Introduction
Venous thromboembolism (VTE) is a common disease
with high prevalence and various etiological factors. In developed
countries, the annual incidence of VTE is ~1–2 events/1,000
individuals (1). Surgery, fracture,
malignancy, immobilization, elevated homocystein, protein C-,
protein S- or antithrombin-deficiency, and prothrombin G20210A
variation are considered established risk factors for VTE (1). However, a large number of patients
develop VTE without specific reasons and are regarded as idiopathic
VTE patients who comprise >30% of the total VTE events (2).
Arterial thromboemboli are white thrombi that exist
in the high shear and platelet-rich area, while venous thrombosis
are red thrombi that are generally characterized by red cells under
hypercoagulation and venous stasis (3). The thrombi are considered independent
entities with different pathological mechanisms and therapeutic
consequences. However, since idiopathic VTE and atherosclerotic
vascular disorders may share some common risk factors, obesity,
dyslipidemia, metabolic syndrome, smoking, hypertension, estrogen
and other risk factors have been investigated to determine the
association. Findings of previous studies (4,5) on
obesity, metabolic syndrome and high-density lipoprotein
cholesterol (HDL-C) are inconsistent, with metabolic syndrome and
HDL-C being the most controversial. Ageno et al (6) conducted a meta-analysis indicating an
obvious correlation between low HDL-C levels, high triglyceride
levels and VTE. A randomized trial (JUPITER trial) showed that
rosuvastatin significantly reduced the occurrence of symptomatic
VTE in a healthy population (7).
However, two large population-based prospective studies
demonstrated that metabolic syndrome, diabetes, hypertension and
other traditional atherosclerotic risk factors did not contribute
to VTE but that only abdominal obesity was relevant (4,8).
Besides dyslipidemia, hyperglycemia and
hypertension, hyperuricemia has been considered an independent risk
factor for cardiovascular disease (CVD) (9), although it is rarely associated with
venous thrombosis. A retrospective case-control study was therefore
conducted to investigate the correlation of serum uric acid (SUA)
and idiopathic VTE in a Chinese population. In the hospital-based
case-control study, we focused on the risk of idiopathic VTE
correlated with SUA, as well as blood lipids, fasting blood glucose
(FBG), current smoking status and hypertension.
Patients and methods
Patients and diagnostic criteria
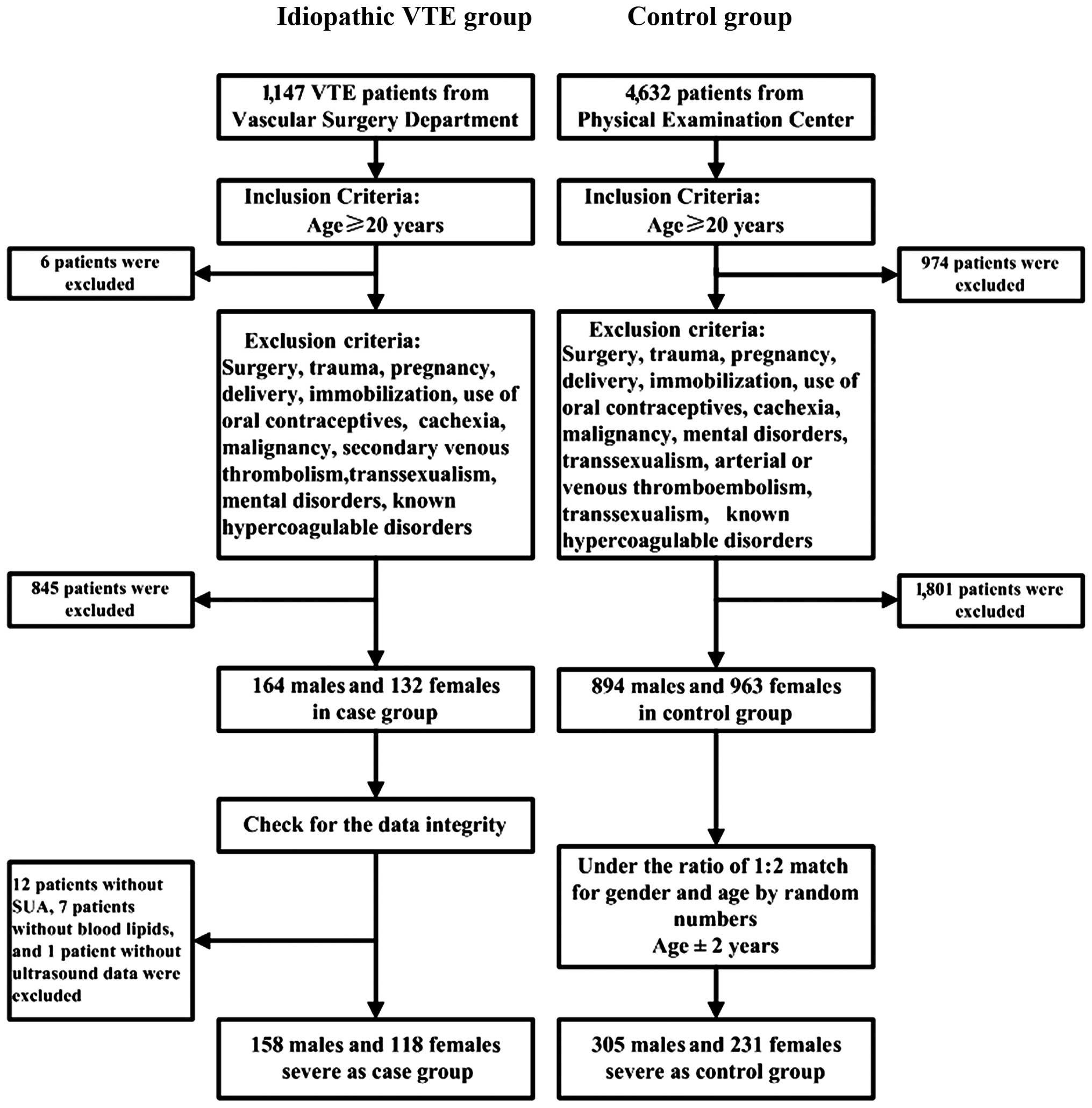
The study was conducted between January 2012 and
June 2015, and 1,147 VTE patients treated in the Department of
Vascular Surgery (Union Hospital, Tongji Medical College, Huazhong
University of Science and Technology, Wuhan, China) were enrolled.
Six patients were excluded due to age restriction (<20 years).
In addition, 845 patients were excluded based on the exclusion
criteria mentioned below. After checking for the integrity of the
data, there were 12 patients without SUA, 7 patients without blood
lipids and 1 patient without ultrasonography information; these
patients were also excluded. A total of 276 patients diagnosed as
idiopathic VTE with objective diagnosis of deep vein thrombosis
(DVT) or pulmonary embolism (PE) were included in the study.
Intervals carried out from clinical onset to diagnosis occurred
within 1 month. DVT was diagnosed by color Doppler ultrasonography
and PE was diagnosed by computed tomography angiography (CTA).
Idiopathic VTE was defined as the absence of risk factors including
surgery, trauma, pregnancy, delivery, cachexia, immobilization,
malignancy, secondary VTE, use of oral contraceptives,
transsexualism, mental disorders and known hypercoagulable
disorders. As many as 4,632 control subjects with the same ethnic
background from the Physical Examination Center of Union Hospital,
Tongji Medical College, Huazhong University of Science and
Technology were included in the study. However, 974 subjects, aged
<20 years were excluded, as well as 894 men and 963 women. After
randomly matching for gender and age at a ratio of 1:2, 305 males
and 231 females served as the control group. The exclusion criteria
for the control group included surgery, trauma, pregnancy,
delivery, cachexia, immobilization, malignancy, use of oral
contraceptives, transsexualism, mental disorders, arterial or VTE
and known hypercoagulable disorders (Fig. 1).
Data collection
The following clinical data were collected from the
patients and controls: Age, gender, site of thrombosis, smoking
status, blood pressure, serum levels of SUA, HDL-C, low-density
lipoprotein cholesterol (LDL-C), triglyceride, total cholesterol,
and FBG. Hyperuricemia was defined as SUA >420 µmol/l (7.0
mg/dl) for male or >357 µmol/l (6.0 mg/dl) for female patients.
Dyslipidemia was defined as HDL-C <1.036 mmol/l (40 mg/dl) for
male or <1.295 mmol/l (50 mg/dl) for female patients, and/or
LDL-C >3.1 mmol/l (120 mg/dl), and/or total cholesterol >5.18
mmol/l (200 mg/dl), and/or triglyceride >1.7 mmol/l (150 mg/dl).
Hyperglycemia was defined as FBG >6.1 mmol/l (110 mg/dl).
Smoking status was divided into current smoker or non-smoker, and
hypertension was defined as systolic blood pressure >140 mmHg
and/or diastolic blood pressure >90 mmHg. HDL-C, total
cholesterol, triglyceride and FBG were determined in fresh plasma.
The LDL-C was measured by the Friedewald equation except for
patients with plasma triglycerides >400 mg/dl. FBG was tested
after 6 h of fasting. After 10 min rest, blood pressure was
measured in the patient's right radial artery in the sitting
position using an electronic sphygmomanometer. Measurements were
repeated after 5 and 10 min from the initial measurement. The mean
value of the three measurements was recorded. Age for control
subjects was matched ± 2 years for idiopathic VTE patients.
Statistical analysis
Group differences were examined with regard to
gender, age (years), HDL-C (mmol/l), LDL-C (mmol/l), triglyceride
(mmol/l), total cholesterol (mmol/l), FBG (mmol/l), SUA (µmol/l),
current smoker (yes or no), and hypertension (yes or no) in
idiopathic VTE patients. The descriptive measures were expressed as
frequency and proportion for categorical variables, and mean ±
standard deviations (SDs) or median and interquartile for
continuous variables. The differences between groups were analyzed
using the Chi-square test for categorical variables and the t-test
(normal distribution) or Kruskal-Wallis rank sum test (non-normal
distribution) for continuous variables (Table I).
 | Table I.Characteristics of participants with
or without an idiopathic VTE. |
Table I.
Characteristics of participants with
or without an idiopathic VTE.
| Characteristics | Idiopathic VTE (n=
276) | Control (n=536) | P-value |
|---|
| Age (years) | 52.9±14.4 | 52.7±14.5 | 0.901 |
| Man | 158 (57.2%) | 309 (57.6%) | 0.912 |
| HDL-C (mmol/l) | 1.30±0.38 | 1.36±0.32 | 0.028 |
| LDL-C (mmol/l) | 2.60±0.79 | 2.40±0.74 | <0.001 |
| Triglyceride
(mmol/l) | 1.21 (0.88–1.66) | 1.14 (0.83–1.59) | 0.066 |
| Total cholesterol
(mmol/l) | 4.58±0.98 | 4.39±0.94 | 0.006 |
| FBG (mmol/l) | 5.76±1.84 | 5.21±1.44 | <0.001 |
| SUA (μmol/l) | 320.5±101.3 | 309.8±87.9 | 0.121 |
| Current smoker | 83 (30.1%) | 128 (23.6%) | 0.057 |
| Hypertension | 44 (15.9%) | 82 (15.3%) | 0.810 |
Conditional logistic regression was employed to
estimate the odds ratios (ORs) and 95% confidence intervals (CIs)
for the association between SUA per-SD (92.67 µmol/l) increase and
idiopathic VTE. Univariate analysis was performed to examine the
effect of each parameter on idiopathic VTE (Table II).
 | Table II.Univariate analysis was performed to
examine the influence of each parameter on idiopathic VTE. |
Table II.
Univariate analysis was performed to
examine the influence of each parameter on idiopathic VTE.
| Characteristics | P-value | OR (95%
CI)a |
|---|
| Gender |
0.740 |
|
| Male |
| Ref. |
|
Female |
|
1.59
(0.103–24.55) |
| Age (years) |
0.842 | 1.01 (0.93–1.09) |
| SUA per-SD
increase |
0.068 | 1.16
(0.989–1.36) |
| HDL-C (mmol/l) |
0.022 | 0.588
(0.374–0.925) |
| LDL-C (mmol/l) | <0.001 | 1.43 (1.17–1.75) |
| Triglyceride
(mmol/l) |
0.277 | 1.10 (0.93–1.29) |
| Total cholesterol
(mmol/l) |
0.005 | 1.25
(1.07–1.46) |
| FBG (mmol/l) | <0.001 | 1.24
(1.12–1.37) |
| Current smoker |
0.036 |
|
| No |
| Ref. |
|
Yes |
| 1.49
(1.03–2.15) |
| Hypertension |
0.900 |
|
| No |
| Ref. |
|
Yes |
|
1.030
(0.674–1.57) |
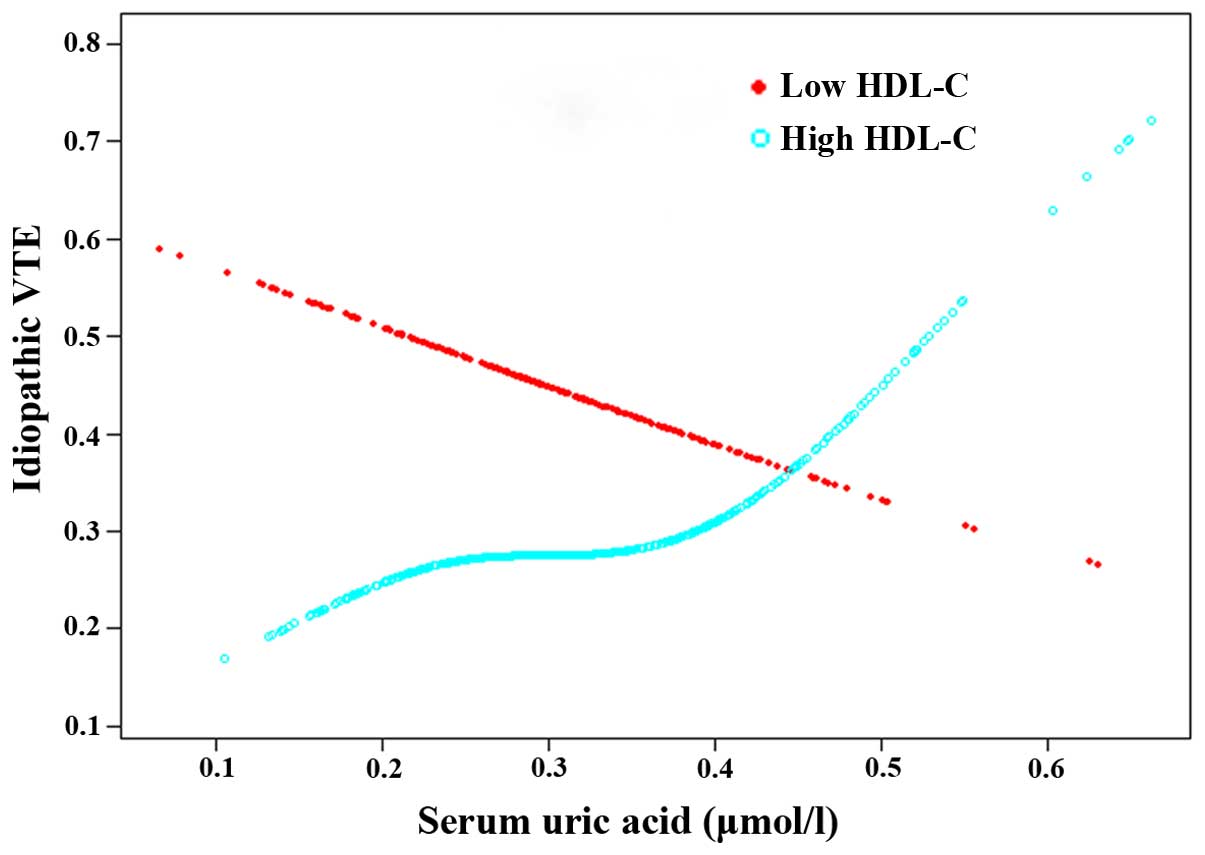
The association between SUA and idiopathic VTE was
examined using the smoothing plot, with an adjustment for gender,
age, LDL-C, total cholesterol, FBG, and stratification by high
HDL-C (HDL-C >1.036 mmol/l for men or >1.295 mmol/l for
women) (Fig. 2). The interaction
analysis was applied to confirm the effect of high HDL-C levels on
SUA for idiopathic VTE (Table
III). An initial model was adjusted only for matching
variables. Additionally, a second model was adjusted for gender,
age, LDL-C, total cholesterol, and FBG.
 | Table III.Association of SUA per-SD increase
with the risk of idiopathic VTE in different HDL-C levels. |
Table III.
Association of SUA per-SD increase
with the risk of idiopathic VTE in different HDL-C levels.
|
| Low HDL-C | High HDL-C |
|
|---|
|
|
|
|
|
|---|
| Model | OR (95% CI) | P-value | OR (95% CI) | P-value | P for
interaction |
|---|
| Model 1 | 0.865
(0.522–1.43) | 0.574 | 1.39
(1.12–1.73) | 0.003 | 0.0040 |
| Model 2 | 0.852
(0.513–1.42) | 0.538 | 1.38
(1.11–1.72) | 0.004 | 0.0041 |
| Model 3 | 0.813
(0.471–1.40) | 0.455 | 1.29
(1.02–1.64) | 0.032 | 0.0026 |
P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
Empower (R) (http://empowerstats.com/en/; X&Y Solutions, Inc.,
Boston, MA, USA) and R (https://www.R-project.org).
Ethical issues and
confidentiality
The present study was approved by the Medical Ethics
Committee of Wuhan Union Hospital (Hubei, China). Under the
provision that no personal identifiers be recorded, approval for
using data from medical records was only for retrospective study
analysis. Thus, no individual consent was obtained.
Results
Patient characteristics
A total of 276 patients were included in the
idiopathic VTE group and 536 gender- and age-matched subjects were
included in the control group. The mean ages in the case and
control groups were 52.9±14.4 and 52.7±14.5 years. Idiopathic VTE
patients and controls were Chinese and the basic characteristics of
the study population are provided in Table I. There were no statistically
significant differences of the study population in baseline
regarding age, gender, SUA, triglyceride, current smoking, and
hypertension. The number of thrombotic events was higher in the
male (158 males) than in the female (118 females) participants. The
sites of VTE were 189 patients (68.48%) in the left lower
extremity, 77 patients (27.9%) in the right lower extremity, PE in
33 patients (11.96%), 7 patients in the two lower extremities, 2
patients in the left upper extremity, 1 patient in the right upper
extremity and inferior vena cava thrombosis in 1 patient. This
result also confirmed the finding that the general population
usually develop DVT in the left lower limb due to right common
iliac artery compression anatomically.
Systemic findings associated with
idiopathic VTE
The univariate analysis revealed that HDL-C, LDL-C,
total cholesterol, FBG, and current smoking status were
significantly associated with idiopathic VTE (Table II). HDL-C showed a negative
correlation with the increased risk of idiopathic VTE, a strong
protective factor [P=0.022, OR=0.588 (95% CI: 0.374–0.925)].
Additionally, LDL-C and total cholesterol were positively
correlated with idiopathic VTE, P<0.001, OR=1.43 (95% CI:
1.17–1.75); P=0.005, OR=1.25 (95% CI: 1.07–1.46), respectively. In
addition to recognized atherosclerosis risk factors, hyperglycemia
and current smoking status presented a similar close association
with the disease, P<0.001, OR=1.24 (95% CI: 1.12–1.37); P=0.036,
OR=1.49 (95% CI: 1.03–2.15). In the univariate analysis, we did not
identify any correlations of SUA per-SD increase [P=0.068, OR=1.16
(95% CI: 0.989–1.36)], triglyceride [P=0.277, OR=1.10 (95% CI:
0.93–1.29)], and hypertension [P=0.900, OR=1.030 (95% CI:
0.674–1.57)] to idiopathic VTE.
Interaction analysis for SUA and HDL-C
in Idiopathic VTE
To determine whether an irrelevant relationship of
SUA and idiopathic VTE was caused by the interactions among other
risk factors, we conducted the interaction analysis to scan for
interaction factors. After checking for each parameter, we found
only the interaction of HDL-C to be significant (P<0.001 for
interaction), but not for age, gender, FBG, LDL-C and total
cholesterol.
HDL-C was stratified into the high and low groups
(men, <1.036 mmol/l and women, <1.295 mmol/l) to examine its
interaction with SUA for idiopathic VTE (Table III). In the total HDL-C population,
there was no obvious association between SUA and idiopathic VTE.
However, following stratification of HDL-C into two groups, a
significant association of SUA to idiopathic VTE was identified in
the high HDL-C population (P=0.003 for crude and P=0.032 for
adjusted), while this correlation was not observed in the low HDL-C
population (Fig. 2).
Discussion
In this retrospective case-control study, we
determined the value of SUA, HDL-C, LDL-C, total cholesterol,
triglyceride, FBG, current smoking and hypertension for the
correlation of idiopathic VTE. The results showed that there were a
close association of HDL-C, LDL-C, total cholesterol, FBG and
current smoking with the disease. Notably, the association between
SUA and idiopathic VTE varied with the HDL-C level, in which SUA
was significantly associated with idiopathic VTE at a high HDL-C
level, while the correlation was no longer present when the HDL-C
level was decreased.
The effect of HDL-C on the risk of VTE is not as
obvious as that for arterial thrombosis. However, previous findings
revealed an association between HDL and VTE (3,10).
According to size and density, HDL can be classified into the
subcategories: i) HDL3, smaller with a high density of 1.125–1.21
g/ml; and ii) HDL2, larger with a low density of 1.063–1.125 g/ml,
and each subgroup can be subdivided by their apolipoprotein (apo)
composition. Generally, HDL2 are apoA-I- and apoE-rich particles,
while HDL3 are apoA-I- and apoA-II-rich but apoE less particles
(3). It has been suggested that the
HDL that is enriched in apoA-I may be more cardioprotective than
apoA-I/-II-dominated HDL; therefore, HDL2 may have an advantage
over HDL3 on the protective effects of the cardiovascular system
(11). Deguchi et al
(5) found that in the DVT patients
with or without PE, the HDL2, HDL-C and apoA-I levels were
distinctly low. Correspondingly, a subsequent study of VTE
indicated that for each increase of 0.1 mg/ml plasma apoA-I, there
would be a 0.87 (95% CI: 0.80–0.94) relative risk of VTE recurrence
(10). Furthermore, the
antithrombolic function of HDL enhances the anticoagulant protein C
pathway to attenuate the expression of the tissue factor and reduce
thrombin generation, and promotes the synthesis of prostacyclin
(12). Additionally, the size of
HDL-C and HDL was negatively correlated with the plasminogen
activator inhibitor-I level, an inhibitor of tissue plasminogen and
plasminogen activators, indicating that HDL particles may be
involved in the promotion of plasmin generation and fibrinolysis to
reduce the occurrence of thrombotic events (13).
As the end product of purine, SUA has been
investigated to be closely associated with CVD. Numa et al
(14) identified that for the
nonvalvular atrial fibrillation patients, the SUA level was
considered an independent indicator of transesophageal
echocardiography risk, which includes thrombi and aortic
atherosclerosis. Tang et al (15) also reached a similar conclusion
whereby the hyperuricemia was a modest predictor for left atrial
thromboembolism occurrence among nonvalvular atrial fibrillation
patients. The mechanisms of SUA damage for CVD are considered
mainly as oxidative stress and inflammation. Some investigators
suggested that there are three mechanisms that may induce UA injury
to the vessel endothelium: i) Aggravated inflammatory reaction in
endothelial cells and proliferation in vascular smooth muscle cells
as a consequence of active oxygen species produced in UA
metabolism; ii) UA crystals that stimulate the production of
multinucleate cells and inflammation mediators leading to damage in
the vascular endothelium; and iii) UA is transported into vascular
endothelial and smooth muscle cells to trigger the cascade reaction
leading to fetal intracellular damages (16). The above mechanisms partially
explained the association between SUA and endothelium injury, which
is an essential aspect in Virchow's triad for VTE formation.
Recently, Zhang et al (17) conducted a study in coronary artery
disease (CAD) patients on the association between SUA level and
lipoprotein subfractions, which are considered promising new
measurements of CAD risk assessment on blood plasma lipids. From
their study, the SUA level was confirmed to be correlated with
small dense LDL-C positively and large HDL-C negatively, and this
correlation may have an impact on the risk prediction of SUA for
CAD to a certain extent. In addition, it has been suggested that
compared with the quantity, the quality of subfractions is more
important; thus, a decreased large HDL-C may be more atherogenic
although the entire HDL-C level remains normal (18).
In the present study, HDL-C manifested a strong
protective effect on the disease, and the correlation of SUA and
idiopathic VTE was only detected in the high HDL-C population. This
is likely due to SUA attenuating large HDL-C quality albeit its
total amount of HDL-C remains at a high level and its correlation
to idiopathic VTE was obvious under the specific conditions.
However, for the low HDL-C population, the decreased number of
HDL-C has become a confounding factor and the relationship between
SUA and idiopathic VTE was not distinct in this population.
Additionally, it has been suggested that the
excessively produced or decreased clearance of UA is a potential
etiology of metabolic syndrome and type 2 diabetes mellitus
(19), in which the inflammation
markers are correlated with an increased number and activity of
platelets and fibrinogen, leading to a prothrombotic state. Those
findings also indicated that the overburdened UA in hypertension is
as critical as insulin resistance, contributing to circulating
insulin accumulation and the acceleration of UA intracellular
transportation and resorption (20).
Considering the interaction of SUA, HDL-C and blood glucose in the
disease, we hypothesize that the controversial results obtained in
previoius studies on the association of metabolic syndrome and
idiopathic VTE was caused by SUA leading to different degrees of
HDL-C reduction. As for the number of metabolic syndrome patients,
their HDL-C remained at a normal level, although the protective
function, and quality of large HDL-C, was already weakened by SUA
and these patients were prone to develop VTE events. Therefore,
taking total HDL-C as a risk factor for idiopathic VTE, and not
distinguishing its levels or components, may cause the interaction,
leading to inconsistent conclusions. To the best of our knowledge,
few studies considered SUA as s risk factor for idiopathic VTE and
none assessed the interaction analysis between HDL-C and SUA in the
disease.
Although the harmful role of smoking in arterial
thrombosis is well-established, its effect on venous thrombus
remains to be determined. The currently known adverse effect of
smoking on the vascular system is mainly on endothelial damage
which may up-regulate the vessel prothrombotic and pro-inflammatory
states, disturb platelet function, damage the vascular wall by
increasing inflammatory mediators, and reduce the endothelial NO
production to make hemodynamic changes (21). Sweetland et al (22) conducted a large prospective study of
women with a smoking habit in England, and the results showed that
the current smokers had a significantly increased incidence of VTE
compared with never-smokers, and the risk was more significant in
heavier compared to lighter smokers. However, Enga et al
(23) who carried out the Tromsø
study concluded that heavy smoking is a risk factor only limited to
provoking VTE. When taking cancer and myocardial infarction into
account, the obvious association was no longer present, suggesting
that the risk of VTE was altered by the development of malignancy
or myocardial infarction. Another two large population-based case
control studies (24,25) were in agreement with the results of
the Tromsø study whereby smoking itself was a weak risk factor and
did not significantly impact the development of idiopathic VTE. Our
results corroborate the previous finding regarding an association
between smoking and idiopathic VTE. At the same time, our results
were consistent with those of the study on longitudinal
investigation of thromboembolism etiology (7), in which hypertension was not relevant
to VTE.
Thus, SUA may act as an important connection between
atherosclerosis and idiopathic VTE by influencing the functional
HDL-C and it should be considered a critical loop in the progress
of pro-inflammatory and pro-thrombotic state in the disease. Taking
the role of hyperuricemia, dyslipidemia and hyperglycemia in the
pathogenesis of VTE together, it is reasonable to consider
idiopathic VTE as a consequence of metabolic disorders.
There are some limitations to the present study.
Firstly, diagnosed cancer patients were excluded from the study,
albeit some patients with idiopathic VTE may have had occult cancer
during the time of investigation since not all the patients
underwent screening of tumor markers. The effect of occult cancer
on the dyslipidemia, hyperglycemia and hyperuricemia is unknown
while its impact on the results of our analysis is probably low.
Secondly, since idiopathic VTE patients were defined without using
the most common known risk factors, thrombophilia (such as Factor V
Leiden) and some heterogeneity (such as prothrombin G20210A
mutation) were not investigated in the present study. However, this
is unlikely to have had a significant influence on our results as
there is currently no exact evidence suggesting that
hypercoagulation would result in dyslipidemia, hyperglycemia or
hyperuricemia (26). Since this is a
retrospective study, the patients in our control group enrolled
according to their detailed history but not the performance of
ultrasound or CTA to screen out asymptomatic VTE, which may not
appropriately represent a generally healthy population. However,
the current prevalence of dyslipidemia, hyperglycemia and
hyperuricemia in the control group were slightly higher than that
reported in the general Chinese population (27–29),
indicating that following selection of a different control group
the ORs may have been even greater than those reported in the
current study.
In conclusion, our findings suggest that a decreased
HDL-C level, increased total cholesterol, FBG, and current smoking
are closely associated with idiopathic VTE. SUA was associated with
increased risk of idiopathic VTE in the high HDL-C population,
which may act as a pivotal connection between atherosclerosis and
venous thrombus. However, additional studies are to be conducted to
confirm these findings.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81270391). The
investigators are grateful to Professor Sun Yi, Dr Chen Xinglin, Dr
Yang Chong and Dr Chen Qijun for their invaluable assistance and
advice.
References
|
1
|
Kearon C: Epidemiology of venous
thromboembolism. Semin Vasc Med. 1:7–26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heit JA: Venous thromboembolism
epidemiology: Implications for prevention and management. Semin
Thromb Hemost. 28(Suppl 2): 3–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Stoep M, Korporaal SJ and Van Eck
M: High-density lipoprotein as a modulator of platelet and
coagulation responses. Cardiovasc Res. 103:362–371. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brækkan SK, Hald EM, Mathiesen EB,
Njølstad I, Wilsgaard T, Rosendaal FR and Hansen JB: Competing risk
of atherosclerotic risk factors for arterial and venous thrombosis
in a general population: The Tromso study. Arterioscler Thromb Vasc
Biol. 32:487–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deguchi H, Pecheniuk NM, Elias DJ, Averell
PM and Griffin JH: High-density lipoprotein deficiency and
dyslipoproteinemia associated with venous thrombosis in men.
Circulation. 112:893–899. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ageno W, Becattini C, Brighton T, Selby R
and Kamphuisen PW: Cardiovascular risk factors and venous
thromboembolism: A meta-analysis. Circulation. 117:93–102. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glynn RJ, Danielson E, Fonseca FA, Genest
J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ,
MacFadyen JG, et al: A randomized trial of rosuvastatin in the
prevention of venous thromboembolism. N Engl J Med. 360:1851–1861.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Steffen LM, Cushman M, Peacock JM,
Heckbert SR, Jacobs DR Jr, Rosamond WD and Folsom AR: Metabolic
syndrome and risk of venous thromboembolism: Longitudinal
investigation of thromboembolism etiology. J Thromb Haemost.
7:746–751. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao G, Huang L, Song M and Song Y:
Baseline serum uric acid level as a predictor of cardiovascular
disease related mortality and all-cause mortality: A meta-analysis
of prospective studies. Atherosclerosis. 231:61–68. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eichinger S, Pecheniuk NM, Hron G, Deguchi
H, Schemper M, Kyrle PA and Griffin JH: High-density lipoprotein
and the risk of recurrent venous thromboembolism. Circulation.
115:1609–1614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Umaerus M, Rosengren B, Fagerberg B,
Hurt-Camejo E and Camejo G: HDL2 interferes with LDL association
with arterial proteoglycans: A possible athero-protective effect.
Atherosclerosis. 225:115–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mineo C, Deguchi H, Griffin JH and Shaul
PW: Endothelial and antithrombotic actions of HDL. Circ Res.
98:1352–1364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asselbergs FW, Williams SM, Hebert PR,
Coffey CS, Hillege HL, Navis G, Vaughan DE, van Gilst WH and Moore
JH: Gender-specific correlations of plasminogen activator
inhibitor-1 and tissue plasminogen activator levels with
cardiovascular disease-related traits. J Thromb Haemost. 5:313–320.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Numa S, Hirai T, Nakagawa K, Ohara K,
Fukuda N, Nozawa T and Inoue H: Hyperuricemia and transesophageal
echocardiographic thromboembolic risk in patients with atrial
fibrillation at clinically low-intermediate risk. Circ J.
78:1600–1605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang RB, Dong JZ, Yan XL, Du X, Kang JP,
Wu JH, Yu RH, Long DY, Ning M, Sang CH, et al: Serum uric acid and
risk of left atrial thrombus in patients with nonvalvular atrial
fibrillation. Can J Cardiol. 30:1415–1421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feig DI, Kang DH and Johnson RJ: Uric acid
and cardiovascular risk. N Engl J Med. 359:1811–1821. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Xu RX, Li S, Zhu CG, Guo YL, Sun
J and Li JJ: Lipoprotein subfractions partly mediate the
association between serum uric acid and coronary artery disease.
Clin Chim Acta. 441:109–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martin SS, Jones SR and Toth PP:
High-density lipoprotein subfractions: Current views and clinical
practice applications. Trends Endocrinol Metab. 25:329–336. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakagawa T, Hu H, Zharikov S, Tuttle KR,
Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta
J, et al: A causal role for uric acid in fructose-induced metabolic
syndrome. Am J Physiol Renal Physiol. 290:F625–F631. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zapolski T, Waciński P, Kondracki B,
Rychta E, Buraczyńska MJ and Wysokiński A: Uric acid as a link
between renal dysfunction and both pro-inflammatory and
prothrombotic state in patients with metabolic syndrome and
coronary artery disease. Kardiol Pol. 69:319–326. 2011.PubMed/NCBI
|
|
21
|
Barua RS and Ambrose JA: Mechanisms of
coronary thrombosis in cigarette smoke exposure. Arterioscler
Thromb Vasc Biol. 33:1460–1467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sweetland S, Parkin L, Balkwill A, Green
J, Reeves G and Beral V: Million Women Study Collaborators:
Smoking, surgery, and venous thromboembolism risk in women: United
Kingdom cohort study. Circulation. 127:1276–1282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Enga KF, Braekkan SK, Hansen-Krone IJ, le
Cessie S, Rosendaal FR and Hansen JB: Cigarette smoking and the
risk of venous thromboembolism: The Tromsø Study. J Thromb Haemost.
10:2068–2074. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blondon M, Wiggins KL, McKnight B, Psaty
BM, Rice KM, Heckbert SR and Smith NL: The association of smoking
with venous thrombosis in women. A population-based, case-control
study. Thromb Haemost. 109:891–896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blondon M, Wiggins KL, Van Hylckama Vlieg
A, McKnight B, Psaty BM, Rice KM, Heckbert SR and Smith NL:
Smoking, postmenopausal hormone therapy and the risk of venous
thrombosis: A population-based, case-control study. Br J Haematol.
163:418–420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Woodward M, Lowe GD, Rumley A,
Tunstall-Pedoe H, Philippou H, Lane DA and Morrison CE:
Epidemiology of coagulation factors, inhibitors and activation
markers: The Third Glasgow MONICA Survey. II. Relationships to
cardiovascular risk factors and prevalent cardiovascular disease.
Br J Haematol. 97:785–797. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun GZ, Li Z, Guo L, Zhou Y, Yang HM and
Sun YX: High prevalence of dyslipidemia and associated risk factors
among rural Chinese adults. Lipids Health Dis. 13:1892014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, et al: China National Diabetes and
Metabolic Disorders Study Group: Prevalence of diabetes among men
and women in China. N Engl J Med. 362:1090–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao B, Zhou J, Ge J, Zhang Y, Chen F, Lau
WB, Wan Y, Zhang N, Xing Y, Wang L, et al: Association of maximum
weight with hyperuricemia risk: A retrospective study of 21,414
Chinese people. PLoS One. 7:e511862012. View Article : Google Scholar : PubMed/NCBI
|