Introduction
Alcoholic liver disease (ALD) is the major cause of
chronic liver disease, and can lead to fatty liver, hepatitis and
cirrhosis (1,2). In developed countries, ALD is a major
cause of end-stage liver disease that requires treatment with liver
transplantation (3). However, the
scarcity of donor organs and complications associated with
immunosuppression and transplant rejection limit the availability
and clinical utility of liver transplantation (4). Traditional standard treatments of ALD,
which include abstinence from alcohol, nutritional support and
corticosteroid administration, have not been modified for at least
40 years, despite the poor outcomes of these methods (3,5,6). Therefore, novel therapies are urgently
required, particularly for patients with severe forms of ALD (such
as alcoholic hepatitis), as well as for patients not achieving
alcohol abstinence (3).
In recent decades, the use of herbal medicine in the
prevention of ALD has attracted increased attention since it is
multitargeted and presents less toxic effects (5,7).
Linderae radix (LR; known as Wuyao in Chinese), the dried root of
Lindera aggregata (Sims) Kosterm., has been frequently used
in traditional Chinese medicine for treating various diseases,
including abdominal pain and frequent urination (8,9). LR
consists of alkaloids, volatile oils and sesquiterpene esters, with
alkaloids being the main active component (8,10,11).
Previous studies have found that total alkaloids in LR exert an
anti-inflammatory effect by blocking the nuclear factor-κB (NF-κB)
and mitogen-activated protein kinase (MAPK) signaling pathways
(10,11). In addition, a study by Yamahara et
al (12) revealed that the
sesquiterpene in LR had a protective effect on liver injury induced
by CCl4 or ethionine, and inhibited the increase in
serum transaminase levels. A water extract of LR has been found to
exert a cytoprotective activity against ethanol-induced acute
gastric injury, and this protective activity was possibly mediated
by endogenous prostaglandins and the vagus nerve (13). Certain previous studies have
identified the active ingredients of LR and their pharmacological
effects, such as the anti-inflammatory effect of alkaloids in the
cardiovascular system and the anti-fatigue effect of flavonoids
(8–11,14);
however, further research focusing on the protective effects of LR
on the liver has rarely been reported.
Therefore, in the present study, LR was extracted
using various solvents in order to evaluate the therapeutic effects
and potential mechanisms of LR extracts on alcoholic liver injury.
These extracts were then administrated to a rat model of
ethanol-induced liver injury that was established in the current
study. The blood biochemistry and oxidation indices, as well as the
mRNA expression levels of NF-κB, tumor necrosis factor-α (TNF-α),
interleukin (IL)-1β and cytochrome P450 2E1 (CYP2E1), were
determined to investigate the therapeutic potential and potential
mechanisms of LR extracts on alcoholic liver injury.
Materials and methods
Animals
A total of 90 healthy male Sprague-Dawley (SD) rats
(age, 6 weeks; weight, 170–200 g) were obtained from the
Experimental Animal Center of Zhejiang Academy of Medical Sciences
(Hangzhou, China). The rats were housed at 25±2°C and exposed to a
12/12 h light-dark cycle, with free access to food and water. For
12 h before and 2 h after drug administration, the animals were
fasted, but had free access to water. All experimental procedures
were in accordance with the guidelines established by the Ethics
Committee and Animal Management Committee of People's Hospital of
Tiantai County (Tiantai, China).
Preparation of LR extracts using
different solvents
Dried roots of Lindera aggregata (Sims) Kosterm.
were provided by Zhejiang Biological Engineering Co., Ltd. (China).
Dried root (200 g) extract was obtained by heating with water
(dilution, 1:12) at 100°C for 1.5 h, followed by leaching to obtain
a decoction. The remaining dregs were extracted again by heating
with 1:10 water at 100°C for a further 1 h, and then leached. The
decoction obtained during the two extraction processes was
concentrated to 1 g/ml under vacuum conditions and labeled as
LR-water extract (LR-W). In addition, an LR-ethanol extract (LR-E)
was obtained using a similar process, in which the same volume of
75% ethanol replaced water at room temperature and ethanol was
removed under the reduced pressure conditions.
Subsequently, LR-E was successively extracted with
petroleum ether, ethyl acetate and water-saturated butanol. Each
extraction process was completed when the solution turned
colorless, followed by collection and concentration of the
extraction layer. The LR was divided into four components:
Petroleum ether (LR-E1), ethyl acetate (LR-E2), butanol (LR-E3) and
residual water (LR-E4). With the exception of LR-E1, the other
extraction layers were directly diluted with water to obtain 1 ml
of solution equal to 1 g crude drug. LR-E1 was ground with addition
of Tween-80 (Shanghai Dazhong Pharmaceutical Factory, Shanghai,
China), mixed with water on a magnetic stirrer and diluted with
water to obtain 1 ml of solution corresponding to 1 g crude drug.
The aforementioned solutions were diluted with water to a final
concentration of 0.5 g/ml and stored until further use.
Animal treatment
The 90 healthy male SD rats were randomly divided
into nine groups (n=10 in each) as follows: Normal control, ALD
model, positive control, LR-W, LR-E, LR-E1, LR-E2, LR-E3 and LR-E4
groups. All the rats were given the basal diet and water ad
libitum. Rats in the normal control group were administrated with 1
ml distilled water. Rats in other groups were intragastrically
administrated with 1 ml liquor (52% ethanol; Beijing Red Star Co.,
Ltd., Beijing, China) per 100 g body weight (bw) in gradient
concentrations (days 1–2, 30% v/v; days 3–4, 40% v/v; days 5–10,
50% v/v), two times per day for 10 continuous days. After 10 days,
an acute alcoholic liver injury model was established. As shown in
Table I, the serum alanine
aminotransferase (ALT) and aspartate aminotransferase (AST) of rats
in model group were significantly higher compared with those in the
normal control group (P<0.05), suggesting the successful
establishment of the liver injury rat model.
 | Table I.Effects of Linderae radix extracts on
the blood biochemistry index of acute alcoholic liver injury
rats. |
Table I.
Effects of Linderae radix extracts on
the blood biochemistry index of acute alcoholic liver injury
rats.
| Group | Dose (g/kg) | ALT (U/l) | AST (U/l) | TC (mmol/l) | TG (mmol/l) |
|---|
| Normal control | – | 64.6±10.5 | 165.2±20.0 | 1.48±0.22 | 0.326±0.063 |
| Model | – |
87.9±29.0a |
191.1±16.8b |
1.85±0.42a |
0.453±0.093b |
| Positive control | 0.0225 | 70.3±8.6c |
174.3±12.4c |
1.39±0.19d | 0.523±0.122 |
| LR-W | 2 | 75.6±16.3 | 188.0±33.8 |
1.53±0.14c | 0.418±0.121 |
| LR-E | 2 |
61.2±10.2d |
166.9±13.0d |
1.51±0.37c |
0.361±0.106c |
| LR-E1 | 2 |
58.8±8.6d |
165.6±15.6d |
1.57±0.27c | 0.380±0.181 |
| LR-E2 | 2 |
65.9±7.1c | 172.9±30.9 | 1.63±0.36 | 0.454±0.188 |
| LR-E3 | 2 |
63.0±11.1c | 181.6±35.2 | 1.69±0.27 |
0.374±0.078c |
| LR-E4 | 2 |
69.3±13.0c | 174.2±31.6 |
1.59±0.14c | 0.455±0.136 |
During 10 days of liquor administration, rats in the
normal control and model groups were fed daily with 1 ml/100 g bw
distilled water, while rats in the positive control were treated
with 2.25 mg/ml dimethyl diphenyl bicarboxylate (DDB; Zhejiang
Wanbang Pharmaceutical Co., Ltd., Taizhou, China), which is a
widely used hepatoprotectant (15–17).
Furthermore, rats in the LR treatment groups were orally
administered with 1 ml/100 g bw LR extract once daily for 10 days.
Subsequent to the last administration, blood samples were obtained
from the inferior vena cava for biochemical assay. The rats were
anesthetized and sacrificed using 5% chloral hydrate (Shanghai
Chemical Agent Cooperation, Shanghai, China), and liver tissue was
immediately removed for oxidation index and histopathological
analyses.
Blood biochemistry index assay
Blood obtained from the rats was centrifuged at
1,006 × g for 15 min at room temperature to obtain serum. Next, the
serum levels of ALT, AST, triglycercides (TG) and total cholesterol
(TC) were measured using a TBA-120FR automatic biochemistry
analyzer (Toshiba Medical System Co., Ltd., Tochigi, Japan), and
according to the manufacturer instructions of the kits (Ningbo
Meikang Biotechnology Co., Ltd., Ningbo, China). The details for
the four kits were: ALT assay kit (cat. no. 20130517); AST assay
kit (cat. no. 20130608); TC assay kit (cat. no. 20130626); and TG
assay kit (cat. no. 20130719). Normal serum reference values for
the analytes were as follows: ALT, 54.6–71.9 U/l; AST, 154.0–183.8
U/l; TC, 1.35–1.69 mmol/l; and TG, 0.27–0.38 mmol/l.
Oxidation index determination
Liver homogenate was prepared by grinding 0.5 g
liver tissue in 4.5 g physiological saline. The levels of
malondialdehyde (MDA) and superoxide dismutase (SOD) in the serum
and liver tissue were investigated using a PowerWave 340 microplate
reader (Bio-Tek Instruments, Inc., Winooski, VT, USA), according to
the manufacturer instructions of the MDA (cat. no. 20130819) and
SOD (cat. no. 20130517) kits (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). Normal serum reference values: MDA,
3.46–3.79 nmol/ml; SOD, 136.4–149.1 U/ml.
Histopathological examination
The left lobe of the liver was fixed with 10%
neutral formalin, embedded in paraffin and stained with
hematoxylin-eosin (Merck KGaA, Darmstadt, Germany). Tissue slides
were observed under a B5-223IEP light microscope (Motic China Group
Co., Ltd., Guangzhou, China) coupled with a Advanced 3.2 image
analysis system (Motic China Group Co., Ltd., Xiamen, China).
Expression levels of NF-κB, TNF-α and
IL-1β in liver tissue
The expression levels of NF-κB, TNF-α and IL-1β in
liver tissue were detected using the labeled streptavidin-biotin
method, according to the kit instructions (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The monoclonal antibody
catalogue numbers were as follows: Anti-mouse TNF-α, (sc-8436);
anti-mouse NF-κB; (sc-8436); and anti-rabbit IL-1β, (sc-7884).
Cells with yellow/brown particles were determined as having a
positive expression.
Expression of CYP2E1 mRNA determined
by reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
The expression of CYP2E1 can be induced following
ethanol consumption (18), thus this
was determined in the present study. The total RNA in liver tissue
was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA). Next, total RNA was reverse
transcribed into cDNA (RevertAid First Strand cDNA Synthesis kit;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The mRNA
expression of CYP2E1 in liver tissue was detected by qPCR coupled
with a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific,
Inc.) and LightCycler 480 Instrument II (Roche Diagnostics, Basel,
Switzerland). PCR was performed using a Fermentas First Strand cDNA
Synthesis kit (cat. no. K1621; Thermo Fisher Scientific, Inc.,
Vilnius, Lithuania) and a Premix Ex Taq™ master mix kit (cat. no.
RR390Q; Takara Bio, Inc., Tokyo, Japan), with β-actin used as an
internal control. The primer sequences used in qPCR are shown in
Table II. The PCR mixture consisted
of 10 µl Premix Ex Taq (Probe qPCR), 0.4 µl forward primer, 0.4 µl
reverse primer, 1.5 µl cDNA, 0.4 µl MGB probe and 7.3 µl
nuclease-free water (total volume, 20 µl. The qPCR cycling
conditions were as follows: 94°C for 30 sec, followed by 35 cycles
at 94°C for 30 sec, 62°C for 30 sec, 72°C for 30 sec, and extension
at 72°C for 7 min. Subsequently, 10 ml PCR product was subjected to
electrophoresis in a 1.5% agarose gel stained with ethidium bromide
(Sigma-Aldrich, St. Louis, MO, USA).
 | Table II.Sequences of primers used for reverse
transcription-polymerase chain reaction. |
Table II.
Sequences of primers used for reverse
transcription-polymerase chain reaction.
| Gene | Primer
direction | Primer
sequence |
|---|
| β-actin | Upstream |
5′-CATCATGAAGTGTGACGTTGAC-3′ |
|
| Downstream |
5′-TCAGGAGGAGCAATGATCTTGA-3′ |
|
| Probe |
5′-TGCTGTCTGGTGGCAC-3′ |
| Cytochrome P450
2E1 | Upstream |
5′-GATATGTCATCCCCAAGGGTA-3′ |
|
| Downstream |
5′-CACACACACGCTTTCCTGC-3′ |
|
| Probe |
5′-CAGATCCAGAGAAGTTT-3′ |
Statistical analysis
All measurements are expressed as the mean ±
standard deviation, and SPSS 16.0 software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. Values in different
groups were compared with one-way analysis of variance, and the
analysis between groups was performed with the least significant
difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of LR extracts on serum enzyme
levels
The levels of serum enzymes detected in the present
study are presented in Table I. The
serum levels of ALT and AST in the model group rats were
significantly higher compared with those in the normal control
group rats (P<0.05), suggesting the successful establishment of
the liver injury rat model. Compared with the model group, serum
ALT levels in the positive control, LR-E (P<0.01), LR-E1
(P<0.01), LR-E2 (P<0.05), LR-E3 (P<0.05) and LR-E4
(P<0.05) groups were significantly lower. In addition, serum AST
levels in the positive control, LR-E and LR-E1 groups were
significantly lower (P<0.05) compared with those in the model
group. The results suggest that LR extracts suppress the increases
of serum AST and ALT induced by alcoholic liver injury.
Effect of LR extracts on serum lipid
parameters
The results of serum lipid parameter levels (TC and
TG) are shown in Table I. TC and TG
levels in the model control group were significantly higher when
compared with those in the normal control group (P<0.05).
Compared with the model group, serum TC levels were significantly
lower in the positive control, LR-W, LR-E, LR-E1 and LR-E4 groups.
Furthermore, the serum TG levels in LR-E and LR-E3 groups were
significantly lower when compared with those in the model control
group (P<0.05). These results suggest that LR extracts inhibit
increases of serum TG induced by alcoholic liver injury.
Effect of LR extracts on oxidation
indices
As shown in Table
III, MDA levels in the serum and liver tissue of the model
control group was significantly higher compared with the normal
control levels, while SOD levels were significantly lower
(P<0.05). Compared with the model group, the serum MDA levels in
the LR-E and LR-E4 groups were significantly decreased, while the
serum SOD levels in the positive control, LR-W, LR-E, LR-E3 and
LR-E4 groups were significantly increased (P<0.05). In addition,
the liver SOD levels in the positive control, LR-E1, LR-E3 and
LR-E4 groups were significantly increased (P<0.05) compared with
the model group; however, no statistically significant difference
was observed in the liver MDA levels among the various treatment
groups. These results indicate that LR extracts reduce alcoholic
liver injury-related decreases in SOD levels.
 | Table III.Effects of Linderae radix extracts on
the serum oxidation index of acute alcoholic liver injury rats. |
Table III.
Effects of Linderae radix extracts on
the serum oxidation index of acute alcoholic liver injury rats.
| Group | Dose (g/kg) | Liver MDA (nmol/mg
protein) | Liver SOD (U/mg
protein) | Serum MDA
(nmol/ml) | Serum SOD
(U/ml) |
|---|
| Normal control | – | 5.80±1.04 | 194.7±20.4 | 3.62±0.19 | 143.5±7.5 |
| Model | – |
8.61±3.21a |
155.3±15.0b |
4.46±0.30b |
126.2±6.6b |
| Positive
control | 0.0225 | 7.66±3.38 |
169.0±12.2c | 4.29±1.02 |
138.4±10.3d |
| LR-W | 2 | 6.57±3.17 | 162.9±7.80 | 4.46±0.59 |
138.1±4.7d |
| LR-E | 2 | 7.75±4.13 | 165.7±12.8 |
3.87±0.44d |
136.3±10.4c |
| LR-E1 | 2 | 7.88±3.26 |
167.7±10.0c | 4.50±0.64 | 127.3±9.6 |
| LR-E2 | 2 | 7.26±3.68 | 160.1±12.5 | 4.50±1.22 | 125.8±5.8 |
| LR-E3 | 2 | 7.98±2.65 |
169.3±10.0c | 4.22±0.80 |
133.3±10.1c |
| LR-E4 | 2 | 6.96±2.07 |
167.1±13.1c |
3.78±0.58d |
140.2±6.4d |
Histopathological changes in liver
tissue of ALD model rats
Visual inspection of the liver tissue obtained from
the ALD rats revealed that the tissues were of normal color, the
liver capsule was shiny, and no adhesion was identified between
abdominal intestinal canals in the normal control group. By
contrast, the liver color in the ALD model group rats was slightly
yellow. The liver color in the various treatment groups was found
to be similar to that in the normal control group.
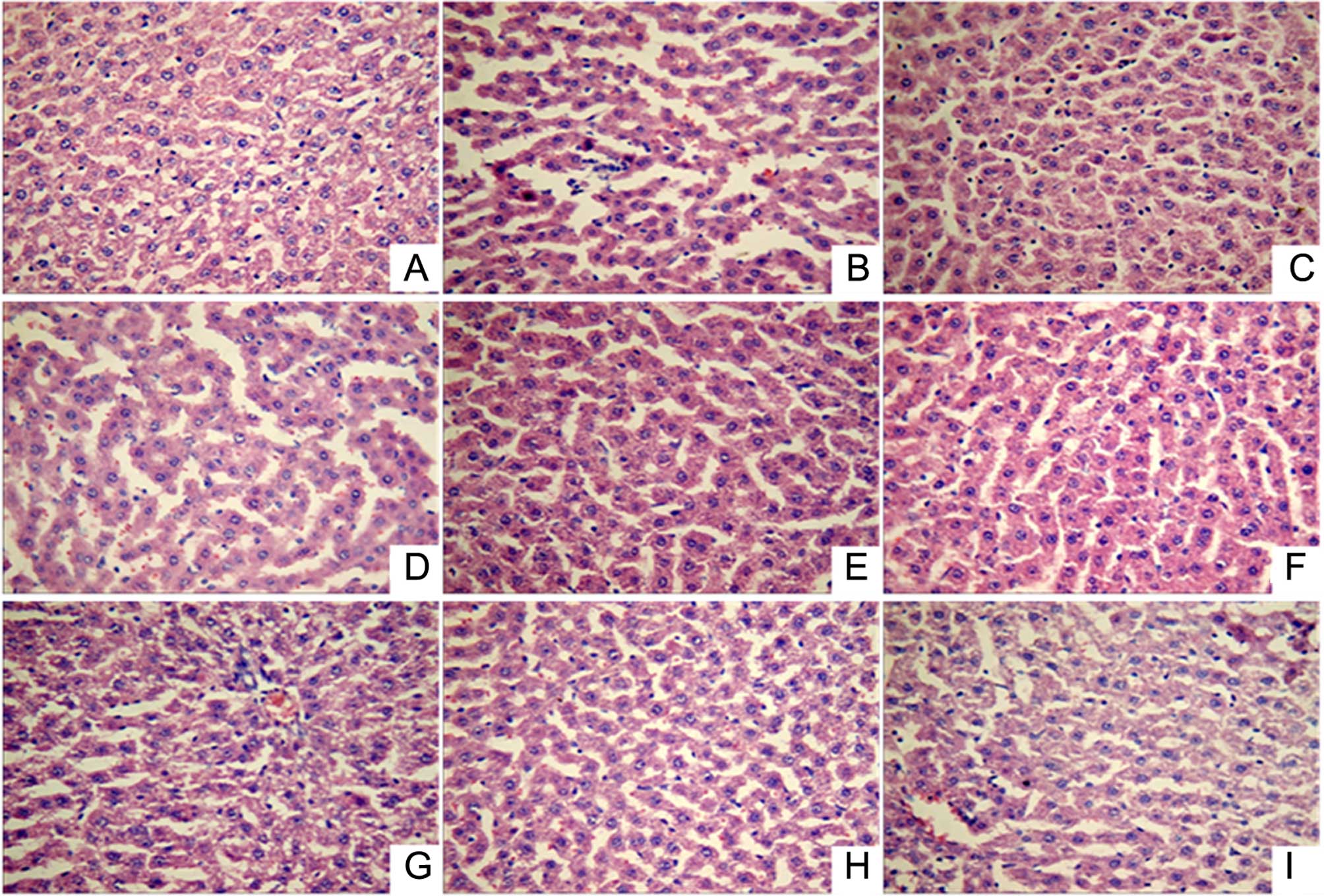
Histopathological examination (Fig. 1) showed that the liver lobular
structure was clear in the normal control group, with the hepatic
cords arranged neatly by radiating from the central vein, while the
liver cell outline was clear and polygonal. In addition, there was
no hepatocellular necrosis, no significant pathological changes,
and a small number of inflammatory cells was identified throughout
the entire liver. However, in the acute alcoholic liver injury
model rats, fuzzy hepatic lobule boundaries and scattered liver
cells within the lobular in spotty necrosis were observed, the
portal area showed inflammatory cell infiltration, certain cell
nuclei had disappeared, and the liver cell cord was disordered. By
contrast, in all the treatment groups (positive control and LR
extract groups), the lobular boundaries were clear and hepatic cord
cells were arranged neatly, while the inflammatory cell
infiltration, necrosis and pathological changes in the liver tissue
were significantly reduced. Thus, LR extract treatment appears to
mitigate alcoholic liver injury.
Effect of LR extracts on the
expression levels of NF-κB, TNF-α and IL-1β in liver tissues
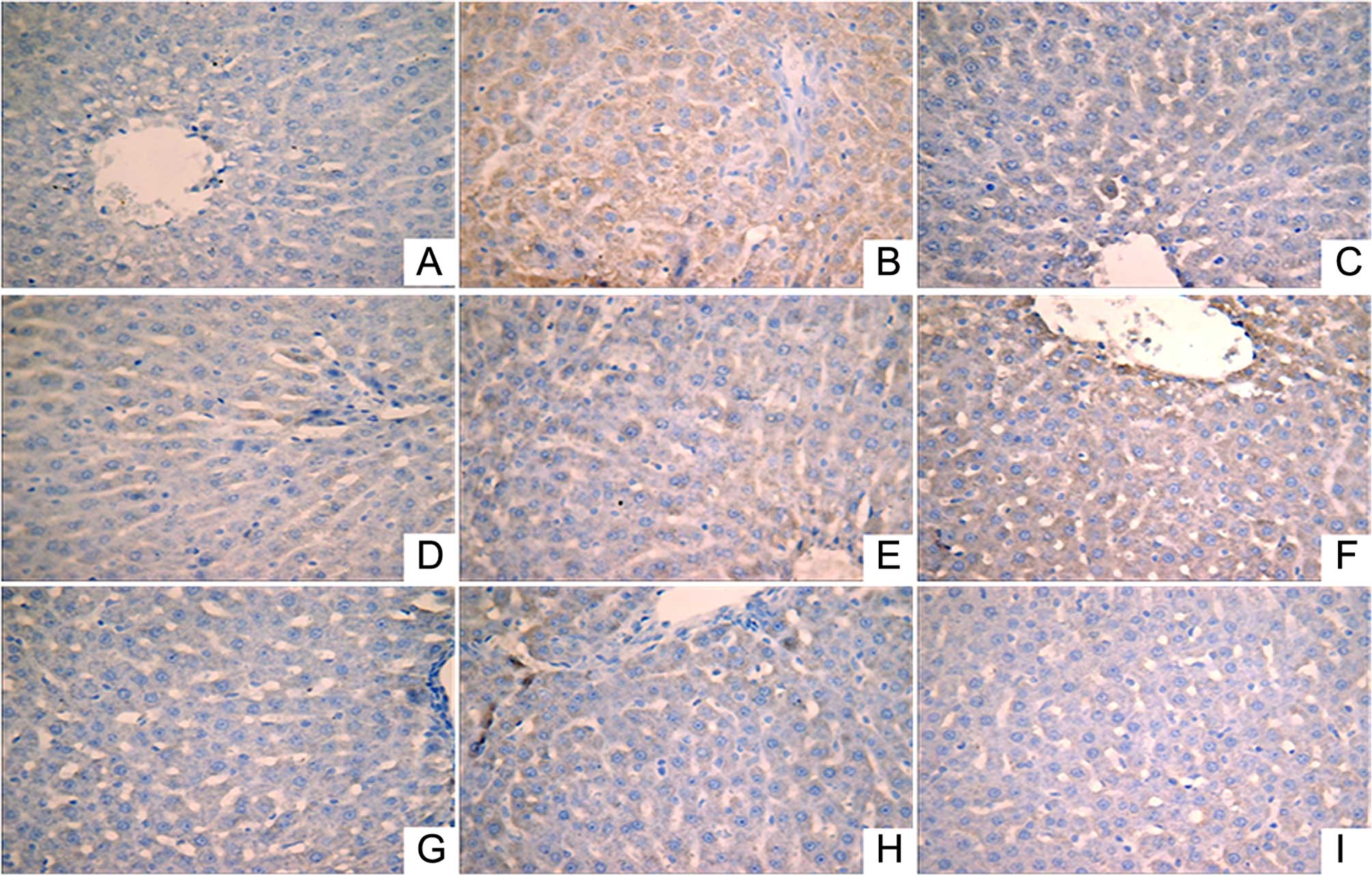
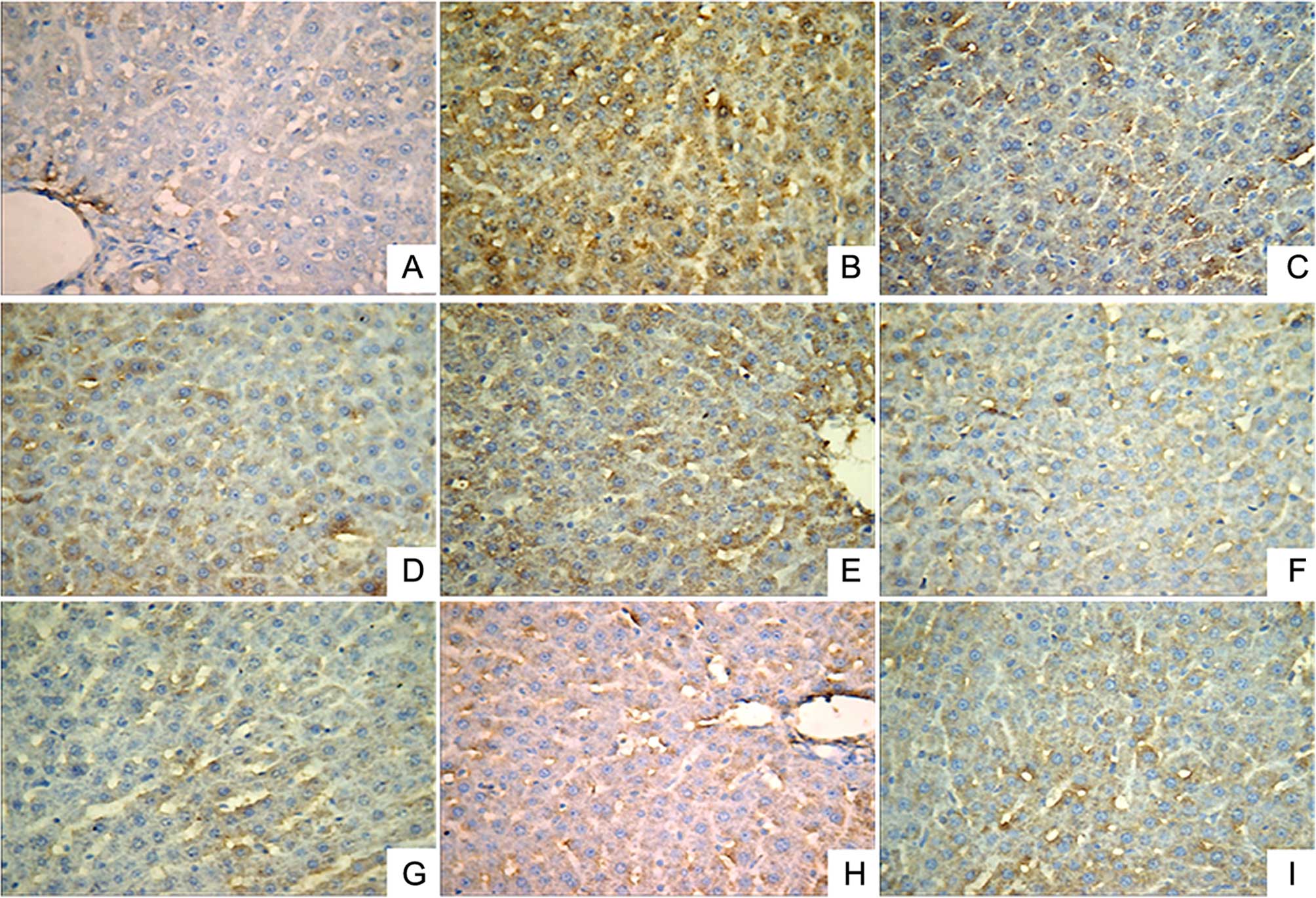
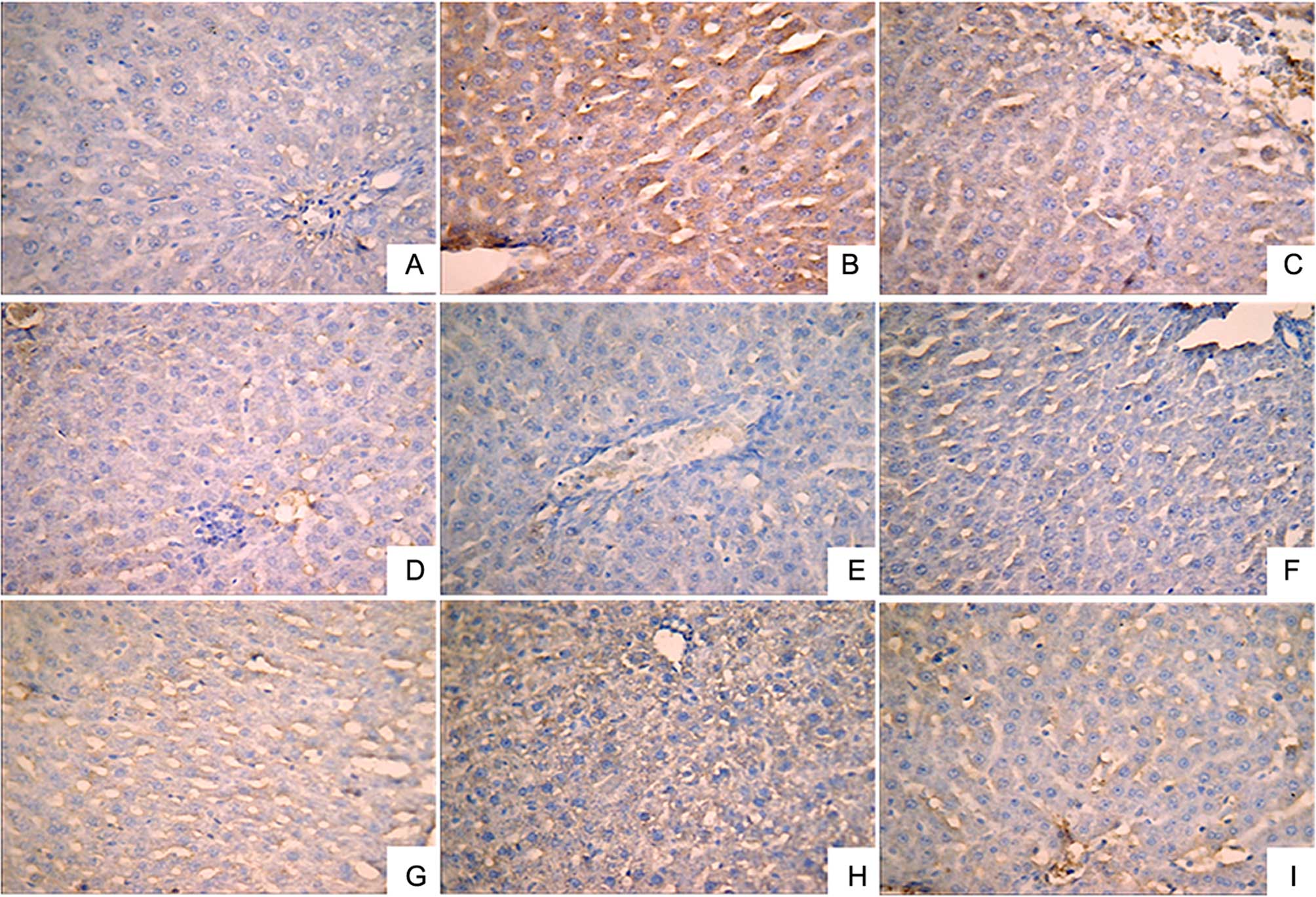
Immunohistochemical analysis was performed to
investigate the expression levels of IL-1β (Fig. 2), NF-κB (Fig. 3) and TNF-α (Fig. 4). A small number of yellow/brown
areas were observed in liver cells of rats in the normal control
group, suggesting low expression levels of IL-1β, NF-κB and TNF-α.
Compared with the normal control group, the expression levels of
IL-1β, NF-κB and TNF-α in the model group were significantly
increased. Treatment with DDB and the various LR extracts decreased
the high expression levels induced by alcohol stimulation. These
findings inidicate that LR extracts prohibit increases in the
expression of NF-κB, TNF-α and IL-1β induced by alcohol
consumption.
Effect of LR extracts on the
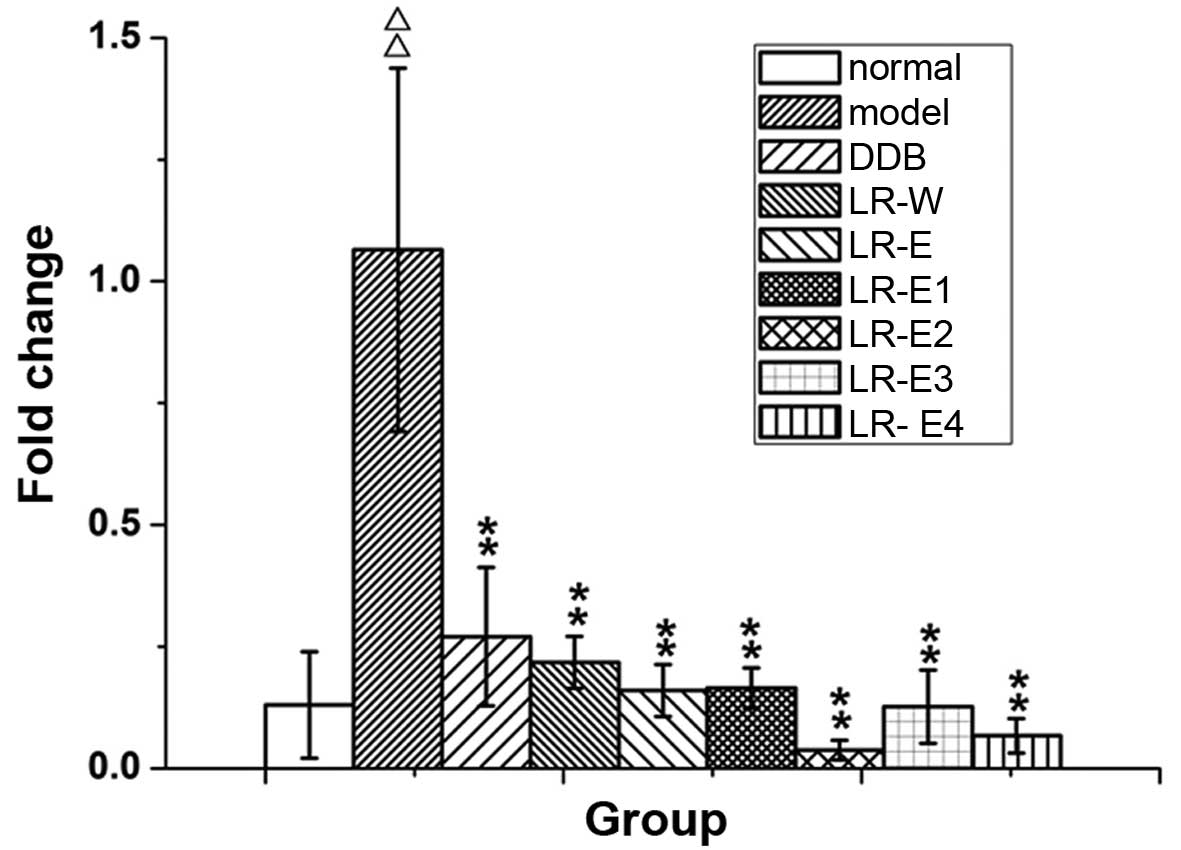
expression of CYP2E1 mRNA in liver tissues
The expression of CYP2E1 mRNA was detected by
RT-qPCR (Fig. 5). Compared with the
normal control group, CYP2E1 mRNA expression in liver tissue from
rats in the model group was significantly enhanced (P≤0.01).
Treatment with DDB and all LR extracts effectively reduced the
expression of CYP2E1 mRNA that was induced by excessive alcohol
intake (P≤0.01). The results indicate that LR extracts mitigate the
increase of CYP2E1 detected in the alcoholic liver injury rat
model.
Discussion
ALD is a common consequence of excessive alcohol
abuse and remains a major cause of morbidity and mortality
worldwide (19). LR has been widely
used in traditional Chinese medicine, and extracts of LR have been
reported to exert a therapeutic effect on various diseases,
including abdominal pain and frequent urination (20,21). In
the present study, we successfully established an acute alcoholic
liver injury model by gavage, and studied the preventive effect and
the potential mechanisms of various LR extracts on the rat model.
The results revealed that LR extracts had preventive effect on
alcoholic liver injury by inhibiting serum ACT and AST levels, and
this beneficial effect may be associated with anti-inflammation and
anti-oxidation.
LR has been used in the treatment of various
diseases (8), with certain studies
reporting that LR extracts have a protective effect on
ethanol-induced liver injury (12,20,22). The
activities of ALT and AST are commonly used as reliable markers for
clinical monitoring of liver injury or liver function (23,24).
However, in the current study, the active component in each LR
extract were not isolated and identified, which is a limitation of
the study. Thus, further experiments isolating and identifying
these active components in LR extracts may contribute to the
development of novel treatments against ALD.
Inflammation serves a critical role in the
pathogenesis and progression of ALD (7), while Kupffer cells play a key role in
hepatic inflammation in liver injury (25). Excessive alcohol intake can activate
Kupffer cells, resulting in the secretion of various inflammatory
mediators, such as cytokines (TNF-α and IL-1β) and NF-κB, which
then further promotes inflammation and liver injury (25,26). LR
has been proven to exhibit anti-inflammation by preventing the
production of inflammatory mediators (10,27).
Similarly, in the present study, LR extracts were found to inhibit
the expression levels of NF-κB, TNF-α and IL-1β. Thus, this may
suggest that LR extracts exhibit an anti-inflammatory effect on
alcoholic liver injury by inhibiting the expression levels of
NF-κB, TNF-α and IL-1β.
Ethanol-induced oxidative stress serves a
significant role in the mechanisms underlying the development of
ALD (28). CYP2E1 seems to be the
main contributor to ethanol-induced oxidant stress (29). Expression of CYP2E1 has been shown to
be significantly elevated upon exposure to ethanol, and CYP2E1
induces the production of reactive oxygen species (ROS), which are
toxic to cells and result in lipid peroxidation (LPO) (29). MDA, the end-product of LPO, has been
widely used as an indicator of LPO and a marker for the status of
oxidative stress (30). Furthermore,
SOD is essential for the endogenous anti-oxidative defense system
to scavenge ROS and maintain the cellular redox balance (31). The RT-qPCR results showed that LR
extracts may inhibit CYP2E1 expression in liver tissue, decrease
the serum MDA levels, and elevate serum and liver tissue SOD
activity to varying degrees. Thus, it is suggested that LR extracts
may effectively protect the liver from ethanol-induced oxidative
stress.
In conclusion, the results of the present study
suggest that LR extracts exhibit a protective effect on alcoholic
liver injury, and the mechanism may be attributed to the
anti-inflammatory and anti-oxidative action. However, isolating and
identifying the active components in LR extract is an indispensable
step for the further clinical application of LR.
Acknowledgements
This study was supported by grants from the Project
of Technology Application for Public Welfare in Zhejiang Province
(grant no. 2013C33222), Medical and Health Project in Zhejiang
Province (grant no. 2015DTA021), the Youth Project of National
Natural Science Foundation (grant no. 81503328) and Project of
Technology Application for Public Welfare in Zhejiang Province
(grant no. 2016C33184).
References
|
1
|
Gao B and Bataller R: Alcoholic liver
disease: Pathogenesis and new therapeutic targets.
Gastroenterology. 141:1572–1585. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie YD, Feng B, Gao Y and Wei L:
Characteristics of alcoholic liver disease and predictive factors
for mortality of patients with alcoholic cirrhosis. Hepatobiliary
Pancreat Dis Int. 12:594–601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Altamirano J and Bataller R: Alcoholic
liver disease: Pathogenesis and new targets for therapy. Nat Rev
Gastroenterol Hepatol. 8:491–501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levine P, McDaniel K, Francis H, Kennedy
L, Alpini G and Meng F: Molecular mechanisms of stem cell therapy
in alcoholic liver disease. Dig Liver Dis. 46:391–397. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Woo GA and O'Brien C: Long-term management
of alcoholic liver disease. Clin Liver Dis. 16:763–781. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orman ES, Odena G and Bataller R:
Alcoholic liver disease: Pathogenesis, management and novel targets
for therapy. J Gastroenterol Hepatol. 28(Suppl 1): 77–84. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding RB, Tian K, He CW, Jiang Y, Wang YT,
Wan JB and Huang LL: Herbal medicines for the prevention of
alcoholic liver disease: A review. J Ethnopharmacol. 144:457–465.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng XL, Ma SC, Wei F, Wang GL, Xiao XY
and Lin RC: A new sesquiterpene isolated from Lindera
aggregata (SIMS) Kosterm. Chem Pharm Bull (Tokyo).
55:1390–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Y, Zheng Y, Liu X, Han Z, Ren Y, Gan L,
Zhou C and Luan L: Separation and quantitative determination of
sesquiterpene lactones in Lindera aggregata (Wu-yao) by
ultra-performance LC-MS/MS. J Sep Sci. 33:1072–1078.
2010.PubMed/NCBI
|
|
10
|
Luo Y, Liu M, Yao X, Xia Y, Dai Y, Chou G
and Wang Z: Total alkaloids from Radix Linderae prevent the
production of inflammatory mediators in
lipopolysaccharide-stimulated RAW 264.7 cells by suppressing
NF-kappaB and MAPKs activation. Cytokine. 46:104–110. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Dai Y, Yang J, Chou G, Wang C and
Wang Z: Treatment with total alkaloids from Radix Linderae reduces
inflammation and joint destruction in type II collagen-induced
model for rheumatoid arthritis. J Ethnopharmacol. 111:322–328.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamahara J, Matsuda H, Sawada T and
Kushida H: Biologically active principles of crude drugs preventive
effect of sesquiterpenoid components of the root of Lindera
strychinifolia on experimental liver damage. Shōyakugaku
Zasshi. 37:84–86. 1983.(In Japanese).
|
|
13
|
Zhu M, Luk CT and Lew TH: Cytoprotective
Effect of Lindera aggregata roots against ethanol-induced
acute gastric injury. Pharm Biol. 36:222–226. 1998. View Article : Google Scholar
|
|
14
|
Yi YN, Cheng XM, Liu LA, Hu GY, Wang ZT,
Deng YD, Huang KL, Cai GX and Wang CH: Simultaneous determination
of synephrine, arecoline and norisoboldine in Chinese patent
medicine Si-Mo-Tang oral liquid preparation by strong cation
exchange high performance liquid chromatography. Pharm Biol.
50:832–838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El-Beshbishy HA: The effect of dimethyl
dimethoxy biphenyl dicarboxylate (DDB) against tamoxifen-induced
liver injury in rats: DDB use is curative or protective. J Biochem
Mol Biol. 38:300–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao M, Zhang J and Liu G: Effect of
diphenyl dimethyl bicarboxylate on concanavalin A-induced liver
injury in mice. Liver Int. 25:904–912. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abdel-Hameid NA: Protective role of
dimethyl diphenyl bicarboxylate (DDB) against erythromycin induced
hepatotoxicity in male rats. Toxicol In Vitro. 21:618–625. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kessova I and Cederbaum AI: CYP2E1:
Biochemistry, toxicology, regulation and function in
ethanol-induced liver injury. Curr Mol Med. 3:509–518. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albano E: Oxidative mechanisms in the
pathogenesis of alcoholic liver disease. Mol Aspects Med. 29:9–16.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohno T, Takemura G, Murata I, Kagawa T,
Akao S, Minatoguchi S, Fujiwara T and Fujiwara H: Water extract of
the root of Lindera strychnifolia slows down the progression
of diabetic nephropathy in db/db mice. Life Sci. 77:1391–1403.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noda Y and Mori A: Antioxidant activities
of uyaku (Lindera strychnifolia) leaf extract: A natural
extract used in traditional medicine. J Clin Biochem Nutr.
41:139–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu M, Luk C and Lew T: Cytoprotective
effect of Lindera aggregata roots against ethanol-induced
acute gastric injury. Pharm Biol. 36:222–226. 1998. View Article : Google Scholar
|
|
23
|
Giannini EG, Testa R and Savarino V: Liver
enzyme alteration: A guide for clinicians. CMAJ. 172:367–379. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Recknagel RO, Glende EA Jr, Dolak JA and
Waller RL: Mechanisms of carbon tetrachloride toxicity. Pharmacol
Ther. 43:139–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luckey SW and Petersen DR: Activation of
Kupffer cells during the course of carbon tetrachloride-induced
liver injury and fibrosis in rats. Exp Mol Pathol. 71:226–240.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thurman RG: II. Alcoholic liver injury
involves activation of Kupffer cells by endotoxin. Am J Physiol.
275:G605–G611. 1998.PubMed/NCBI
|
|
27
|
Luo Y, Liu M, Xia Y, Dai Y, Chou G and
Wang Z: Therapeutic effect of norisoboldine, an alkaloid isolated
from Radix Linderae, on collagen-induced arthritis in mice.
Phytomedicine. 17:726–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Das SK and Vasudevan DM: Alcohol-induced
oxidative stress. Life Sci. 81:177–187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu Y and Cederbaum AI: CYP2E1 and
oxidative liver injury by alcohol. Free Radic Biol Med. 44:723–738.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Del Rio D, Stewart AJ and Pellegrini N: A
review of recent studies on malondialdehyde as toxic molecule and
biological marker of oxidative stress. Nutr Metab Cardiovasc Dis.
15:316–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raychaudhuri SS and Deng XW: The role of
superoxide dismutase in combating oxidative stress in higher
plants. Botanical Review. 66:89–98. 2000. View Article : Google Scholar
|