Introduction
Mast cells, first identified by Paul Ehrlich in
1878, originate from multilineage hematopoietic progenitors that
migrate to the organs and tissues and mature, ultimately residing
under the effect of local cytokines and stem cell factor (1–4). Mast
cells are predominantly localized at sites that are in close
contact with the external environment, including the respiratory
tract, gastrointestinal tract and skin. These cells function as
important sentinel cells, which identify risk, and initiate and
coordinate an inflammatory response following their activation
(5–7). Mast cells are considered to be
multifunctional cells that can be produced and stored, and
specifically recognize and respond to various stimuli by releasing
an array of biologically active mediators (1,4).
Therefore, mast cells participate in various biological processes,
including the maintenance of homeostasis, angiogenesis, innate and
adaptive immunity and immune tolerance. They also serve a
significant role in several diseases, such as bronchial asthma,
chronic skin inflammation (8),
autoimmune diseases including rheumatoid arthritis (2,9),
atherosclerosis (10), cancer
(11) and fibrotic diseases
(4,7). A correlation between disease severity
and the number of mast cells has been previously identified in
certain of the aforementioned diseases (2). Thus, controlling the mast cell numbers
may serve as an attractive therapeutic intervention in mast
cell-associated diseases, while a feasible strategy to counteract
mast cell-dependent disease is to selectively induce mast cell
apoptosis (5,12,13).
Mast cells have been demonstrated to serve a primary role in asthma
(14), and previous studies have
reported that miR-223 increased remarkably in the serum of children
with asthma. Similar findings have been observed in an OVA-induced
murine asthma model (15).
MicroRNAs (miRNAd or miRs) are a group of small,
non-coding, single-stranded RNA molecules with a length of ~22
nucleotides. MiRNAs bind to the 3′-untranslated region (3′-UTR) of
mRNAs, which then results in mRNA degradation or translational
inhibition, thus post-transcriptionally regulating the translation
of target genes (16–19). These target genes regulate a wide
variety of biological processes, including differentiation,
proliferation, maturation, apoptosis and tumorigenesis (16–19).
Insulin-like growth factor 1 receptor (IGF-1R) is a
transmembrane receptor tyrosine kinase that is widely expressed in
numerous cell lines and cell types, which is very important for
cellular proliferation in vivo. The primary components of
the IGF-1R pathway include IGF-1R and its highly structurally
conserved family member, the insulin receptor. Both receptors
consist of two half-receptors, each comprising one extracellular
α-subunit and one transmembrane β-subunit that possesses tyrosine
kinase activity. IGF-1R signaling cascades begin at the cell
surface with IGF ligands (IGF-1 and IGF-2) binding to several
transmembrane receptors, namely IGF-1R, IGF-2R and the IR, which
serves an important role in cell growth, transformation, and the
protection of cells from a variety of apoptotic stimuli (20–23). The
relationship between microRNA and IGF-1R has attracted increasing
attention. It has been reported that miR-7 (24), miR-320a (25) and miR-503 (26) modulate glioma cell functions
including proliferation, apoptosis, migration, invasion and
tumorigenesis by targeting IGF-1R. In addition, researches have
demonstrated that upregulation of miR-150, miR-630 (27) and miR-497 (28) inhibit cell proliferation and enhance
apoptosis in pancreatic cancer cells by targeting IGF-1R. More
importantly, studies have confirmed that miR-223 serves an
important role in cell proliferation and apoptosis by targeting the
IGF-1R in other cell types (19,29).
Apoptosis regulated by a specific gene is also known
as programmed cell death. Previous studies have confirmed several
components via which mast cell apoptosis is regulated, including
growth factors, monomeric IgE, Toll-like receptors, tumor necrosis
factor-α receptors and proteins of the Bcl-2 family (2,30).
However, the underlying mechanism via which miR-223 regulates the
apoptosis of mast cells remains unclear. In the present study, the
role of miR-223 in cell apoptosis was investigated to determine the
potential mechanism by which the miR-223-induced increase in cell
apoptosis is mediated.
Materials and methods
Cell culture and transfection
Rat basophilic leukemia (RBL) cells were obtained
from the Shanghai Institute of Biochemistry and Cell Biology
(Chinese Academy of Science, Beijing, China). The RBL cells, were
cultured in Eagle's minimal essential medium (EMEM) supplemented
with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.,
Carlsbad, CA, USA). The cells were incubated at 37°C in a
humidified atmosphere supplemented with 5% CO2. The
cells were transfected with an miR-223 mimic the following day by
seeding using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.).
Lipofectamine 2000 alone was used as the control (empty vector). In
order to induce miR-223 overexpression, 50 nM miR-223 mimic was
used (Thermo Fisher Scientific, Inc.). The cells were harvested 24
h after transfection, and total RNA was extracted for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis. Furthermore, cell lysates were prepared for western
blotting. Cell apoptosis assays were also performed subsequent to
inducing serum starvation for 24 h.
RT-qPCR
Total RNA was extracted from RBL cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Next, total
RNA was reverse transcribed into cDNA using a TaqMan MicroRNA
Reverse Transcription Kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
qPCR was performed using an Applied Biosystems 7500 Fast Real-Time
PCR system (Thermo Fisher Scientific, Inc.) and a TaqMan MicroRNA
Assay (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
following thermal conditions were used for all qPCR reactions:
Amplification at 95°C for 5 min, 40 cycles at 95°C for 10 sec, at
60°C for 30 sec and at 72°C for 10 sec. All reactions were
performed in triplicate with a final volume of 20 µl (10 µl TaqMan
master mix, 1 µl cDNA, 1 µl miR-223/U6RNA probe and 8 µl
diethylpyrocarbonate). The primers used in the PCR experiment were
as follows: miR-223 forward, 5′-GTGCAGGGTCCGAGGT-3′, and reverse,
5′-CGGGCTGTCAGTTTGTCA-3′; U6RNA forward, 5′-CTCGCTTCGGCAGCACA-3′,
and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. The miRNA expression was
determined using the 2−ΔΔCq method (31). U6RNA (Invitrogen; Thermo Fisher
Scientific, Inc.) was used as an internal control.
Transfection
RBL cells were plated into a 96-well plate at a
density of 3,000 cells/well; Lipofectamine 2000 was used as the
vector, and RBL cells were transfected with an miR-223 mimic.
Briefly, one day prior to transfection, RBL cells were placed in
EMEM supplemented with 10% FBS until 30–50% confluence. For miR-223
overexpression, 50 nM mimic miR-223 was used. Cells were incubated
at 37°C in a CO2 incubator for 24–48 h; the growth
medium was replaced of EMEM supplemented with 10% FBS after 4–6
h.
Cell viability assay
RBL cells were transfected with 50 nM miR-223
mimics, and cell viability was assessed 24 h post-transfection
after serum starvation for 24 h. For the cell viability assay, 10
µl Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) solution was added to each well of 96-well
plates (3,000 cells/well) and the plates were incubated for 1 h
with EMEM supplemented with 10% FBS. The optical density (OD) at
450 nm was recorded using a microplate reader (DNM-9602; Beijing
Perlong New Technology Co., Ltd., Beijing, China). The OD value
representing the cell viability and number in each group was
calculated and summarized based on the results of three independent
experiments.
Apoptosis assay
Apoptosis of RBL cells was detected using an Annexin
V-fluorescein isothiocyanate (FITC)/propidium-iodide (PI) staining
assay. In order to induce cell apoptosis, the cells were grown to
50–60% confluence, then washed twice with FBS-free EMEM and starved
in EMEM without 10% FBS for 24 h. Flow cytometric analysis of
apoptotic cells was performed using a FITC Annexin V Apoptosis
Detection Kit I (BD Biosciences; Franklin Lakes, NJ, USA).
Subsequent to washing with cold phosphate buffered saline (PBS),
the cells were resuspended in binding buffer and stained with
Annexin V-FITC/PI for 15 min in darkness at room temperature.
Apoptotic cells were then evaluated by gating PI- and Annexin
V-positive cells on a BD FACSCalibur flow cytometer (BD
Biosciences). All the experiments were performed in triplicate. The
distribution of cells was analyzed using Cell Quest Pro software
(version 4.01; BD Biosciences) within 1 h of staining. Data from
10,000 cells were collected for each data file, and the number of
cells in each category was expressed as a percentage of the total
number of stained cells.
Dual luciferase reporter assay
A luciferase reporter assay was performed on
HEK-293T cells (Shanghai Institute of Biochemistry and Cell Biology
(Chinese Academy of Science) in order to confirm that IGF-1R is the
target gene of miR-223. At 48 h after transfection, the transfected
cells were lysed using 100 µl passive lysis buffer (Promega Corp.,
Madison, WI, USA), subsequent to washing twice in PBS. Next, 20 µl
lysate was used to measure the luciferase activity with a
Dual-Luciferase Reporter Assay system (Promega Corp.). The data
were calculated based on the results of four independent
experiments. The mutated psiCHECK™-2-IGF-1R 3′-UTR (China Anping
Kang Biological Technology Co., Ltd., Shenzhen, China) was also
transfected under the same conditions. Subsequently, the miR-223
inhibitor and the control were used at a final concentration of 100
nM in order to determine the inhibitory effect of miR-223 on the
3′-UTR of IGF-1R.
Western blot analysis
RBL cells were cultured in 6-well plates
(3×105 cells/well) and lysed in ice-cold
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Nantong, China). The total protein concentration was
determined using an enhanced BCA protein assay kit (Beyotime
Institute of Biotechnology). An equal amount of protein was then
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto nitrocellulose filter
membranes (Merck Millipore, Billerica, MA, USA). Next, the blots
were blocked with 5.0% non-fat milk in PBS with Tween 20 (PBST) for
2 h at room temperature. The blots were then incubated overnight at
4°C with specific primary antibodies against the following: IGF-1R
(rabbit monoclonal; 9750), protein kinase B (Akt; rabbit
polyclonal; 9272) and phosphorylated-Akt (Ser473; p-Akt; rabbit
monoclonal; 4060) at dilutions of 1:1,000, 1:1,000 and 1:2,000 (all
three from Cell Signaling Technology, Inc., Beverly, MA, USA); and
B-cell lymphoma-2 (Bcl-2; dilution, 1:200; sc-7382) and GAPDH
(rabbit polyclonal; dilution, 1:1,000; sc-25778) (both purchased
from Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Subsequently, the membranes were washed three times with PBST and
then incubated with horseradish peroxidase-conjugated goat
anti-rabbit or anti-mouse antibodies (1:5,000; ZB-2305; Zhongshan
Golden Bridge Biotechnology Co., Ltd., Zhongshan, China) for 2 h at
room temperature. Signals were detected on a gel imaging system
using the Pierce ECL Western blotting substrate (Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Statistical analysis
Data were analyzed using SPSS software version 20.0
(IBM Corp., Armonk, NY, USA). The Student's t-test was used for the
analysis of statistical differences between two independent groups.
Quantitative data are expressed as the mean ± standard deviation of
at least three independent experiments. Differences were considered
as statistically significant at P<0.05.
Results
Upregulation of miR-223 in mast
cells
In order to elucidate the role of miR-223 in mast
cells, a model of overexpressed miR-223 was established by
transfecting the cells with miR-223 mimics using Lipofectamine
2000. Subsequently, in order to identify miR-223 expression in mast
cells, RT-qPCR was performed, and the results showed that miR-223
expression was significantly higher in cells transfected with
miR-223 compared with the expression in the control group (Table I).
 | Table I.miR-223 overexpressed in mast
cells. |
Table I.
miR-223 overexpressed in mast
cells.
| Group | miRNA | Fold change | P-value |
|---|
| Control | miR-223 |
1.08 |
|
| miR-223 | miR-223 | 3549.66 | <0.001 |
miR-223 suppression of mast cell
viability
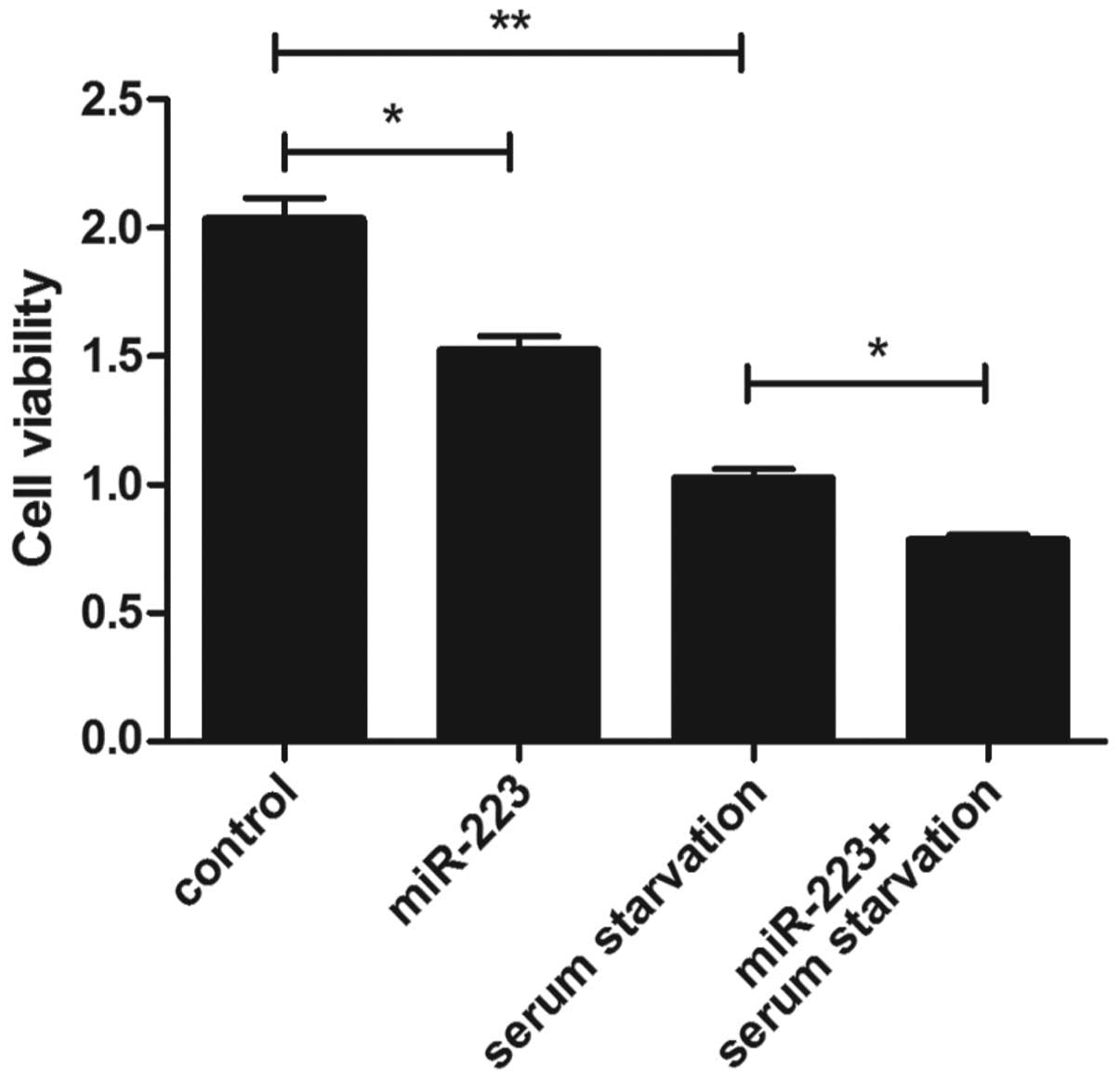
A CCK-8 assay was performed in order to investigate
the viability of mast cells. At 24 h after serum starvation, a
significant decrease in cell viability was detected when compared
with that in the control group (Fig.
1). As shown in Fig. 1, the cell
viability was decreased in the miR-223 group compared with that in
the control group, and the same result was observed for the
miR-223-transfected cells under the serum starvation
conditions.
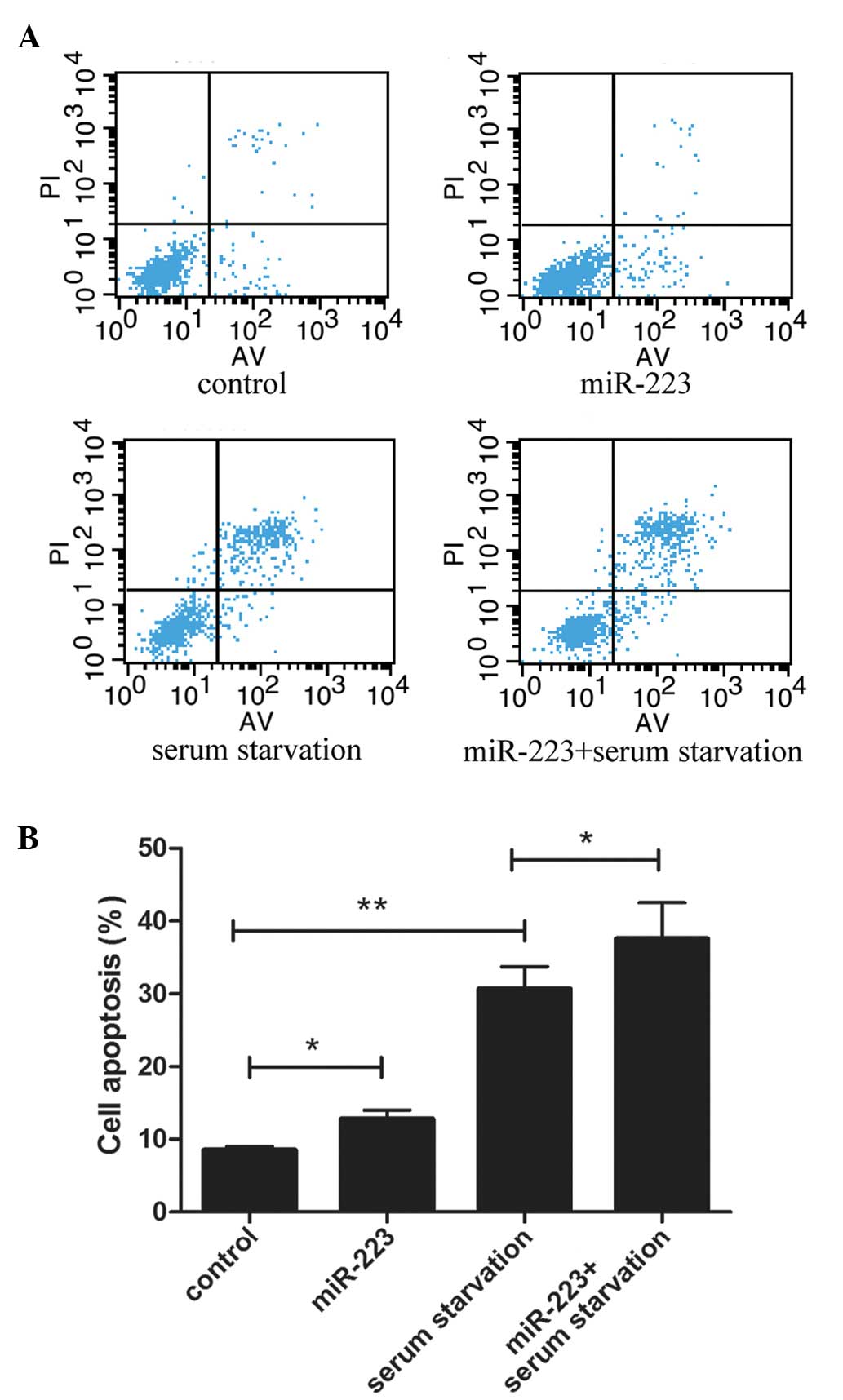
miR-223 promotes cell apoptosis
To detect the effect of miR-223 on the apoptosis of
mast cells, a cytofluorometric apoptosis assay was performed and
the flow cytometry results are shown in Fig. 2A. Previous studies have shown that
serum starvation can effectively induce cell apoptosis (32,33). In
the current study, serum starvation conditions were induced on
cultured mast cells for 24 h in order to initiate cell apoptosis.
As shown in Fig. 2B, incubation of
the cells with miR-223 significantly increased the serum
starvation-induced apoptosis of mast cells.
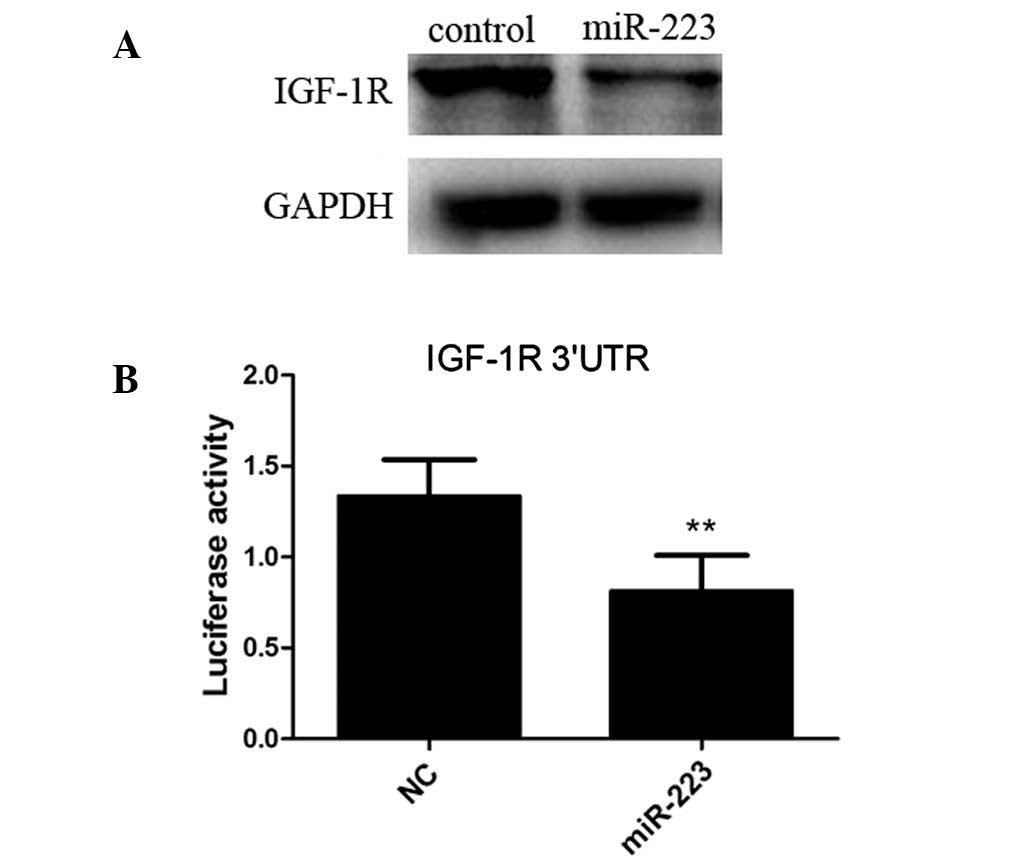
IGF-1R is targeted by miR-223
An increasing number of studies are investigating
IGF-1R, which has been demonstrated to serve a significant role in
the regulation of cell proliferation and apoptosis (34–37).
Therefore, in order to further investigate the underlying mechanism
through which miR-223 promotes the apoptosis of mast cells, IGF-1R
was selected in the present study. The results demonstrated that
the IGF-1R protein expression levels, as detected by western
blotting, decreased in the cells overexpressing miR-223 compared
with those in the control group (Fig.
3A). A 3′-UTR reporter assay was then conducted to determine
whether miR-223 binds to the 3′-UTR of IGF-1R. The results showed a
decrease in luciferase activity in the cells transfected with an
miR-223 expression vector compared with the cells transfected with
the control vector (Fig. 3B),
indicating that IGF-1R was inhibited in IGF-1R 3′-UTR by
miR-223.
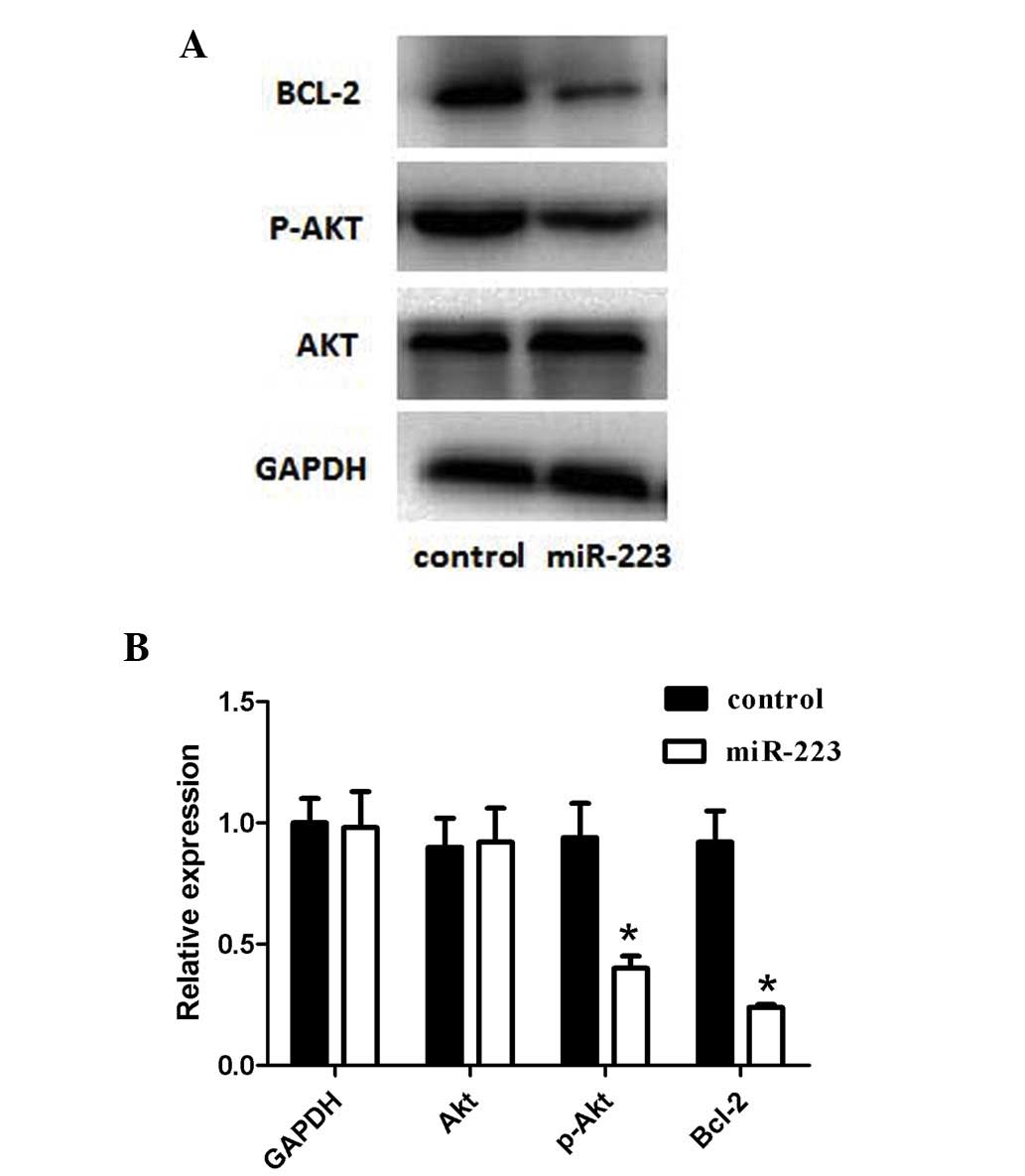
miR-223 inhibition of PI3K/Akt
signaling pathway
The expression levels of Akt, an essential protein
kinase in the PI3K/Akt signaling pathway downstream of IGF-1R, and
of its active form (p-Akt) were determined to assess whether the
IGF-1R-mediated downstream signaling pathway was also inhibited by
miR-223. The results revealed that the expression of p-Akt was
significantly decreased compared with that of the control group;
however, the expression of total Akt was unaffected (Fig. 4). Furthermore, Bcl-2 (an
anti-apoptotic regulator) is one of the downstream molecules, which
are normally promoted by p-Akt. In the present study, the protein
expression of Bcl-2 was downregulated upon miR-223 transfection,
when compared with that in the control group (Fig. 4).
Discussion
In the present study, increasing apoptosis was
observed in mast cells with an overexpression of miR-223. miRNAs
serve a vital regulatory role in various biological processes by
targeting mRNAs for cleavage or translational repression (16,19). In
recent years, accumulating evidence has demonstrated that the
myeloid-specific miR-223 can affect multiple targets and modulate
different cellular processes, ranging from the regulation of cell
proliferation and cancer development to hematopoietic
differentiation, particularly in myeloid lineage development, and
immune cell function (38–40). A number of studies have demonstrated
that miR-223, an evolutionarily conserved miRNA, may represent a
potential biomarker for various diseases, including recurrent
ovarian cancer (41), psoriasis
(42) and bladder cancer (43). It was also reported that miR-223
functions as an oncogene in human colorectal cancer (CRC) cells,
and reducing the miR-223 expression may result in decreased cell
proliferation, migration and invasion of CRC cells (44). Therefore, elevated expression of
miR-223 leads to a poor prognosis in patients with CRC (45). Similar effects have been observed in
osteosarcoma (46,47) and Helicobacter
pylori-associated gastric cancer (48). Furthermore, studies have confirmed
that miR-223 targets the transcription factor CEBP-α (49), glutamate receptors (GluR2 and NR2B)
(50), stathmin1 (51) and IGF-1R (52), contributing to the biological
function in other cell types.
Two pathways are known to be involved in regulating
mast cell apoptosis: The extrinsic and the intrinsic pathways. The
Bcl-2 protein family plays a key role in the intrinsic pathway of
apoptosis (2). This protein family
contains pro- and anti-apoptotic members, and the balance between
these members determines cell survival and apoptosis (5). As shown in Fig. 4, the expression of Bcl-2 protein
decreased in cells overexpressing miR-223 when compared with that
in the control cells. In addition, identification of the
pro-apoptotic Bcl-2 homology 3-only proteins has also been
reported, including p53 upregulated modulator of apoptosis and
Bcl-2-like 11, which are critical for the induction of mast cell
apoptosis following cytokine deprivation (2,12).
Furthermore, mast cell apoptosis can be triggered by tumor necrosis
factor-related apoptosis-inducing ligand receptor, which is
involved in the extrinsic pathway of apoptosis (2). Therefore, induction of apoptosis may
provide a novel intervention by disrupting the development of mast
cell-associated disorders.
In the present study, a model of miR-223
overexpression was established by transfecting cells using
Lipofectamine 2000, and the miR-223 suppression of proliferation
and promotion of apoptosis was observed in mast cells. The present
results suggest that miR-223 functioned as a negative regulator of
cell growth. In order to determine the underlying mechanisms and
target genes that were responsible for the suppressive function of
miR-223, a luciferase reporter assay was performed. The results
demonstrated that overexpression of miR-223 resulted in a
significant decrease in the luciferase activity of the
IGF-1R-3′-UTR reporter. Furthermore, western blotting detected that
the IGF-1R protein expression was significantly downregulated
following overexpression of miR-223 in cells. All the
aforementioned results indicate that IGF-1R is a functional target
gene of miR-223.
The IGF-1R signaling pathway is involved in the
normal growth and development of cells, and serves a crucial role
in the regulation of cell proliferation following IGF-1 binding to
IGF-1R through activation of the PI3K/Akt signaling pathway
(52–54). The upstream PI3K/Akt signaling
pathway has been shown to mediate dynamic changes in the actin
cytoskeleton, integrin signaling and cell survival (37,55). The
PI3K/Akt signaling pathway is important in miR-223 regulation of
cell apoptosis, and the results of the present study showed that
the PI3K/Akt signaling pathway was suppressed by overexpression of
miR-223, which is consistent with bioinformatical predictions. The
current study revealed that miR-223 regulates the apoptosis of mast
cells by targeting IGF-1R and its downstream PI3K/Akt signaling
pathway.
In conclusion, the present study indicated that
increasing the expression of miR-223 can promote apoptosis in mast
cells. IGF-1R, as the target gene of miR-223, and the PI3K/Akt
signaling pathway were found to be involved in the regulation of
mast cell apoptosis.
Acknowledgements
The current research was supported by the National
Natural Science Foundation of China (grant no. 81200012; awarded to
Feng Liu) and the National Natural Science Foundation of China
(grand no. 81370132; awarded to Deyu Zhao). The authors would also
like to thank the Medical School of Nanjing University for its
technical support.
References
|
1
|
da Silva EZ, Jamur MC and Oliver C: Mast
cell function: A new vision of an old cell. J Histochem Cytochem.
62:698–738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ekoff M and Nilsson G: Mast cell apoptosis
and survival. Adv Exp Med Biol. 716:47–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valent P, Akin C, Arock M, Brockow K,
Butterfield JH, Carter MC, Castells M, Escribano L, Hartmann K,
Lieberman P, et al: Definitions, criteria and global classification
of mast cell disorders with special reference to mast cell
activation syndromes: A consensus proposal. Int Arch Allergy
Immunol. 157:215–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wygrecka M, Dahal BK, Kosanovic D,
Petersen F, Taborski B, von Gerlach S, Didiasova M, Zakrzewicz D,
Preissner KT, Schermuly RT and Markart P: Mast cells and
fibroblasts work in concert to aggravate pulmonary fibrosis: Role
of transmembrane SCF and the PAR-2/PKC-alpha/Raf-1/p44/42 signaling
pathway. Am J Pathol. 182:2094–2108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karlberg M, Ekoff M, Huang DC, Mustonen P,
Harvima IT and Nilsson G: The BH3-mimetic ABT-737 induces mast cell
apoptosis in vitro and in vivo: Potential for
therapeutics. J Immunol. 185:2555–2562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Picard M, Giavina-Bianchi P, Mezzano V and
Castells M: Expanding spectrum of mast cell activation disorders:
Monoclonal and idiopathic mast cell activation syndromes. Clin
Ther. 35:548–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Overed-Sayer C, Rapley L, Mustelin T and
Clarke DL: Are mast cells instrumental for fibrotic diseases? Front
Pharmacol. 4:1742014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harvima IT and Nilsson G: Stress, the
neuroendocrine system and mast cells: Current understanding of
their role in psoriasis. Expert Rev Clin Immunol. 8:235–241. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benoist C and Mathis D: Mast cells in
autoimmune disease. Nature. 420:875–878. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun J, Sukhova GK, Wolters PJ, Yang M,
Kitamoto S, Libby P, MacFarlane LA, Mallen-St Clair J and Shi GP:
Mast cells promote atherosclerosis by releasing proinflammatory
cytokines. Nat Med. 13:719–724. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Soucek L, Lawlor ER, Soto D, Shchors K,
Swigart LB and Evan GI: Mast cells are required for angiogenesis
and macroscopic expansion of Myc-induced pancreatic islet tumors.
Nat Med. 13:1211–1218. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Melo FR, Waern I, Rönnberg E, Åbrink M,
Lee DM, Schlenner SM, Feyerabend TB, Rodewald HR, Turk B,
Wernersson S and Pejler G: A role for serglycin proteoglycan in
mast cell apoptosis induced by a secretory granule-mediated
pathway. J Biol Chem. 286:5423–5433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lessene G, Czabotar PE and Colman PM:
BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov.
7:989–1000. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bradding P, Walls AF and Holgate ST: The
role of the mast cell in the pathophysiology of asthma. J Allergy
Clin Immunol. 117:1277–1284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu C, Mei J, Li D, Liu J, Qin H, Liu F and
Zhao D: Expression of microRNA in murine model induced by
ovalbumin. Shi Yong Er Ke Lin Chuang Za Zhi. 21:1655–1657. 2012.(In
Chinese).
|
|
16
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ebert MS and Sharp PA: Roles for microRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: MicroRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan Y, Liang H, Liu H, Li D, Chen X, Li L,
Zhang CY and Zen K: Platelet-secreted microRNA-223 promotes
endothelial cell apoptosis induced by advanced glycation end
products via targeting the insulin-like growth factor 1 receptor. J
Immunol. 192:437–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
LeRoith D and Roberts CT Jr: The
insulin-like growth factor system and cancer. Cancer Lett.
195:127–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pollak MN, Schernhammer ES and Hankinson
SE: Insulin-like growth factors and neoplasia. Nat Rev Cancer.
4:505–518. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Samani AA, Yakar S, LeRoith D and Brodt P:
The role of the IGF system in cancer growth and metastasis:
Overview and recent insights. Endocr Rev. 28:20–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adams TE, Epa VC, Garrett TP and Ward CW:
Structure and function of the type 1 insulin-like growth factor
receptor. Cell Mol Life Sci. 57:1050–1093. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang B, Sun F, Dong N, Sun Z, Diao Y,
Zheng C, Sun J, Yang Y and Jiang D: MicroRNA-7 directly targets
insulin-like growth factor 1 receptor to inhibit cellular growth
and glucose metabolism in gliomas. Diagn Pathol. 19:2112014.
View Article : Google Scholar
|
|
25
|
Guo T, Feng Y, Liu Q, Yang X, Jiang T,
Chen Y and Zhang Q: MicroRNA-320a suppresses in GBM patients and
modulates glioma cell functions by targeting IGF-1R. Tumor Biol.
35:11269–11275. 2014. View Article : Google Scholar
|
|
26
|
Zhang Y, Chen X, Lian H, Liu J, Zhou B,
Han S, Peng B, Yin J, Liu W and He X: MicroRNA-503 acts as a tumor
suppressor in glioblastoma for multiple antitumor effects by
targeting IGF-1R. Oncology Reports. 31:1445–1452. 2014.PubMed/NCBI
|
|
27
|
Farhana L, Dawson MI, Murshed F, Das JK,
Rishi AK and Fontana JA: Upregulation of miR-150* and miR-630
induces apoptosis in pancreatic cancer cells by targeting IGF-1R.
PLoS One. 8:e610152013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu JW, Wang TX, You L, Zheng LF, Shu H,
Zhang TP and Zhao YP: Iusulin-like growth factor 1 receptor
(IGF-1R) as a target of miR-497 and plasma IGF-1R levels associated
with TNM stage of pancreatic cancer. PloS One. 9:e928472014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang K, Dong X, Sui C, Hu D, Xiong T,
Liao S and Zhang H: MiR-223 suppresses endometrial carcinoma cells
proliferation by targeting IGF-1R. Am J Transl Res. 6:841–849.
2014.PubMed/NCBI
|
|
30
|
Gerbaulet A, Hartmann K and Mekori YA:
Mast cell apoptosis. Methods Mol Biol. 315:407–423. 2006.PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hara K, Ueda S, Ohno Y, Tanaka T, Yagi H,
Okazaki S, Kawahara R, Masayuki T, Enomoto T, Hashimoto Y, et al:
NIH3T3 cells overexpressing CD98 heavy chain resist early G1 arrest
and apoptosis induced by serum starvation. Cancer Sci.
103:1460–1466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang W, Ye D, Dai L, Shen Y and Xuu J:
Overexpression of hTERT extends replicative capacity of human
nucleus pulposus cells, and protects against serum
starvation-induced apoptosis and cell cycle arrest. J Cell Biochem.
113:2112–2121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen J, Hou R, Zhang X, Ye Y, Wang Y and
Tian J: Calycosin suppresses breast cancer cell growth via
ERβ-dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways.
PloS One. 9:e912452012. View Article : Google Scholar
|
|
35
|
Ohtani M, Numazaki M, Yajima Y and
Fujita-Yamaguchi Y: Mechanisms of antibody-mediated insulin-like
growth factor I receptor (IGF-IR) down-regulation in MCF-7 breast
cancers. Biosci Trends. 3:131–138. 2009.PubMed/NCBI
|
|
36
|
Zhao Y, Wang Z, Jiang Y and Yang C:
Inactivation of Rac 1 reduces Trastuzumab resistance in PTEN
deficient and insulin-like growth factor I receptor overexpressing
human breast cancer SKBR3 cells. Cancer Lett. 313:54–63. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pollak M: The insulin and insulin-like
growth factor receptor family in neoplasia: An update. Nat Rev
Cancer. 12:159–169. 2012.PubMed/NCBI
|
|
38
|
Johnnidis JB, Harris MH, Wheeler RT,
Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD and
Camargo FD: Regulation of progenitor cell proliferation and
granulocyte function by microRNA-223. Nature. 451:1125–1129. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haneklaus M, Gerlic M, O'Neill LA and
Masters SL: MiR-223: Infection, inflammation and cancer. J Intern
Med. 274:215–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vian L, Di Carlo M, Pelosi E, Fazi F,
Santoro S, Cerio AM, Boe A, Rotilio V, Billi M, Racanicchi S, et
al: Transcriptional fine-tuning of microRNA-223 levels directs
lineage choice of human hematopoietic progenitors. Cell Death
Differ. 21:290–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Laios A, O'Toole S, Flavin R, Martin C,
Kelly L, Ring M, Finn SP, Barrett C, Loda M, Gleeson N, et al:
Potential role of miR-9 and miR-223 in recurrent ovarian cancer.
Mol Cancer. 7:352008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Løvendorf MB, Zibert JR, Gyldenløve M,
Røpke MA and Skov L: MicroRNA-223 and miR-143 are important
systemic biomarkers for disease activity in psoriasis. J Dermatol
Sci. 75:133–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gottardo F, Liu CG, Ferracin M, Calin GA,
Fassan M, Bassi P, Sevignani C, Byrne D, Negrini M, Pagano F, et
al: Micro-RNA profiling in kidney and bladder cancers. Urol Oncol.
25:387–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang J, Luo X, Li H, Yue X, Deng L, Cui Y
and Lu Y: MicroRNA-223 functions as an oncogene in human colorectal
cancer cells. Oncol Rep. 32:115–120. 2014.PubMed/NCBI
|
|
45
|
Li ZW, Yang YM, Du LT, Dong Z, Wang LL,
Zhang X, Zhou XJ, Zheng GX, Qu AL and Wang CX: Overexpression of
miR-223 correlates with tumor metastasis and poor prognosis in
patients with colorectal cancer. Med Oncol. 31:2562014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang H, Yin Z, Ning K, Wang L, Guo R and
Ji Z: Prognostic value of microRNA-223/epithelial cell transforming
sequence 2 signaling in patients with osteosarcoma. Hum Pathol.
45:1430–1436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li G, Cai M, Fu D, Chen K, Sun M, Cai Z
and Cheng B: Heat shock protein 90B1 plays an oncogenic role and is
a target of microRNA-223 in human osteosarcoma. Cell Physiol
Biochem. 30:1481–1490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ma L, Chen Y, Zhang B and Liu G: Increased
microRNA-223 in Helicobacter pylori-associated gastric cancer
contributed to cancer cell proliferation and migration. Biosci
Biotechnol Biochem. 78:602–608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pulikkan JA, Dengler V, Peramangalam PS,
Peer Zada AA, Müller-Tidow C, Bohlander SK, Tenen DG and Behre G:
Cell-cycle regulator E2F1 and microRNA-223 comprise an
autoregulatory negative feedback loop in acute myeloid leukemia.
Blood. 115:1768–1778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Harraz MM, Eacker SM, Wang X, Dawson TM
and Dawson VL: MicroRNA-223 is neuroprotective by targeting
glutamate receptors. Proc Natl Acad Sci USA. 109:18962–18967. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kang W, Tong JH, Chan AW, Lung RW, Chau
SL, Wong QW, Wong N, Yu J, Cheng AS and To KF: Stathmin1 plays
oncogenic role and is a target of microRNA-223 in gastric cancer.
PLoS One. 7:e339192012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jia CY, Li HH, Zhu XC, Dong YW, Fu D, Zhao
QL, Wu W and Wu XZ: MiR-223 suppresses cell proliferation by
targeting IGF-1R. PLoS One. 6:e270082011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu LH, Cai QQ, Dong YW, Wang R, He BM, Qi
B, Xu CJ and Wu XZ: Decoy oligonucleotide rescues IGF1R expression
from MicroRNA-223 suppression. PLoS One. 8:e821672013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yu YH, Zhang L, Wu DS, Zhang Z, Huang FF,
Zhang J, Chen XP, Liang DS, Zeng H and Chen FP: MiR-223 regulates
human embryonic stem cell differentiation by targeting the
IGF-1R/Akt signaling pathway. PLoS One. 8:e787692013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rodriguez-Viciana P, Warner PH, Khwaja A,
Marte BM, Pappin D, Das P, Waterfield MD, Ridley A and Downward J:
Role of phosphoinositide 3-OH kinase in cell transformation and
control of the actin cytoskeleton by Ras. Cell. 89:457–467. 1997.
View Article : Google Scholar : PubMed/NCBI
|