Introduction
Extranodal natural killer (NK) cell/T-cell lymphoma
(ENKTL) is a type of non-Hodgkin's lymphoma that presents
significantly different distributions patterns according to
population and geography, and is relatively common in Asia and
South America (1,2). The incidence of patients with lymphoma
is as high as 11% in China (3).
NK/T-cell lymphomas usually occur in middle-aged patients, and
their features are characterized by localized disease in
approximately two-thirds of patients, along with frequent adjacent
tissue invasion, a high frequency of ‘B’ symptoms, despite
apparently limited disease, and rare bone marrow involvement
(4–6). In addition, ENKTL is highly invasive,
resistant to conventional chemotherapy, highly malignant,
associated with short survival time and rapid clinical progression,
and has a relatively poor prognosis (7–9).
ENKTL is thought to arise from NK cells or
occasionally from a subset of γδd or αβ cytotoxic T-cells, and is
strongly associated with Epstein-Barr virus infection (10,11). The
ENKTL phenotype is characterized by the expression of cytoplasmic
CD3ε in the absence of surface CD3, T-cell receptor expression, or
T-cell receptor gene rearrangement, as well as an activated
cytotoxic profile with expression of perforin, granzyme B, TIA-1,
and frequent CD56 expression (12,13).
As ENKTL is rare, and prospective group studies are
limited, no standard treatment regimen is currently available.
Early NK/T-cell lymphoma is sensitive to radiotherapy if confined
to the nasal cavity; however, recurrence at the primary site or
distant sites occurred in 50% of patients who received
radiotherapy, with a five-year survival rate of 29.8–66% (14–17).
Since the multidrug resistance (MDR) gene is highly expressed in
ENKTL tumor cells, the tumors are insensitive to conventional
chemotherapy regimens (such as CHOP) that are applied to other
pathological types of non-Hodgkin's lymphoma (18). Asparaginase is not regulated by the
MDR gene, and numerous studies have demonstrated that the
application of asparaginase to relapsed or refractory ENKTL induced
promising responses and high survival rates (19–22). Few
clinical studies on asparaginase-based regimens as first-line
treatments for newly diagnosed ENKTL are available (22,23),
and, to the best of our knowledge, no previous studies have
compared the short-term efficacy and long-term survival impacts of
asparaginase and CHOP treatment regimens in patients with ENKTL.
Therefore, the present study investigated the effects of
asparaginase in patients with previously untreated ENKTL. The
clinical characteristics of 181 patients with newly-diagnosed
pathologically ENKTL were retrospectively analyzed. The patients
were divided into CHOP treatment regimen and asparaginase-based
chemotherapy groups according to the administered treatment
regimen. Short-term efficacy, long-term survival, and safety were
compared in the two groups and the results are reported herein.
Materials and methods
Clinical data
A total of 181 patients with newly diagnosed ENKTL
received either CHOP treatment or L-asparaginase-based chemotherapy
along with radiotherapy between October 2003 and June 2013 at the
Henan Tumor Hospital, First Affiliated Hospital of Henan University
of Science and Technology (Luoyang, China), the Xinzheng City
People's Hospital (Xinzheng, China), and the Jiaozuo City Second
People's Hospital (Jiaozuo, China). Of these patients, 69 were
included in the CHOP treatment group and 112 were included in the
L-asparaginase treatment group. All patients were diagnosed via
imaging studies and pathological analyses. Differences in the main
clinical characteristics between the two groups of patients were
not statistically significant (P>0.05), as shown in Table I.
 | Table I.Clinical characteristics of patients
with newly diagnosed ENKTL. |
Table I.
Clinical characteristics of patients
with newly diagnosed ENKTL.
| Characteristic | CHOP | L-asparaginase | P-value |
|---|
| Gender |
|
| 0.685 |
|
Male | 47 | 73 |
|
|
Female | 22 | 39 |
|
| Age, years |
|
| 0.571 |
|
≤60 | 59 | 99 |
|
|
>60 | 10 | 13 |
|
| Ann Arbor
stage |
|
| 0.89 |
|
I/II | 58 | 95 |
|
|
III/IV | 11 | 17 |
|
| IPI score |
|
| 0.679 |
|
0–1 | 52 | 92 |
|
| ≥2 | 17 | 20 |
|
| ECOG performance
status |
|
| 0.88 |
|
0–1 | 67 | 109 |
|
| ≥2 | 2 | 3 |
|
| B symptoms |
|
| 0.229 |
|
Yes | 30 | 59 |
|
| No | 39 | 53 |
|
| ENKTL subtype |
|
| 0.639 |
|
UNKTL | 66 | 107 |
|
|
EUNKTL | 3 | 5 |
|
| Bony
perforation | 18 | 28 | 0.87 |
| Elevated serum LDH
(>245 IU/l) | 42 | 53 | 0.076 |
| Elevated
β2-microglobulin (>2.2 U/l) | 52 | 76 | 0.281 |
| Leukocytopenia
(<4.0×109/l) | 15 | 26 | 0.818 |
| Haemoglobin
(<110 g/l) | 14 | 21 | 0.799 |
|
CD56+ | 57 | 97 | 0.463 |
|
EBV+ | 43 | 63 | 0.421 |
| Ki67 expression
(median) | 60 | 60 | 0.846 |
Prognostic factors
The International Prognostic Index (IPI)clinical
tool was used to predict the prognosis of patients (24). IPI factors include: Age, >60
years; serum lactate dehydrogenase (LDH) level above normal;
performance status, 2–4; stage of disease, III or IV; and
extranodal involvement, >1 site (24). In addition, the gender, B-symptoms
(including unexplained fever, >10% weight loss or sweating),
Eastern Cooperative Oncology Group performance status and primary
anatomic sites of lymphomatous involvement for all patients were
evaluated for their potential prognostic importance. The serum LDH
and β2-microglobulin levels were analyzed using an
automated biochemical analyzer (Modular P800; Hitachi, Ltd., Tokyo,
Japan), and the expression of Ki67 in ENKTL tumor samples were
assessed by immunohistochemistry. Briefly, the tumor samples were
cut into sections using a microtome (Leica RM2235; Leica
Microsystems GmbH, Wetzlar, Germany), followed by incubation with
mouse anti-Ki67 monoclonal antibody (1:200; cat. no. M7240; Dako,
Glostrup, Denmark), and then ready-to-use EnVision reagent (cat.
no. K5007; Dako). Subsequently, positive cells (yellow/tan color)
were counted in 10 fields of view under a microscope (Olympus IX81;
Olympus Corporation, Tokyo, Japan). Phosphate-buffered saline was
used as a negative control.
Treatment
The CHOP treatment regimen included 750
mg/m2 (d1) cyclophosphamide (Baxter Oncology
GmbH, Halle, Germany), 50 mg/m2 (d1)
adriamycin (Pfizer, New York, USA), 1.4 mg/m2
(d1) vincristine (Zhejiang Hisun Pharmaceutical, Co.,
Ltd., Taizhou, China) and 40 mg/m2 (d1–5)
prednisone (Zhejiang Xianju Pharmaceutical Co., Ltd., Taizhou,
China), which was administered to 69 patients. L-asparaginase
(Guangzhou Baiyun Shan Ming Xing Pharmaceutical, Co., Ltd.,
Guangzhou, China) treatment was given to 112 patients. The patients
who received L-asparaginase treatment were subdivided into the
following treatment groups: CHOP-L (65 patients), VLP (22
patients), COP-L (10 patients), or L-asparaginase alone (15
patients). The patients in the CHOP-L group received the entire
CHOP treatment regimen, whereas the patients in the VLP group
received the vincristine and prednisone only, and the patients in
the COP-L group received the cyclophosphamide, vincristine and
prednisone only. All patients received 6,000 U/m2
(d1–7) L-asparaginase and the other drugs at the
reference doses described for the CHOP treatment. The patients who
responded positively to an L-asparaginase skin test (25 patients),
which was indicated by the presence of erythemas or wheals, were
treated with 2,500 U/m2 pegaspargase (Jiang Su Heng Rui
Medicine, Co., Ltd., Lianyungang, China). During chemotherapy, the
heart, liver and kidneys were protected by intravenous injection
with 5 mg tropisetron hydrochloride (Jiang Su Heng Rui Medicine,
Co., Ltd.) 30 min prior to chemotherapy, hydration (250–300 ml/m2
H2O) and 1.25% sodium bicarbonate (Tianjin
Pharmaceuticals Group Co., Ltd., Tianjin, China) intravenous
injection. Following chemotherapy, patients with leukocytopenia
(white blood cells count, <4.0×109/l) were treated
with granulocyte colony-stimulating factor (Harbin Pharmaceutical
Group Bioengineering, Co., Ltd., Harbin, China) to elevate their
white blood cell counts, patients with anaemia were injected with
red blood cells, and patients with low platelets were transfused
with platelets (both Henan Red Cross Blood Centre, Zhengzhou,
China). All patients underwent involved-field radiation therapy
(TrueBeam™ Radiotherapy System; Varian Medical Systems, Inc., Palo
Alto, CA, USA) following two cycles of chemotherapy.
Three-dimensional radiotherapy counting was performed with a 6–8 MV
photon ray at a median dose of 52 Gy (range, 40–60 Gy) and was
administered in fractionated doses of 2 Gy five times/week. The
radiotherapy target covered the edge of the lesion and the
infiltration. Subsequent chemotherapy continued until completion
following radiotherapy. All patients provided signed
written-informed consent prior to treatment.
Evaluation of short-term efficacy and
adverse reactions
All patients underwent at least six cycles of
chemotherapy combined with radiotherapy. Following two cycles of
chemotherapy and the entire treatment regimen, the efficacies of
the treatments in all patients were evaluated by a physical
examination, B-ultrasound and computed tomography (CT; GE
LightSpeed Pro 32; GE Healthcare Life Sciences, Chalfont, UK) or
positron emission/CT (GE Discovery STE 16; GE Healthcare Life
Sciences). Clinical efficacy was assessed according to World Health
Organization (WHO) criteria (25),
which classify responses as complete remission (CR), partial
remission (PR), stable disease and progressive disease. The overall
response rate (ORR) was calculated as CR + PR. Based on the WHO
evaluation criteria for adverse reactions, the adverse reactions
were divided into 0-IV degrees of severity. Toxicity assessments
were conducted at the end of each chemotherapy cycle.
Evaluation of survival
Overall survival (OS) was defined as the time
between the date of diagnosis and the date on which the patient
succumbed to the disease or the end of the follow-up period.
Progression-free survival (PFS) was defined as the time between the
date of diagnosis and the date of tumor progression/relapse, the
date on which the patient succumbed to the disease, or the end of
the follow-up period. For patients who were lost to follow-up,
survival ended at the time of the last follow-up.
Statistical analysis
Statistical analysis was conducted with SPSS 18.0
software (SSPS, Inc., Chicago, IL, USA). Categorical variables were
compared between the groups using a χ2 test, and
continuous variables were compared between groups using a t-test
for independent samples. Kaplan-Meier survival analysis was applied
to calculate the patient survival rates. The survival rates were
compared between groups using a log-rank test. Variables with
P<0.05 were included in the Cox proportional hazards regression
model for multivariate analysis. P<0.05 was considered to
indicate a statistically significant result.
Results
Short-term efficacy and long-term
survival
Treatment efficacies were evaluated in the 181
patients, and the overall efficacies in the two treatment groups
are shown in Table II. The 69
patients in the CHOP treatment group included 10 cases of CR and 22
cases of PR for an ORR of 46.4% following two cycles of
chemotherapy; 29 cases of CR and 21 cases of PR for an ORR of
72.46% was shown at the end of the treatment. The χ2
test results (Table III)
demonstrated that among the various clinical factors, patient age,
clinical stage, and IPI score significantly affected the ORR
(P<0.001) at the end of the treatment period. The 112 patients
in the asparaginase group included 38 cases of CR and 38 cases of
PR for an ORR of 67.9% following two cycles of chemotherapy, and 72
cases of CR and 29 cases of PR for an ORR of 90.18% at the end of
the treatment period. Among the various clinical factors, clinical
staging, IPI score, ECOG score, primary lesion site, and
leukocytopenia affected the ORR (P<0.05).
 | Table II.Response assessments in the two
treatment groups at various time points. |
Table II.
Response assessments in the two
treatment groups at various time points.
|
|
| Following the
second cycle | Following all
cycles and radiation |
|---|
|
|
|
|
|
|---|
| Group | N | CR | ORR | CR | ORR |
|---|
| CHOP | 69 | 10 (14.5%) | 32 (46.4%) | 29 (42.03%) | 50 (72.46%) |
| L-asp | 112 | 38 (31.3%) | 76 (67.9%) | 72 (64.28%) | 101 (90.18%) |
| P-value |
| 0.011 | 0.004 | 0.003 | 0.002 |
 | Table III.Clinical characteristics and response
rates following CHOP and L-asparaginase-based treatment regimens
followed by radiation. |
Table III.
Clinical characteristics and response
rates following CHOP and L-asparaginase-based treatment regimens
followed by radiation.
|
| CHOP | L-asparaginase |
|---|
|
|
|
|
|---|
| Characteristic | N | ORR | P-value | N | ORR | P-value |
|---|
| Gender |
|
| 0.973 |
|
| 1.000 |
|
Male | 47 | 34 |
| 73 | 66 |
|
|
Female | 22 | 16 |
| 39 | 35 |
|
| Age |
|
| 0.000 |
|
| 0.225 |
|
≤60 | 59 | 48 |
| 99 | 91 |
|
|
>60 | 10 | 2 |
| 13 | 10 |
|
| Ann Arbor
stage |
|
| 0.000 |
|
| 0.012 |
|
I/II | 58 | 48 |
| 95 | 89 |
|
|
III/IV | 11 | 2 |
| 17 | 12 |
|
| IPI score |
|
| 0.000 |
|
| 0.036 |
|
0–1 | 52 | 46 |
| 92 | 86 |
|
| ≥2 | 17 | 4 |
| 20 | 15 |
|
| ECOG performance
status |
|
| 0.073 |
|
| 0.025 |
|
0–1 | 67 | 50 |
| 109 | 100 |
|
| ≥2 | 2 | 0 |
| 3 | 1 |
|
| B symptoms |
|
| 0.344 |
|
| 0.896 |
|
Yes | 30 | 20 |
| 53 | 48 |
|
| No | 39 | 30 |
| 59 | 53 |
|
| ENKTL subtype |
|
| 0.182 |
|
| 0.006 |
|
UNKTL | 66 | 49 |
| 107 | 99 |
|
|
EUNKTL | 3 | 1 |
| 5 | 2 |
|
| Bony
perforation | 18 | 12 | 0.739 | 28 | 26 | 0.855 |
| Elevated LDH
level | 42 | 28 | 0.179 | 53 | 45 | 0.076 |
| Elevated
β2-microglobulin | 52 | 38 | 1.00 | 76 | 68 | 0.981 |
| Leukocytopenia | 15 | 10 | 0.809 | 26 | 20 | 0.027 |
| Haemoglobin | 14 | 7 | 0.076 | 21 | 18 | 0.722 |
|
CD56+ | 57 | 41 | 1.000 | 97 | 88 | 0.980 |
|
EBV+ | 43 | 29 | 0.23 | 63 | 55 | 0.401 |
| Ki67* |
|
| 0.919 |
|
| 0.075 |
|
Low | 37 | 27 |
| 73 | 69 |
|
|
High | 32 | 23 |
| 39 | 32 |
|
| Pegaspargase
replacement | – | – | – | 25 | 22 | 0.973 |
The patients were followed-up until 31 October 2013,
with a median follow-up time of 29 months (range, 6–96 months). A
total of 10 cases were lost to follow-up. In the CHOP treatment
group, the median OS duration was 34 months, the median PFS
duration was 21 months, the one, two, and five-year OS rates were
82.6, 61.9 and 28.4%, respectively, and the one, two, and five-year
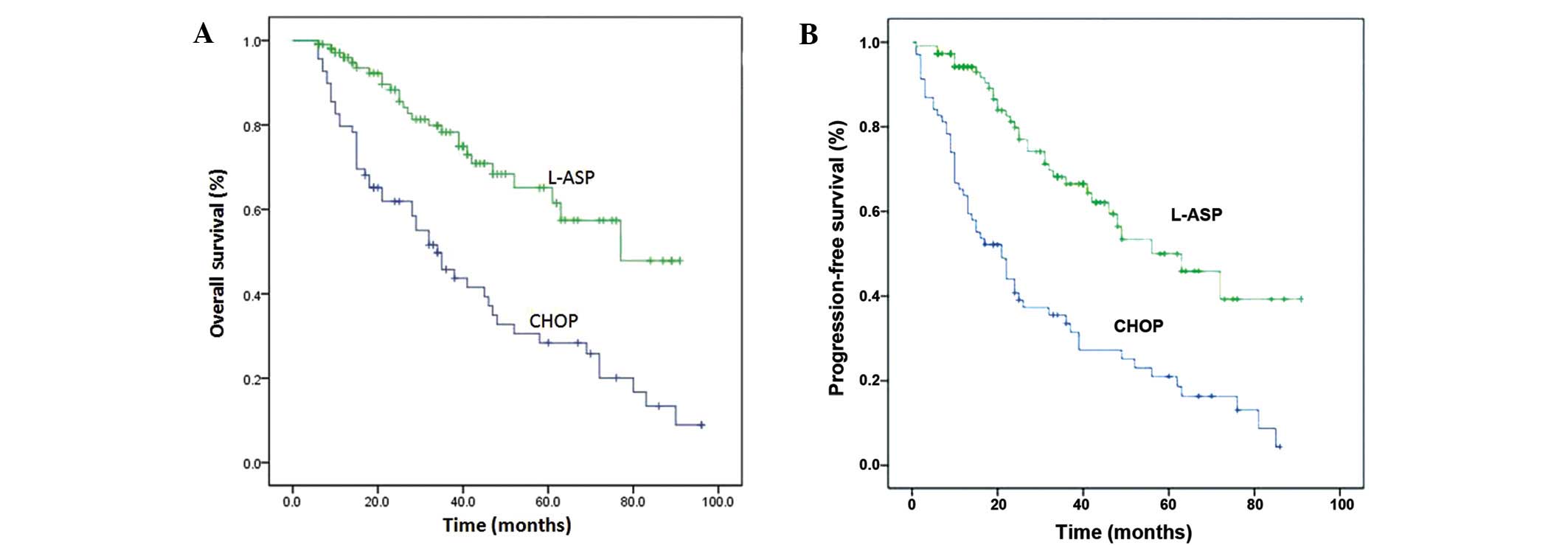
PFS rates were 63.8, 44.0 and 21.0%, respectively (Table IV, Fig.
1A and B). Univariate analysis demonstrated that patient age,
clinical staging, IPI score, ECOG score, primary lesion site, LDH
levels, leukocytopenia, Ki67 levels and early treatment response
significantly affected patient OS and PFS (P<0.05). Other
factors such as gender, B symptoms, and β2-microglobulin
had no impact on the OS and PFS (P>0.05; Table V). Multivariate analysis demonstrated
that early treatment response was an independent predictor of OS
(P<0.001), whereas primary lesion site, early treatment
response, and age were independent predictors of PFS (P=0.010,
P<0.001, and P=0.017, respectively; Table VI). In the asparaginase treatment
group, the median OS duration was 77 months, the median PFS
duration was 56 months, the one, two, and five-year OS rates were
96.0, 88.3 and 65.1%, respectively, and the one, two, and five-year
PFS rates were 94.2, 79.8 and 50%, respectively. Univariate
analysis demonstrated that patient age, clinical stage, IPI score,
ECOG score, primary lesion site, LDH level, leukocytopenia, Ki67
level, and early treatment response affected patient OS and PFS
(P<0.05); other factors such as gender, B symptoms, and
β2-microglobulin had no impact on OS and PFS
(P>0.05). Multivariate analysis demonstrated that early
treatment, clinical staging, LDH levels, and Ki67 levels were
independent predictors of OS and PFS (P<0.001, P=0.001, 0.035
and 0.001, and P=0.011, 0.002, 0.002 and 0.02, respectively).
 | Table IV.Survival data of the two groups. |
Table IV.
Survival data of the two groups.
|
| OS rate (%) | PFS rate (%) |
|---|
|
|
|
|
|---|
| Group | One-year | Two-year | Five-year | One-year | Two-year | Five-year |
|---|
| CHOP | 82.6 | 61.9 | 28.4 | 63.8 | 44.0 | 21.0 |
| L-asp | 96.0 | 88.3 | 65.1 | 94.2 | 79.8 | 50.0 |
| P-value | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
 | Table V.Univariate analysis of prognostic
factors. |
Table V.
Univariate analysis of prognostic
factors.
|
| CHOP | L-asparaginase |
|---|
|
|
|
|
|---|
| Factor | OS | PFS | OS | PFS |
|---|
| Gender | 0.556 | 0.611 | 0.332 | 0.212 |
| Age | 0.000 | 0.000 | 0.027 | 0.038 |
| Ann Arbor
stage | 0.000 | 0.000 | 0.000 | 0.000 |
| IPI score | 0.000 | 0.000 | 0.005 | 0.018 |
| ECOG performance
status | 0.000 | 0.000 | 0.000 | 0.000 |
| B symptoms | 0.575 | 0.439 | 0.593 | 0.575 |
| ENKTL subtype | 0.000 | 0.000 | 0.000 | 0.000 |
| Bony
perforation | 0.314 | 0.248 | 0.973 | 0.898 |
| Elevated LDH | 0.025 | 0.008 | 0.014 | 0.001 |
| Elevated
β2-MG | 0.300 | 0.356 | 0.550 | 0.242 |
| Leukopenia | 0.034 | 0.006 | 0.006 | 0.020 |
| Haemoglobin | 0.295 | 0.134 | 0.959 | 0.077 |
| CD56 | 0.982 | 0.896 | 0.717 | 0.422 |
| EBV | 0.217 | 0.268 | 0.493 | 0.418 |
| Ki67 | 0.024 | 0.035 | 0.001 | 0.001 |
| Early efficacy | 0.000 | 0.000 | 0.000 | 0.000 |
| Pegaspargase
replacement | – | – | 0.191 | 0.638 |
 | Table VI.Cox proportional hazard regression
analysis for the CHOP and L-asparaginase treatment groups. |
Table VI.
Cox proportional hazard regression
analysis for the CHOP and L-asparaginase treatment groups.
|
| CHOP (95% CI) | L-asparaginase (95%
CI) |
|---|
|
|
|
|
|---|
| Survival | RR | Lower | Upper | P-value | RR | Lower | Upper | P-value |
|---|
| OS |
|
|
|
|
|
|
|
|
| Early
efficacy | 6.596 | 2.365 | 18.397 | 0.000 |
8.760 | 2.879 | 26.657 | <0.001 |
| Ann
Arbor stage | 2.003 | 0.372 | 10.771 | 0.418 | 10.588 | 2.553 | 43.910 | 0.001 |
|
Ki67 | 1.578 | 0.843 |
2.954 | 0.154 |
6.517 | 2.165 | 19.615 | 0.001 |
|
LDH | 1.330 | 0.632 |
2.800 | 0.452 |
3.525 | 1.092 | 11.373 | 0.035 |
| PFS |
|
|
|
|
|
|
|
|
| Early
efficacy | 7.144 | 2.853 | 17.890 | 0.000 |
4.093 | 1.635 | 10.245 | 0.003 |
| ENKTL
subtype | 8.597 | 1.677 | 44.085 | 0.010 |
4.962 | 0.621 | 39.655 | 0.131 |
|
Ki67 | 1.438 | 0.758 |
2.727 | 0.266 |
3.554 | 1.582 |
7.984 | 0.002 |
| Ann
Arbor stage | 1.580 | 0.300 |
8.330 | 0.590 |
7.405 | 2.045 | 26.811 | 0.002 |
|
Age | 5.260 | 1.351 | 20.480 | 0.017 |
5.480 | 0.595 | 50.433 | 0.133 |
|
LDH | 1.348 | 0.646 |
2.811 | 0.426 |
3.554 | 1.582 |
7.984 | 0.001 |
Adverse reactions
The adverse reactions observed in the patients in
the two groups are shown in Table
VII. The primary adverse reactions to chemotherapy included
bone marrow suppression, gastrointestinal reactions, infections,
and liver dysfunction, the majority of which were rapidly relieved
following symptomatic treatment. In the asparaginase treatment
group, 68 cases (60.7%) showed a statistically significant
reduction in fibrinogen levels, as compared with the CHOP treatment
group (P<0.001). Associated bleeding and thrombotic events
occurred in two cases; these were of low severity and improved
following active treatment. During L-asparaginase treatment, a rash
occurred in 29 patients and improved following symptomatic
treatment; 25 of these patients were treated with pegaspargase
subsequent to receiving positive results (detection of erythemas or
wheals) from an L-asparaginase skin test. No treatment
interruptions or treatment-associated mortality occurred in either
group.
 | Table VII.Toxicity profiles of the CHOP and
L-asparaginase treatment groups. |
Table VII.
Toxicity profiles of the CHOP and
L-asparaginase treatment groups.
|
| CHOP | L-asparaginase |
|
|
|---|
|
|
|
|
|
|
|---|
| Toxicity | I–II | III–IV | I–II | III–IV | χ2 | P-value |
|---|
| Leukopenia | 36 (52.2) | 13 (18.8) | 59 (52.7) | 25 (22.3) | 0.348 | 0.555 |
| Anaemia | 13 (18.8) | 2 (2.9) | 24 (21.4) | 5 (4.5) | 0.400 | 0.527 |
|
Thrombocytopenia | 8 (11.6) | 1 (1.4) | 13 (11.6) | 2 (1.8) | 0.005 | 0.946 |
| Increase in
ALT/AST | 11 (15.9) | 3 (4.3) | 31 (27.7) | 5 (4.5) | 3.000 | 0.083 |
| Increase in
bilirubin | 8 (11.6) | 2 (2.9) | 25 (22.3) | 4 (3.6) | 3.283 | 0.070 |
| Increase in
creatinine | 10 (14.5) | 0 | 16 (14.3) | 0 | 0.001 | 0.969 |
| Increase in
BUN | 6 (8.7) | 0 | 11 (9.8) | 0 | 0.064 | 0.801 |
| Anaphylaxis | 3 (4.3) | 0 | 29 (25.9) | 0 | 13.618 | <0.001 |
| Nausea,
vomiting | 41 (59.4) | 3 | 76 (67.9) | 10 (8.9) | 1.647 | 0.199 |
| Infection | 15 (21.7) |
| 23 (20.5) |
| 0.037 | 0.847 |
| Reduction in
FIB | 5 (7.2) |
| 68 (60.7) |
| 50.721 | <0.001 |
Discussion
ENKTL is a type of non-Hodgkin's lymphoma that
accounts for 11% of lymphoma cases in China (3). Given the rapid course of clinical
progression and relatively poor prognosis of ENKTL, the optimal
treatment strategy for this disease remains unclear. Previously,
either CHOP chemotherapy alone or radiotherapy alone was not able
to achieve satisfactory results. Efficacy of the CHOP chemotherapy
regimen for ENKTL is poor (26–29).
Several studies have reported that radiotherapy is an important
treatment method that is beneficial to local lesion control
(15,28,30,31).
However, the rates of recurrence and distant metastasis were high,
and the five-year survival rate was low in the early-stage patients
who received radiotherapy alone (28,32). As
chemoradiotherapy strategies have progressed, chemotherapeutic
regimens based on CHOP-class anthracycline compounds have been
reported (29). However, the
efficacy remained low even following an increase in
cyclophosphamide dose or the combination with other drugs such as
semustine. In addition, these treatment regimens did not
significantly improve patient survival in the early stages of ENKTL
(30).
The insensitivity of ENKTL to conventional
CHOP-class chemotherapy may be associated with tumor cell
overexpression of P-glycoprotein, which is encoded by the MDR1
(33). Doxorubicin and vincristine,
components of the CHOP-class treatment, may be actively pumped out
by P-glycoprotein, thereby reducing the therapeutic effect.
L-asparaginase hydrolyses asparaginase in the serum thereby
reducing the levels of amino acids required in certain tumor cells
that do not produce asparagine synthetase, resulting in the
inhibition of DNA, RNA, and protein synthesis, which acts as an
anti-tumor agent (18,34). In vitro studies have
demonstrated that asparaginase is able to reduce the activity of
normal NK cells and induce apoptosis in NK tumor cells, whereas the
conventional chemotherapy drugs used to treat lymphoma do not have
this property (35,36). Yong et al (37) reported the use of an
L-asparaginase-based treatment as the primary therapy for 11
patients with refractory midline peripheral T-cell lymphoma, and
reported an efficacy of 63.6% and a two-year survival rate of
45.5%. Subsequently, this L-asparaginase treatment was applied in
45 patients with relapsed or refractory ENKTCL and led to a CR rate
of 56%, an ORR of 82%, and a five-year survival rate of 67%
(19). A recent study on CHOP-L
treatment combined with radiotherapy for newly diagnosed ENKTL
demonstrated that, among 38 newly diagnosed patients with ENKTL who
received CHOP-L chemotherapy and sequential radiotherapy, the CR
rate was 81.6%, the ORR rate was 84.2%, and the two-year OS rate
was 80.1% (23). The results of the
present study demonstrated that, compared with the CHOP treatment
group, the ENKTL patient group that received an
L-asparaginase-based treatment combined with radiotherapy had
significantly improved remission rate and improved long-term
survival. Among the 112 patients in the L-asparaginase treatment
group, the overall efficacy following two cycles of chemotherapy
was 67.9%, the overall efficacy at the end of the treatment was
90.18%, the median OS duration was 77 months, the median PFS
duration was 56 months, the one, two, and five-year OS rates were
96.0, 88.3 and 65.1%, respectively, and the one, two, and five-year
PFS rates were 94.2, 79.8 and 50.0%, respectively.
The incidence of allergic reactions in patients who
were treated with L-asparaginase has been reported to be 30%
(38). Studies have reported that
pegaspargase therapy had a similar efficacy to L-asparaginase as a
treatment in children with acute lymphoblastic leukaemia (39,40). In
the present study, allergic reactions occurred in 29 patients in
the asparaginase treatment group (25.9%) during the application of
L-asparaginase, and 25 of these patients (22.3%) switched to
treatment with pegaspargase following a positive L-asparaginase
skin test results. The statistical results indicated that switching
to pegaspargase did not affect the CR rate, OS, or PFS. A study on
treatment with pegaspargase in newly diagnosed ENKTL patients is
currently on-going in China. Pegaspargase may be another treatment
option for ENKTL patients.
Prognostic factors that have been reported to be
closely associated with ENKTL include patient age, B symptoms, ECOG
score, regional lymph node invasion, clinical staging, CR rate,
leukocytopenia, IPI score and Ki67 expression levels (41–43). In
the present study, a univariate analysis of the 112 patients in the
asparaginase regimen group demonstrated that patient age, clinical
staging, IPI score, ECOG score, primary lesion site, LDH,
leukocytopenia, Ki67 levels, and early treatment response affected
patient OS and PFS (P<0.05). Other factors such as gender, B
symptoms, and β2-microglobulin had no impact on OS and
PFS (P>0.05). Multivariate analysis demonstrated that early
treatment response (P<0.001), clinical staging (P<0.001), and
LDH level (P=0.006) were independent predictors of OS, whereas Ki67
expression levels (P=0.011), early treatment response (P=0.001),
clinical staging (P=0.002), and LDH level (P=0.001) were
independent predictors of PFS.
In the present study the primary adverse reactions
to treatment experienced by the patients in the two groups included
bone marrow suppression, gastrointestinal reactions, infections,
and liver dysfunction, most of which were rapidly relieved
following symptomatic treatment. The incidence of
hypofibrinogenemia was significantly higher in the asparaginase
treatment group compared with the CHOP treatment group, although
associated bleeding and thrombotic events occurred in only two
cases and at a low severity, and no treatment-associated mortality
was recorded.
In conclusion, L-asparaginase-based chemotherapy
combined with radiotherapy is effective as a first-line treatment
for ENKTL. The patients who received this type of treatment showed
significantly increased improvements compared with those treated
with the CHOP regimen combined with radiotherapy, with regard to
short-term efficacy and long-term survival, as well as safety
performance.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81170520),
the Henan Department of Health/Provincial Departmental
collaboration project (grant no. 2011010014), and the National
Natural Science Foundation of Henan Province (grant nos. 81170520
and 81000921).
References
|
1
|
Oshimi K: Progress in understanding and
managing natural killer-cell malignancies. Br J Haematol.
139:532–544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suzuki R, Takeuchi K, Ohshima K and
Nakamura S: Extranodal NK/T-cell lymphoma: Diagnosis and treatment
cues. Hematol Oncol. 26:66–72. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun J, Yang Q, Lu Z, He M, Gao L, Zhu M,
Sun L, Wei L, Li M, Liu C, et al: Distribution of lymphoid
neoplasms in China: Analysis of 4,638 cases according to the world
health organization classification. Am J Clin Pathol. 138:429–434.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim K, Kim WS, Jung CW, Im YH, Kang WK,
Lee MH, Park CH, Ko YH, Ree HJ and Park K: Clinical features of
peripheral T-cell lymphomas in 78 patients diagnosed according to
the revised European-American lymphoma (REAL) classification. Eur J
Cancer. 38:75–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee J, Park YH, Kim WS, Lee SS, Ryoo BY,
Yang SH, Park KW, Kang JH, Park JO, Lee SH, et al: Extranodal nasal
type NK/T-cell lymphoma: Elucidating clinical prognostic factors
for risk-based stratification of therapy. Eur J Cancer.
41:1402–1408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chim CS, Ma SY, Au WY, Choy C, Lie AK,
Liang R, Yau CC and Kwong YL: Primary nasal natural killer cell
lymphoma: Long-term treatment outcome and relationship with the
international prognostic index. Blood. 103:216–221. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakamura S, Katoh E, Koshikawa T, Yatabe
Y, Nagasaka T, Ishida H, Tokoro Y, Koike K, Kagami Y, Ogura M, et
al: Clinicopathologic study of nasal T/NK-cell lymphoma among the
Japanese. Pathol Int. 47:38–53. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi N, Miura I, Chubachi A, Miura AB
and Nakamura S: A clinicopathological study of 20 patients with
T/natural killer (NK)-cell lymphoma-associated hemophagocytic
syndrome with special reference to nasal and nasal-type NK/T-cell
lymphoma. Int J Hematol. 74:303–308. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan JK, Sin VC, Wong KF, Ng CS, Tsang WY,
Chan CH, Cheung MM and Lau WH: Nonnasal lymphoma expressing the
natural killer cell marker CD56: A clinicopathologic study of 49
cases of an uncommon aggressive neoplasm. Blood. 89:4501–4513.
1997.PubMed/NCBI
|
|
10
|
Kluin PM, Feller A, Gaulard P, Jaffe ES,
Meijer CJ, Müller-Hermelink HK and Pileri S: PeripheralT/NK-cell
lymphoma: A report of the IXth Workshop of the European Association
for Haematopathology. Histopathology. 38:250–270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Hakeem DA, Fedele S, Carlos R and
Porter S: Extranodal NK/T-cell lymphoma, nasal type. Oral Oncol.
43:4–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Emile JF, Boulland ML, Haioun C, Kanavaros
P, Petrella T, Delfau-Larue MH, Bensussan A, Farcet JP and Gaulard
P: CD5-CD56+ T-cell receptor silent peripheral T-cell lymphomas are
natural killer cell lymphomas. Blood. 87:1466–1473. 1996.PubMed/NCBI
|
|
13
|
Attygalle AD, Cabeçadas J, Gaulard P,
Jaffe ES, de Jong D, Ko YH, Said J and Klapper W: Peripheral T-cell
and NK-cell lymphomas and their mimics; taking a step
forward-report on the lymphoma workshop of the XVIth meeting of the
European Association for Haematopathology and the society for
Hematopathology. Histopathology. 64:171–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim GE, Cho JH, Yang WI, Chung EJ, Suh CO,
Park KR, Hong WP, Park IY, Hahn JS, Roh JK and Kim BS: Angiocentric
lymphoma of the head and neck: Patterns of systemic failure after
radiation treatment. J Clin Oncol. 18:54–63. 2000.PubMed/NCBI
|
|
15
|
Li YX, Yao B, Jin J, Wang WH, Liu YP, Song
YW, Wang SL, Liu XF, Zhou LQ, He XH, et al: Radiotherapy as primary
treatment for stage IE and IIE nasal natural killer/T-cell
lymphoma. J Clin Oncol. 24:181–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koom WS, Chung EJ, Yang WI, Shim SJ, Suh
CO, Roh JK, Yoon JH and Kim GE: Angiocentric T-cell and NK/T-cell
lymphomas: Radiotherapeutic viewpoints. Int J Radiat Oncol Biol
Phys. 59:1127–1137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Isobe K, Uno T, Tamaru J, Kawakami H, Ueno
N, Wakita H, Okada J, Itami J and Ito H: Extranodal natural
killer/T-cell lymphoma, nasal type: The significance of
radiotherapeutic parameters. Cancer. 106:609–615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamaguchi M, Kita K, Miwa H, Nishii K, Oka
K, Ohno T, Shirakawa S and Fukumoto M: Frequent expression of
P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer.
76:2351–2356. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yong W, Zheng W, Zhu J, Zhang Y, Wang X,
Xie Y, Lin N, Xu B, Lu A and Li J: L-asparaginase in the treatment
of refractory and relapsed extranodal NK/T-cell lymphoma, nasal
type. Ann Hematol. 88:647–652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jaccard A, Petit B, Girault S, Suarez F,
Gressin R, Zini JM, Coiteux V, Larroche C, Devidas A and
Thiéblemont C: L-asparaginase-based treatment of 15 western
patients with extranodal NK/T-cell lymphoma and leukemia and a
review of the literature. Ann Oncol. 20:110–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagafuji K, Fujisaki T, Arima F and
Ohshima K: L-asparaginase induced durable remission of relapsed
nasal NK/T-cell lymphoma after autologous peripheral blood stem
cell transplantation. Int J Hematol. 74:447–450. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Obama K, Tara M and Niina K:
L-asparaginase-Based induction therapy for advanced extranodal
NK/T-cell lymphoma. Int J Hematol. 78:248–250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin N, Song Y, Zheng W, Tu M, Xie Y, Wang
X, Ping L, Ying Z, Zhang C, Deng L, et al: A prospective phase II
study of L-asparaginase- CHOP plus radiation in newly diagnosed
extranodal NK/T-cell lymphoma, nasal type. J Hematol Oncol.
6:442013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
The International Non-Hodgkin's Lymphoma
Prognostic Factors Project: A predictive model for aggressive
non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma
Prognostic Factors Project. N Engl J Med. 329:987–994.
1993.PubMed/NCBI
|
|
25
|
Miller AB, Hoogstraten B, Staquet M and
Winkler A: Reporting results of cancer treatment. Cancer.
47:207–214. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee J, Kim WS, Park YH, Park SH, Park KW,
Kang JH, Lee SS, Lee SI, Lee SH, Kim K, et al: Nasal-type NK/T cell
lymphoma: Clinical features and treatment outcome. Br J Cancer.
92:1226–1230. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim BS, Kim TY, Kim CW, Kim JY, Heo DS,
Bang YJ and Kim NK: Therapeutic outcome of extranodal NK/T-cell
lymphoma initially treated with chemotherapy-result of chemotherapy
in NK/T-cell lymphoma. Acta Oncol. 42:779–783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheung MM, Chan JK, Lau WH, Ngan RK and
Foo WW: Early stage nasal NK/T-cell lymphoma: Clinical outcome,
prognostic factors, and the effect of treatment modality. Int J
Radiat Oncol Biol Phys. 54:182–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim WS, Song SY, Ahn YC, Ko YH, Baek CH,
Kim DY, Yoon SS, Lee HG, Kang WK, Lee HJ, et al: CHOP followed by
involved field radiation: Is it optimal for localized nasal natural
killer/T-cell lymphoma? Ann Oncol. 12:349–352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim SJ and Kim WS: Treatment of localized
extranodal NK/T cell lymphoma, nasal type. Int J Hematol.
92:690–696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang ZY, Li YX, Wang WH, Jin J, Wang H,
Song YW, Liu QF, Wang SL, Liu YP, Qi SN, et al: Primary
radiotherapy showed favorable outcome in treating extranodal
nasal-type NK/T-cell lymphoma in children and adolescents. Blood.
114:4771–4776. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li CC, Tien HF, Tang JL, Yao M, Chen YC,
Su IJ, Hsu SM and Hong RL: Treatment outcome and pattern of failure
in 77 patients with sinonasal natural killer/T-cell or T-cell
lymphoma. Cancer. 100:366–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang B, Li XQ, Ma X, Hong X, Lu H and Guo
Y: Immunohistochemical expression and clinical significance of
P-glycoprotein in previously untreated extranodal NK/T-cell
lymphoma, nasal type. Am J Hematol. 83:795–799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chaudhary PM, Mechetner EB and Roninson
IB: Expression and activity of the multidrug resistance
P-glycoprotein in human peripheral blood lymphocytes. Blood.
80:2735–2739. 1992.PubMed/NCBI
|
|
35
|
Charamella LJ, Meyer C, Thompson GE and
Dimitrov NV: Chemotherapeutic agents and modulation of natural
killer cell activity in vitro. J Immunopharmacol. 7:53–65. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ando M, Sugimoto K, Kitoh T, Sasaki M,
Mukai K, Ando J, Egashira M, Schuster SM and Oshimi K: Selective
apoptosis of natural killer-cell tumours by l-asparaginase. Br J
Haematol. 130:860–868. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yong W, Zhang Y and Zheng W: The efficacy
of L-asparaginase in the treatment of refractory midline peripheral
T-cell lymphoma. Zhong Hua Xue Ye Xue Za Zhi. 21:577–579. 2000.(In
Chinese).
|
|
38
|
Silverman LB, Supko JG, Stevenson KE,
Woodward C, Vrooman LM, Neuberg DS, Asselin BL, Athale UH, Clavell
L, Cole PD, et al: Intravenous PEG-asparaginase during remission
induction in children and adolescents with newly diagnosed acute
lymphoblastic leukemia. Blood. 115:1351–1353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Avramis VI, Sencer S, Periclou AP, Sather
H, Bostrom BC, Cohen LJ, Ettinger AG, Ettinger LJ, Franklin J,
Gaynon PS, et al: A randomized comparison of native Escherichia
coli asparaginase and polyethylene glycol conjugated asparaginase
for treatment of children with newly diagnosed standard-risk acute
lymphoblastic leukemia: A Children's cancer group study. Blood.
99:1986–1994. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cooperation Group of Phase II Clinical
Trial of PEG-Asp: Comparison of polyethylene glycol conjugated
asparaginase and L-asparaginase for treatment of childhood acute
lymphoblastic leukemia. Zhong Hua Xue Ye Xue Za Zhi. 29:29–33.
2008.(In Chinese.).
|
|
41
|
Kim YR, Kim JS, Kim SJ, Jung HA, Kim SJ,
Kim WS, Lee HW, Eom HS, Jeong SH, Park JS, et al: Lymphopenia is an
important prognostic factor in peripheral T-cell lymphoma (NOS)
treated with anthracycline-containing chemotherapy. J Hematol
Oncol. 4:342011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chauchet A, Michallet AS, Berger F,
Bedgedjian I, Deconinck E, Sebban C, Antal D, Orfeuvre H, Corront
B, Petrella T, et al: Complete remission after first-line
radio-chemotherapy as predictor of survival in extranodal NK/T cell
lymphoma. J Hematol Oncol. 5:272012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim SJ, Kim BS, Choi CW, Choi J, Kim I,
Lee YH and Kim JS: Ki-67 expression is predictive of prognosis in
patients with stage I/II extranodal NK/T-cell lymphoma, nasal type.
Ann Oncol. 18:1382–1387. 2007. View Article : Google Scholar : PubMed/NCBI
|