported by a grant from the
Shenzhen Governmental Basic Research Grant (no.
JCYJ20120617115010496), the State Key Laboratory of Bioelectronics
of Southeast University (Nanjing, China) and the Doctor Startup
Foundation of Zunyi Medical College (no. F-746).
References
|
1
|
Roberts TT and Rosenbaum AJ: Bone grafts,
bone substitutes and orthobiologics: The bridge between basic
science and clinical advancements in fracture healing.
Organogenesis. 8:114–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crane GM, Ishaug SL and Mikos AG: Bone
tissue engineering. Nat Med. 1:1322–1324. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Won YH, Kim SG, Oh JS and Lim SC: Clinical
evaluation of demineralized bone allograft for sinus lifts in
humans: A clinical and histologic study. Implant Dent. 20:460–464.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lima CE, Calixto JC and Anbinder AL:
Influence of the association between simvastatin and demineralized
bovine bone matrix on bone repair in rats. Braz Oral Res. 25:42–48.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang HY, Luo JB, Zhou M, Zhang Y and
Huang YL: Biotribological properties at the stem-cement interface
lubricated with different media. J Mech Behav Biomed Mater.
20:209–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Zhang S, Luo J, Liu Y, Qian S,
Liang F and Huang Y: Investigation of protein adsorption mechanism
and biotribological properties at simulated stem-cement interface.
J Tribol. 135:0323012013. View Article : Google Scholar
|
|
7
|
Liu H, Zhang L, Shi P, Zou Q, Zuo Y and Li
Y: Hydroxyapatite/polyurethane scaffold incorporated with
drug-loaded ethyl cellulose microspheres for bone regeneration. J
Biomed Mater Res B Appl Biomater. 95:36–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu C, Su P, Wang Y, Chen X, Meng Y, Liu C,
Yu X, Yang X, Yu W, Zhang X, et al: A novel biomimetic composite
scaffold hybridized with mesenchymal stem cells in repair of rat
bone defects models. J Biomed Mater Res A. 95:495–503. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thomas DB, Brooks DE, Bice TG, DeJong ES,
Lonergan KT and Wenke JC: Tobramycin-impregnated calcium sulfate
prevents infection in contaminated wounds. Clin Orthop Relat Res.
441:366–371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Agarwal A, Goyal RK and Pruthi KK: Fate of
calcium hydroxyapatite blocks with cortico-cancellous autogenous
bone grafting in gap non-union of long bones along with LCP. J Clin
Orthop Trauma. 6:72–73. 2015. View Article : Google Scholar
|
|
11
|
Ajiboye RM, Hamamoto JT, Eckardt MA and
Wang JC: Clinical and radiographic outcomes of concentrated bone
marrow aspirate with allograft and demineralized bone matrix for
posterolateral and interbody lumbar fusion in elderly patients. Eur
Spine J. 24:2567–2572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tavakol S, Khoshzaban A, Azami M, Kashani
IR, Tavakol H, Yazdanifar M and Sorkhabadi SM: The effect of
carrier type on bone regeneration of demineralized bone matrix
in vivo. J Craniofac Surg. 24:2135–2140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turner TM, Urban RM, Hall DJ, Chye PC,
Segreti J and Gitelis S: Local and systemic levels of tobramycin
delivered from calcium sulfate bone graft substitute pellets. Clin
Orthop Relat Res. 437:97–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bakhshalian N, Hooshmand S, Campbell SC,
Kim JS, Brummel-Smith K and Arjmandi BH: Biocompatibility and
microstructural analysis of osteopromotive property of allogenic
demineralized dentin matrix. Int J Oral Maxillofac Implants.
28:1655–1662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jimi E, Hirata S, Osawa K, Terashita M,
Kitamura C and Fukushima H: The current and future therapies of
bone regeneration to repair bone defects. Int J Dentistry.
1482612012.
|
|
16
|
Chen ZW, Liu H and Zhai WL and Zhai WL:
Treatment of infected bone defect with one stage open cancellous
bone grafting. Zhongguo Gu Shang. 21:377–378. 2008.(In Chinese).
PubMed/NCBI
|
|
17
|
García-Gareta E, Coathup MJ and Blunn GW:
Osteoinduction of bone grafting materials for bone repair and
regeneration. Bone. 81:112–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li XD and Hu YY: The treatment of
osteomyelitis with gentamicin-reconstituted bone
xenograft-composite. J Bone Joint Surg Br. 83:1063–1068. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hautamäki MP, Aho AJ, Alander P, Rekola J,
Gunn J, Strandberg N and Vallittu PK: Repair of bone segment
defects with surface porous fiber-reinforced polymethyl
methacrylate (PMMA) composite prosthesis: Histomorphometric
incorporation model and characterization by SEM. Acta Orthop.
79:555–564. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng J, Su Q, Wang C, Cheng G, Zhu R, Shi
J and Yao K: Synthesis and biological evaluation of PMMA/MMT
nanocomposite as denture base material. J Mater Sci Mater Med.
22:1063–1071. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Kawamura K, Kawashita M, Kudo TA,
Kanetaka H and Hiraoka M: In vitro assessment of
poly(methylmethacrylate)-based bone cement containing magnetite
nanoparticles for hyperthermia treatment of bone tumor. J Biomed
Mater Res A. 100:2537–2545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fong N, Poole-Warren LA and Simmons A:
Development of sustained-release antibacterial urinary biomaterials
through using an antimicrobial as an organic modifier in
polyurethane nanocomposites. J Biomed Mater Res B Appl Biomater.
101:310–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quaglino P, Nardò T, Fierro MT, Massaia M,
Orsucci L, Fava P, Marenco F, Marra E, Savoia P, Vitolo U, et al:
Clinicopathologic spectrum of cutaneous diseases in patients with
hematologic malignancies with or without allogeneic bone marrow
transplantation: An observational cohort study in 101 patients. G
Ital Dermatol Venereol. 148:453–463. 2013.PubMed/NCBI
|
|
24
|
Anal AK and Stevens WF: Chitosan-alginate
multilayer beads for controlled release of ampicillin. Int J Pharm.
290:45–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Buranapanitkit B, Srinilta V, Ingviga N,
Oungbho K, Geater A and Ovatlarnporn C: The efficacy of a
hydroxyapatite composite as a biodegradable antibiotic delivery
system. Clin Orthop Relat Res. 424:244–252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Silverman LD, Lukashova L, Herman OT, Lane
JM and Boskey AL: Release of gentamicin from a tricalcium phosphate
bone implant. J Orthop Res. 25:23–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pandey M, Amin M, Ahmad N and Abeer MM:
Rapid synthesis of superabsorbent smart-swelling bacterial
cellulose/acrylamide-based hydrogels for drug delivery. Int J Polym
Sci. 9054712013.
|
|
28
|
Corden TJ, Jones IA, Rudd CD, Christian P,
Downes S and McDougall KE: Physical and biocompatibility properties
of poly-epsilon-caprolactone produced using in situ polymerisation:
A novel manufacturing technique for long-fibre composite materials.
Biomaterials. 21:713–724. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ambrose CG, Clyburn TA, Louden K, Joseph
J, Wright J, Gulati P, Gogola GR and Mikos AG: Effective treatment
of osteomyelitis with biodegradable microspheres in a rabbit model.
Clin Orthop Relat Res. 421:293–299. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mäkinen TJ, Veiranto M, Lankinen P, Moritz
N, Jalava J, Törmälä P and Aro HT: In vitro and in
vivo release of ciprofloxacin from osteoconductive bone defect
filler. J Antimicrob Chemother. 56:1063–1068. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stiller M, Kluk E, Bohner M, Lopez-Heredia
MA, Muller-Mai C and Knabe C: Performance of β-tricalcium phosphate
granules and putty, bone grafting materials after bilateral sinus
floor augmentation in humans. Biomaterials. 35:3154–3163. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoshikawa T, Ohgushi H, Uemura T, Nakajima
H, Ichijima K, Tamai S and Tateisi T: Human marrow cells-derived
cultured bone in porous ceramics. Biomed Mater Eng. 8:311–320.
1998.PubMed/NCBI
|
|
33
|
Hile DD, Amirpour ML, Akgerman A and
Pishko MV: Active growth factor delivery from
poly(D,L-lactide-co-glycolide) foams prepared in supercritical
CO(2). J Control Release. 66:177–185. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang YM, Li BX, Li J, Ma HQ, Zhao YP and
Yuan L: Preparation of porous polylactic-acid/ bone matrix gelatin
composite as scaffold materials for bone-tissue engineering. Nan
Fang Yi Ke Da Xue Xue Bao. 26:1745–1748. 2006.(In Chinese).
PubMed/NCBI
|
|
35
|
Link DP, van den Dolder J, Jurgens WJ,
Wolke JG and Jansen JA: Mechanical evaluation of implanted calcium
phosphate cement incorporated with PLGA microparticles.
Biomaterials. 27:4941–4947. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yandrapu SK, Upadhyay AK, Petrash JM and
Kompella UB: Nanoparticles in porous microparticles prepared by
supercritical infusion and pressure quench technology for sustained
delivery of bevacizumab. Mol Pharm. 10:4676–4686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nofar M, Tabatabaei A and Park CB: Effects
of nano-/micro-sized additives on the crystallization behaviors of
PLA and PLA/CO2 mixtures. Polymer. 54:2382–2391. 2013. View Article : Google Scholar
|
|
38
|
Li Z, Ramay HR, Hauch KD, Xiao D and Zhang
M: Chitosan-alginate hybrid scaffolds for bone tissue engineering.
Biomaterials. 26:3919–3928. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bucholz RW: Nonallograft osteoconductive
bone graft substitutes. Clin Orthop Relat Res. 395:44–52. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Porter NL, Pilliar RM and Grynpas MD:
Fabrication of porous calcium polyphosphate implants by solid
freeform fabrication: A study of processing parameters and in
vitro degradation characteristics. J Biomed Mater Res.
56:504–515. 2001. View Article : Google Scholar : PubMed/NCBI
|