Introduction
Endometrial cancer is the most common gynecological
malignancy and the fourth most common type of cancer in women
(1–3), following breast, lung and colorectal
cancer. The incidence and mortality rate of endometrial cancer is
increasing in developing and developed countries (3–6),
although the rates of several other types of cancer have plateaued
or decreased in the last decade (5).
Over the past decades, much has been discovered about the genetics
and lifestyle and environmental factors that affect endometrial
cancer (7,8). The most significant risk factors for
endometrial cancer and its precursor, endometrial hyperplasia, are
unopposed estrogen, a sedentary lifestyle and obesity (5,9). Obesity
is a major public health issue and continues to increase worldwide.
By 2030, the rate of obesity is anticipated to reach ~50% of the
population of the US and 30% in Europe (10). Obesity not only significantly
increases the risk of insulin resistance and type-2 diabetes but it
has also been identified as a risk factor for several types of
cancer, such as endometrial cancer (10). Previous studies demonstrated that
obesity is one of the strongest risk factors that exists for
numerous types of cancer, including breast, colorectal, kidney,
endometrial, ovarian, pancreatic and liver cancer (5,11–13). In
addition, endometrial cancer was the first type to be shown to be
associated with obesity (5).
Adipose tissue functions as an energy storage and
also as a highly active and large source of endocrine and metabolic
activity (7,10). Adipokines are produced by adipose
tissue and include leptin, resistin, adiponectin (APN), serpin,
plasminogen activator inhibitor-1, retinol binding protein 4 and
other numerous cytokines including tumor necrosis factor-α and
interleukin receptor agonists (IL-1, IL-6, IL-8 and IL-10)
(14,15). APN is the most abundant adipokine
that is secreted mainly from visceral adipose tissue and only from
mature adipocytes. It is an endogenous insulin-sensitizing hormone,
and is linked to obesity, metabolic syndrome, insulin resistance,
type-2 diabetes and inflammation in addition to several other types
of cancer. APN has anti-diabetic, anti-angiogenic,
anti-inflammatory and anti-atherogenic properties (10,14–16),
which are known to inhibit cell proliferation, promote apoptosis
and angiogenesis in tumor biology (17). Furthermore, APN inhibits
carcinogenesis directly and indirectly by regulating hormone and
cytokine levels.
Circulating APN may potentially be a protective
factor for endometrial cancer. Retrospective and prospective
studies have investigated the association of circulating APN levels
with the risk of endometrial cancer. Although the majority of the
studies demonstrated a negative association, several studies
demonstrated that there is no association between the two.
Therefore, the association between the two remains to be clarified.
Nevertheless, the incidence of endometrial cancer has risen in each
year over the past few years and the major problem facing treatment
is its late diagnosis. By the time that it gets diagnosed, the vast
majority of patients have advanced to the late stages of cancer,
and the outcome of clinical treatment is poor. Therefore, is an
interest amongst cancer researchers to identify convenient and
effective molecular markers and methods in order to diagnose cancer
at the early stages. Blood is the most readily available sample and
it is an ideal specimen for identifying molecular markers of
cancer. Circulating APN may be a novel molecular marker or anti
tumor factor that may provide a feasible method for early detection
and prevention of endometrial cancer which may be important in its
diagnosis and therapy. To the best of our knowledge, there have
been a few systematic reviews conducted to evaluate whether low APN
levels increase the risk of endometrial cancer. Therefore, this
meta-analysis was performed to summarize all of the data available
and to provide a quantitative assessment of the risk.
Patients and methods
Characteristics of included
studies
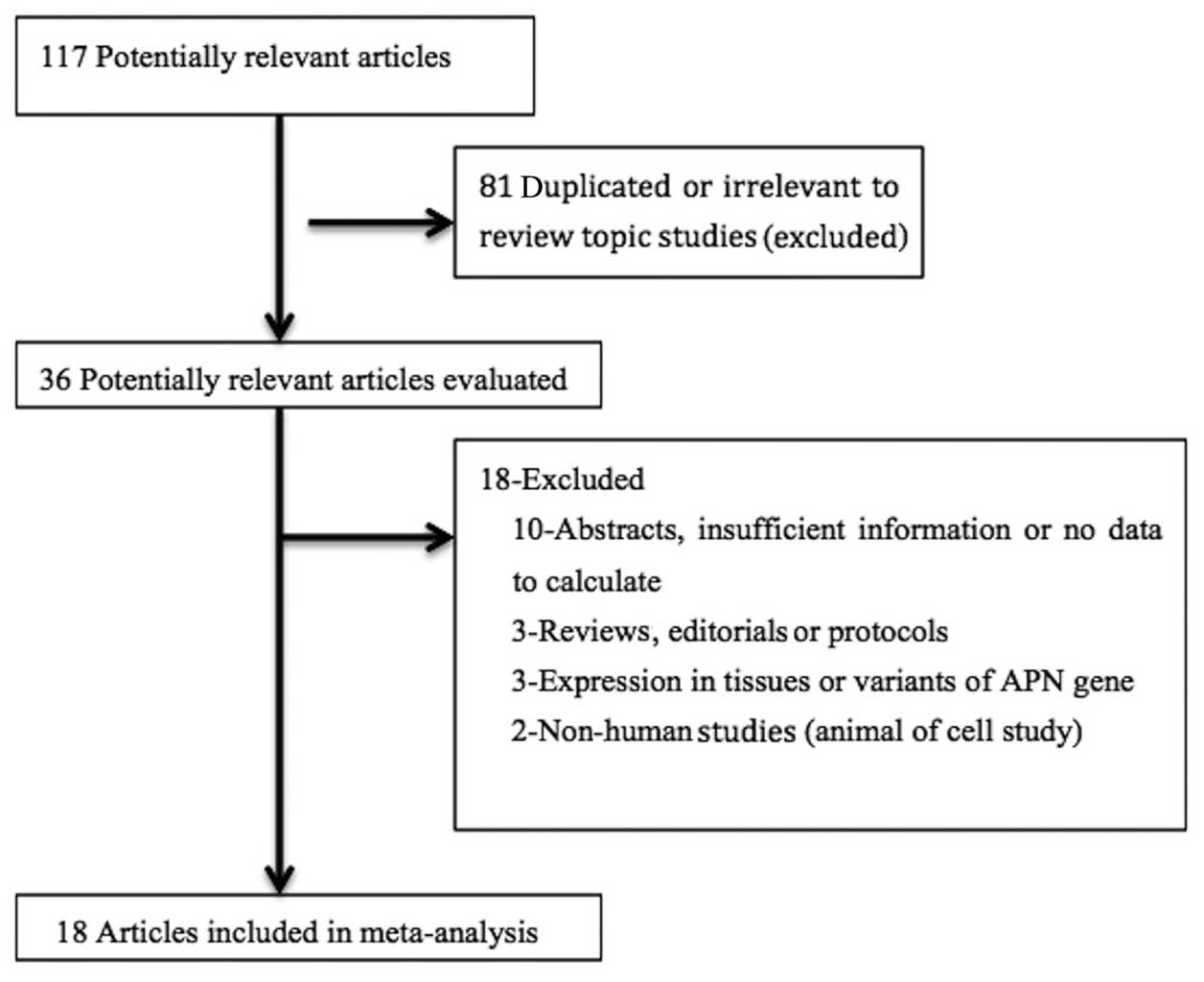
A total of 18 studies were included in the final
analysis (18–35). The detailed steps of the search
results are provided in Fig. 1.
These 18 studies were published between 2003 and 2014, and included
2,337 women with endometrial cancer and 3,355 controls. Out of
those 18 studies, 11 studies were conducted in Asia (China and
Japan), 4 in North America (USA and Canada), and 3 in Europe
(Greece, Poland and Romania). The quality score of the studies
ranged from 5 to 8 stars (Newcastle-Ottawa Scale) (36). There were only 7 studies providing
the cancer grade data (22–24,28,30,32,34). The
main characteristics of the included studies are summarized in
Tables I and II.
 | Table I.Characteristics of eligible studies
in the meta-analysis. |
Table I.
Characteristics of eligible studies
in the meta-analysis.
|
| Adiponectin level,
mean ± SD |
|
|---|
|
|
|
|
|---|
| First author,
year | Ethnicity | Age
differencea | Diagnostic
criteria | No.
cases/controls | Case | Control | Study size | Study quality |
Pre-/post-menopause | Control source and
status | Publication
Language | Assay | Refs. |
|---|
| Friedenreich,
2012 | N Am | None | NS | 514/962 |
11.6±1.6 |
14.6±1.9 | ≥100 | 8 | Both | Population,
healthy | English | ELISA | (18) |
| Ashizawa, 2010 | Asian | None | FIGO | 146/150 |
6.2±0.4 |
9.0±0.4 | ≥100 | 8 | Post- | Hospital,
healthy | English | ELISA | (19) |
| Er, 2011 | Asian | None | FIGO | 49/44 |
8±4 |
13±5 | <100 | 7 | Both | Hospital,
healthy | Chinese | ELISA | (20) |
| Friedenreich,
2011 | N Am | None | NS | 121/262 |
10.1±5.1 |
13.8±7.4 | ≥100 | 8 | Pre- | Population,
healthy | English | ELISA | (21) |
| Friedenreich,
2011 | N Am | None | NS | 394/700 |
14.5±8.5 |
18.1±9.5 | ≥100 | 8 | Post- | Population,
healthy | English | ELISA | (21) |
| Fu, 2012 | Asian | None | NS | 20/30 |
6.7±1.1 |
10.0±1.4 | <100 | 7 | Pre- | Hospital,
healthy | Chinese | ELISA | (22) |
| Fu, 2012 | Asian | None | NS | 15/30 |
9.2±1.0 |
10.0±1.4 | <100 | 7 | Post- | Hospital,
healthy | Chinese | ELISA | (22) |
| Wang, 2013 | Asian | None | FIGO | 135/135 |
15.3±2.3 |
6.8±1.1 | ≥100 | 8 | Both | Hospital,
healthy | Chinese | ELISA | (23) |
| Li, 2011 | Asian | Higher | FIGO | 62/20 |
6.86±1.09 |
15.24±2.32 | <100 | 6 | Both | Hospital,
healthy | Chinese | ELISA | (24) |
| Ma, 2013 | Asian | None | NS | 206/310 |
2330.7±180.5 |
2583.9±147.2 | ≥100 | 8 | Both | Hospital,
healthy | English | ELISA | (25) |
| Mihu, 2013 | European | None | NS | 44/44 |
7374.17±4701.35 |
11045.68±3920.93 | <100 | 5 | Both | Hospital,
Patientsb | English | ELISA | (26) |
| Petridou, 2003 | European | None | NS | 84/84 |
12.37±6.25 |
13.53±5.26 | ≥100 | 7 | Both | Hospital,
Patientsc | English | RIA | (27) |
| Rzepka-Górska,
2008 | European | NS | NS | 37/48 |
15.28±5.74 |
22.7±13.37 | <100 | 5 | Both | Population,
healthy | English | RIA | (28) |
| Soliman, 2006 | N Am | Higher | GOTB | 117/238 |
88.8±63.6 |
148.2±68.3 | ≥100 | 7 | Both | Hospital,
Patientsd | English | ELISA | (29) |
| Tan, 2012 | Asian | None | FIGO | 37/12 |
5.83±2.28 |
15.34±3.05 | <100 | 7 | Both | Hospital,
healthy | Chinese | ELISA | (30) |
| Tang, 2012 | Asian | None | FIGO | 85/85 |
14.2±3.2 |
19.2±5.4 | ≥100 | 8 | Post- | Hospital,
healthy | Chinese | ELISA | (31) |
| Xu, 2014 | Asian | None | FIGO | 56/21 |
5.89±0.96 |
16.12±2.76 | <100 | 6 | Both | Hospital,
healthy | Chinese | ELISA | (32) |
| Yurkovetsky,
2007 | N Am | None | NS | 115/135 |
19.2±0.89 |
23.3±0.68 | ≥100 | 7 | Both | Population,
healthy | English | xMAP, immune
assay | (33) |
| Zhang, 2010 | Asian | Higher | FIGO | 64/25 |
8.2±4.17 |
13.33±5.61 | <100 | 6 | Both | Hospital,
healthy | Chinese | ELISA | (34) |
| Zhou, 2013 | Asian | None | FIGO | 36/20 |
5.43±2.45 |
15.43±2.56 | <100 | 7 | Both | Hospital,
healthy | Chinese | ELISA | (35) |
 | Table II.Characteristics of eligible studies
in this meta-analysis depending on grade. |
Table II.
Characteristics of eligible studies
in this meta-analysis depending on grade.
|
| No. cases | Adiponectin level
(mean ± standard deviation) |
|
|---|
|
|
|
|
|
|---|
| First author,
year | G1 | G2 | G3 | G1 | G2 | G3 | Refs. |
|---|
| Fu, 2012 | 6 | 24 | 5 | 6.1±0.7 | 7.9±1.4 | 9.6±1.2 | (22) |
| Wang, 2013 | 74 | 42 | 19 | 13.2±1.2 | 9.6±1.4 | 6.2±0.9 | (23) |
| Li, 2011 | 17 | 62 | 27 | 6.37±0.61 | 6.89±0.62 | 6.45±0.59 | (24) |
| Rzepka-Górska,
2008 | 12 | 13 | 8 | 19.04±9.43 | 13.48±5.95 | 12.86±1.25 | (28) |
| Tan, 2012 | 12 | 37 | 10 | 7.98±2.18 | 5.49±1.48 | 3.74±0.65 | (30) |
| Xu, 2014 | 27 | 19 | 10 | 6.06±0.72 | 6.83±0.75 | 6.22±0.94 | (32) |
| Zhang, 2010 | 40 | 17 | 7 | 9.31±4.65 | 7.11±2.18 | 4.47±1.35 | (34) |
Search strategy
The US National Library of Medicine's PubMed
database (http://www.ncbi.nlm.nih.gov/pubmed/) was searched
using the terms ‘endometrial cancer’ ‘endometrial carcinoma’
‘adiponectin’ ‘Adipo’ and ‘APN’ to assess the possibility of
conducting the present study. A comprehensive literature search was
conducted in PubMed, SpringerLink (http://link.springer.com/) and Google Scholar
(http://scholar.google.com.hk) up to June
2014 using the terms ‘adiponectin’ or ‘Adipo’ or ‘APN’ and
‘endometrial cancer’ or ‘endometrial carcinoma’. The search was
limited to English and Chinese. Additional studies were also
identified by manually searching references of original studies and
by reviewing articles on the topic. Eligible studies in the current
meta-analysis were those of case-control design, which reported the
correlation between circulating APN levels and endometrial cancer.
The present study was performed in accordance to the MOOSE
guidelines for meta-analysis (37).
Data extraction
Data were independently extracted by two
researchers. For each eligible study, the following information was
recorded: The first author, year of publication, ethnicity, study
size and number of cases, APN measurement method, total APN mean
and standard deviation (38),
menopausal status, control source and health status and language of
publication. For one study (18)
that provided the outcomes in medians and interquartile ranges,
these were converted to the means and standard deviations (39). Any missing data from studies was
obtained by communicating with the authors. The quality of each
study was assessed by Newcastle-Ottawa Scaling (36).
Statistical analysis
The standardized mean differences (SMDs) of the
total circulating APN were calculated for all of the eligible
studies in the meta-analysis, as they were measured in different
units across studies. A fixed effects model was used to pool the
data when the P-value of the Q-test was ≥0.05; otherwise a random
effects model was selected (40).
The Q and I2 statistics were used in order to evaluate the
heterogeneity amongst the results of the studies. When
I2>50%, the heterogeneity was considered significant
(41). A sensitivity analysis was
conducted by exclusion of the studies; subgroup analysis according
to study size, study quality, ethnicity, menopausal status, control
source, language of publication and laboratory assay. Cumulative
meta-analyses were performed in order to observe the change of the
association with time and sample size (42). Furthermore, a funnel plot and Egger's
test was used to assess the publication bias, and P<0.05 was
considered to indicate a statistically significant difference
(43,44). All the analyses were performed using
Stata software version 11.0 (StataCorp LP, College Station, TX,
USA).
Results
Main analysis
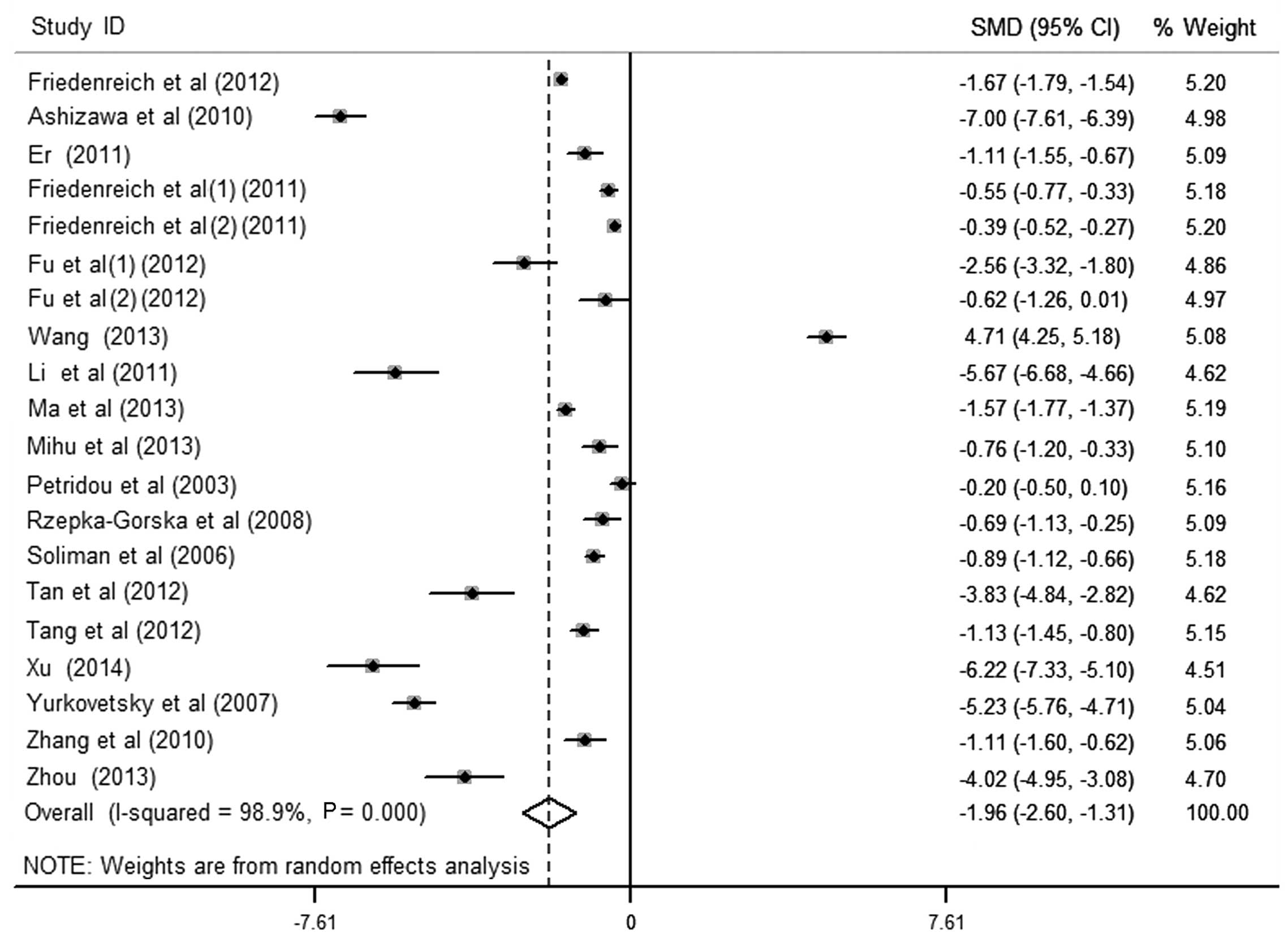
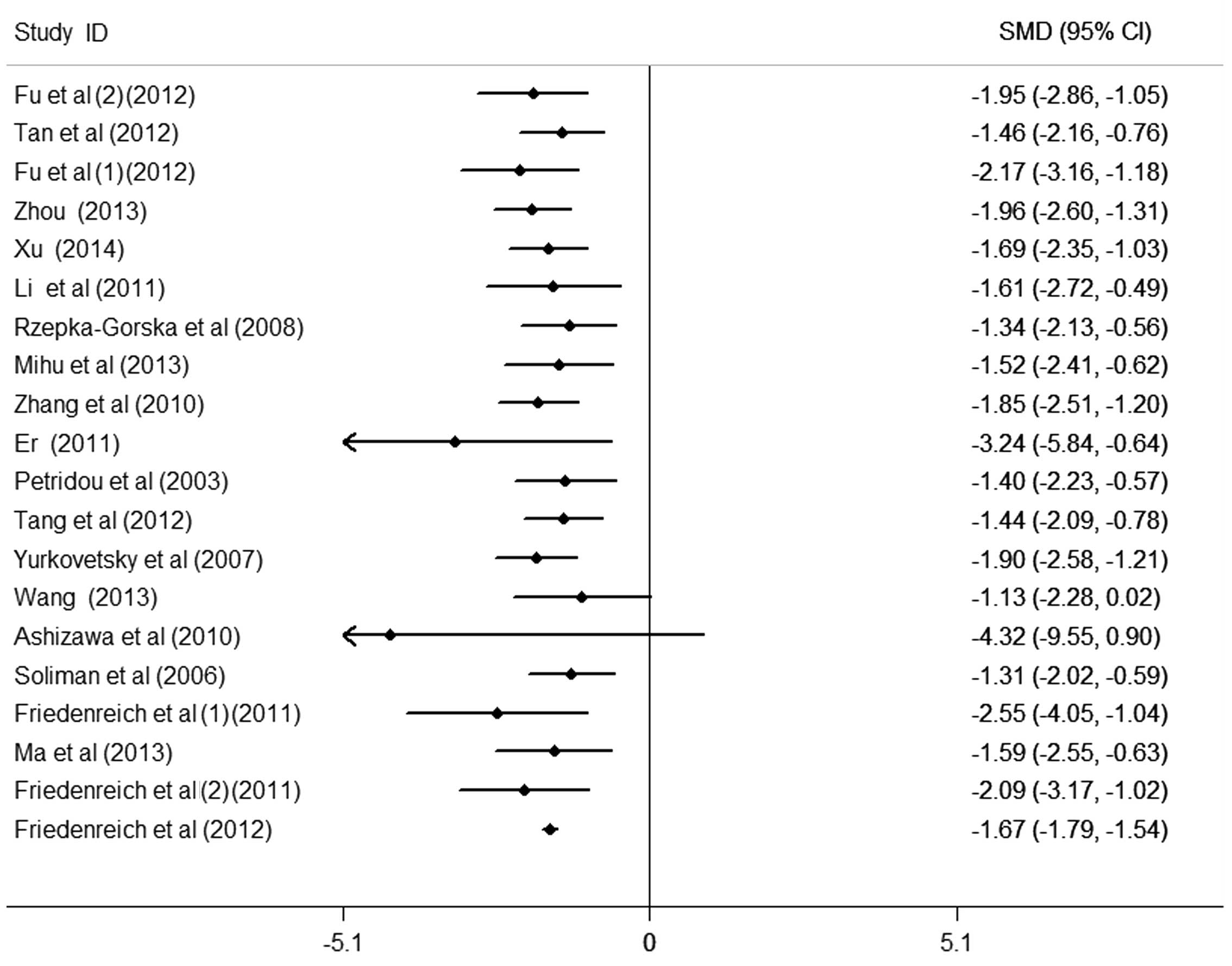
The results of the meta-analysis of the association
between circulating APN levels and endometrial cancer included 20
data sets from 18 studies (Fig. 2).
It was identified that women with endometrial cancer had
significantly lower APN levels in the pooled analysis using a
random-effects model [SMD (95% CI), −1.96 (−2.60, −1.31);
P<0.001], yet with significant heterogeneity among studies
(I2>50%). Egger's test for publication bias did not
provide evidence of significant effect (P>0.05). There were 7
studies providing cancer grade. On comparison of cancer grades, APN
values were not statistically different between the different
grades of endometrial cancer [G1 vs. G3, 1.02 (−0.68, 2.72),
P>0.05; G1 vs. G2, 0.34 (−0.86, 1.54), P>0.05].
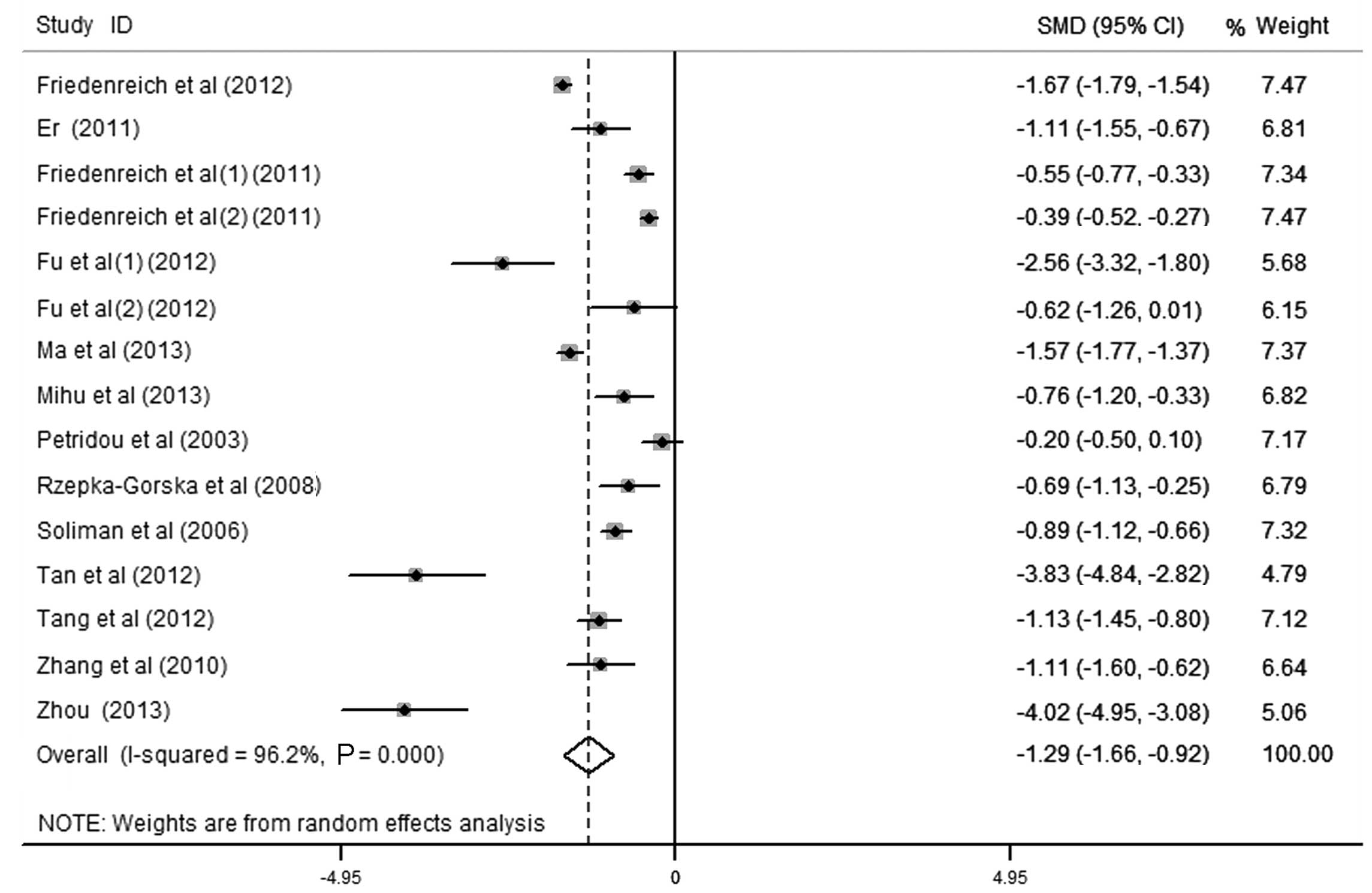
Subgroup/sensitivity analysis
Sensitivity analysis was performed by deleting one
study at a time, and the pooled SMD for the remaining studies was
calculated in order to check the influence of the removed data. The
results revealed that 5 studies were the key contributors to the
heterogeneity among studies (Fig. 3)
(19,23,24,32,33).
Although heterogeneity existed after excluding these studies, the
results revealed no significant alteration with an SMD (95% CI) of
−1.288 (−1.655, −0.922) and P<0.001. To identify potential
sources of heterogeneity between studies, subgroup analysis was
also performed by study size and quality, ethnicity, menopausal
status, language of publication, control source and laboratory
assay. The heterogeneity existed in subgroup analysis, and the
association between small-sized and low-quality studies was
identified to be stronger than in large-sized and high-quality
studies. The present study suggested that the difference in study
size and quality of the cases and the controls was due to the
heterogeneity amongst the studies. The total effect values in Asia,
Europe and North America were −2.48 (−3.97, −0.99), −0.52 (−0.90,
−0.14) and −1.71 (−2.60, −0.82), respectively. The total effect
value of postmenopausal endometrial cancer patients was −2.27
(−4.36, −0.18), which revealed that the high APN level was
associated with decreased endometrial cancer risk in postmenopausal
women (P<0.05). However, there was no association in
premenopausal endometrial cancer [-1.52 (−3.49, 0.45), P>0.05].
Furthermore, the risk of low APN level to endometrial cancer did
not differ substantially by laboratory assay, control sources and
language of publication. These results are presented in Table III.
 | Table III.Stratified meta-analysis of
circulating adiponectin levels and endometrial cancer. |
Table III.
Stratified meta-analysis of
circulating adiponectin levels and endometrial cancer.
|
|
| Endometrial cancer
(N) |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | Data points
(N) | Yes | No | Random effects | P-value |
I2 (%) |
|---|
| All studies | 20 | 2337 | 3355 | −1.96 (−2.60,
−1.31) | 0.000 | 98.9 |
| Study size |
|
|
|
|
|
|
|
<100 | 10 | 420 | 294 | −2.58 (−3.57,
−1.60) | 0.000 | 96.0 |
|
≥100 | 10 | 1917 | 3061 | −1.37 (−2.28,
−0.46) | 0.003 | 99.4 |
| Study quality |
|
|
|
|
|
|
| Low
(score <7) | 5 | 263 | 158 | −2.81 (−4.46,
−1.16) | 0.001 | 97.5 |
| High
(score ≥7) | 15 | 2074 | 3197 | −1.70 (−2.44,
−0.96) | 0.000 | 99.1 |
| Ethnicity |
|
|
|
|
|
|
|
Asia | 12 | 911 | 882 | −2.48 (−3.97,
−0.99) | 0.001 | 99.1 |
|
Europe | 3 | 165 | 176 | −0.52 (−0.90,
−0.14) | 0.007 | 65.1 |
| North
America | 5 | 1261 | 2297 | −1.71 (−2.60,
−0.82) | 0.000 | 99.2 |
|
Pre-/post-menopause |
|
|
|
|
|
|
|
Both | 14 | 1556 | 2098 | −1.95 (−2.81,
−1.08) | 0.000 | 98.9 |
|
Pre- | 2 | 141 | 292 | −1.52 (−3.49,
0.45) | 0.131 | 95.9 |
|
Post- | 4 | 640 | 965 | −2.27 (−4.36,
−0.18) | 0.033 | 99.3 |
| Laboratory
assay |
|
|
|
|
|
|
|
ELISA | 17 | 2101 | 3088 | −1.94 (−2.62,
−1.26) | 0.000 | 98.9 |
|
RIA | 2 | 121 | 132 | −1.96 (−2.60,
−1.31) | 0.086 | 68.6 |
|
xMAP | 1 | 115 | 135 | −5.23 (−5.76,
−4.71) | – | – |
| Control source |
|
|
|
|
|
|
|
Hospital | 15 | 1156 | 1248 | −2.09 (−3.12,
−1.06) | 0.000 | 98.9 |
|
Population | 5 | 1181 | 2107 | −1.68 (−2.63,
−0.72) | 0.001 | 99.2 |
| Control status |
|
|
|
|
|
|
|
Healthy | 17 | 2063 | 3077 | −1.83 (−2.48,
−1.17) | 0.000 | 98.8 |
|
Patients without EC | 3 | 274 | 278 | −2.65 (−6.13,
0.85) | 0.137 | 99.5 |
| Publication
language |
|
|
|
|
|
|
|
English | 10 | 1778 | 2933 | −1.86 (−2.57,
−1.14) | 0.000 | 99.0 |
|
Chinese | 10 | 559 | 422 | −2.13 (−3.91,
−0.34) | 0.019 | 98.9 |
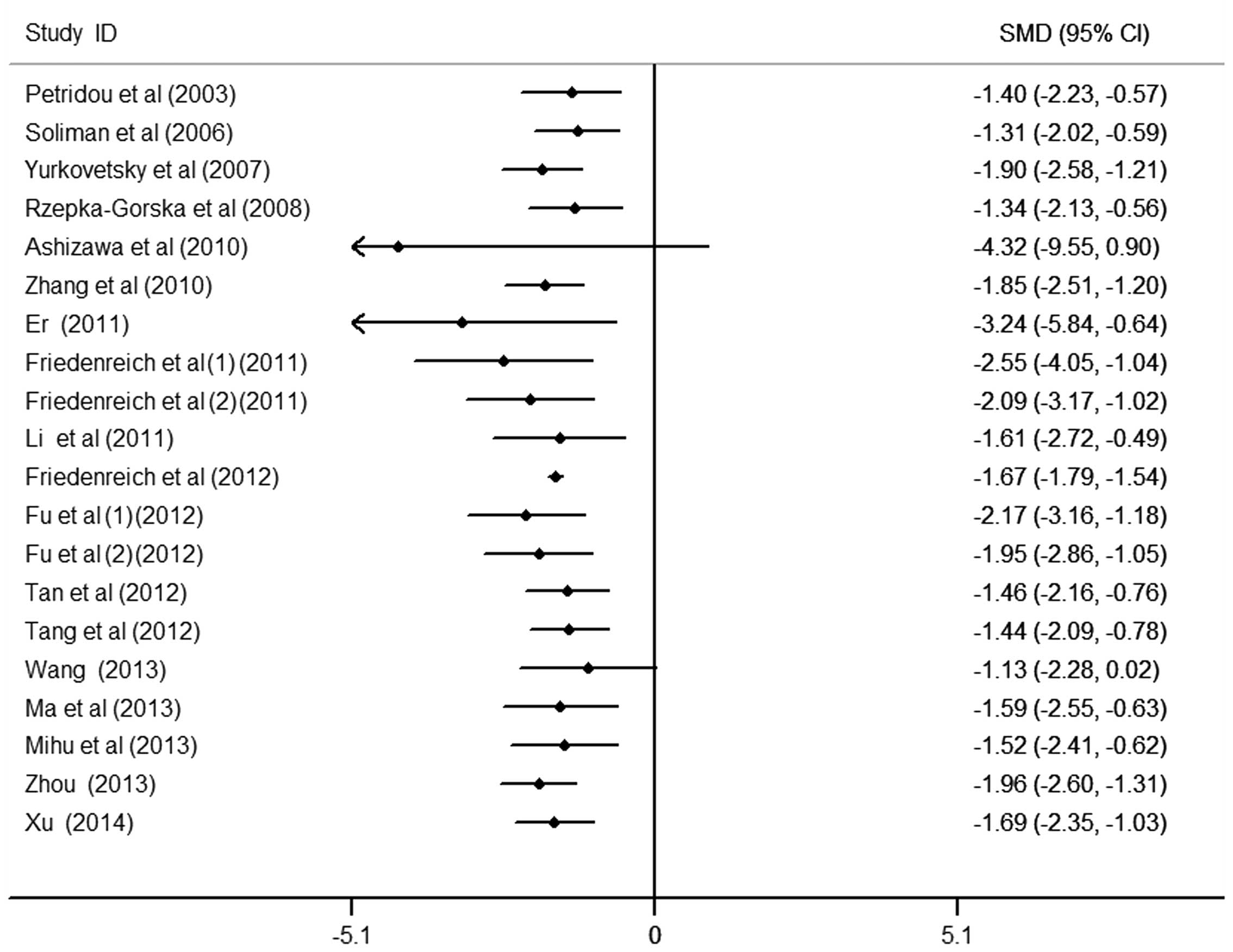
Cumulative meta-analysis
To further observe the change in the association
between circulating APN levels and endometrial cancer, the
cumulative meta-analysis was performed and sorted by year and total
size (Figs. 4 and 5). Data analysis suggests that the risk of
low APN levels to endometrial cancer was not observed all the time
and requires further study in order to confirm this result.
Nevertheless, patients with endometrial cancer were observed to
have markedly lower APN values compared with the controls.
Estimation of publication bias
Begg's funnel plot and Egger's test were performed
to assess the publication bias. The Funnel plot revealed a slight
asymmetry in the distribution but Egger's regression test suggested
no significant asymmetry of the Funnel plot (P=0.229).
Discussion
The majority of previous studies have suggested that
there is an independent and negative association of circulating APN
with endometrial cancer, however, a number of studies indicated no
association (29,45,46).
Since the results of a single study may be affected by numerous
factors, the present study included 18 studies, which comprised a
total of 4,186 participants and 1,823 cases, to further detect the
risk of low APN in endometrial cancer by reducing the bias and by
increasing the efficiency of the statistics. In the present study,
significantly low APN levels were observed in women with
endometrial cancer in the pooled analysis, although there was clear
heterogeneity across the studies. When the cancer grades were
compared, the risk of low APN to endometrial cancer was not
significantly different between grades of endometrial cancer, which
was not identical with previous studies (22–24,28,30,32,34). In
the subgroup and sensitivity analyses, there was significant
heterogeneity among studies. The significant association existed in
all of the pooled analysis, but not in premenopausal women, the
radioimmunoassay or patients without EC from control sources. The
risk was identified to be stronger in small-sized and low-quality
studies than in large-sized and high-quality studies, suggesting
that the effect of APN may be strongly affected by the small-sized
and low-quality studies. A previous study indicated that high APN
levels may decrease the risk of postmenopausal endometrial cancer
(28). However, there was no
significant difference in premenopausal women, which was in
contrast with various other studies (29,47,48).
Since the female gender and hormone levels are recognized risk
factors for endometrial cancer and the mechanism remains unclear,
the risk of low APN levels on endometrial cancer of pre- and
postmenopausal women is difficult to fully investigate. In the
other subgroups, no significant differences were observed in the
risk of low APN to endometrial cancer. In the cumulative
meta-analysis that was undertaken in the present study, the risk of
low APN to endometrial cancer changed with time and sample size.
Therefore, further studies are required in order to identify the
risk.
The mechanisms of action of APN in regards to
endometrial cancer have not yet been fully elucidated. APN is a
link between obesity and obesity-associated malignancies, mainly on
the basis of the finding that APN levels are lower in patients who
suffer with these types of cancer. AdipoR1/AdipoR2 are the two
different receptor isoforms of APN, which are expressed in fat and
muscle tissues in humans. The expression levels of these receptors
are regulated by insulin and peroxisome proliferator-activated
receptors (PPAR)-α and -γ. It has been demonstrated that the
receptors have lower expression levels in subcutaneous and visceral
adipose tissues than in skeletal muscle. Furthermore, previous
studies confirmed that there was a strong, inverse and consistent
association between circulating APN and obesity, insulin resistance
and inflammatory markers, including C-reactive protein and
fibrinogen. Thus, several case-control studies demonstrated that
APN receptors, particularly AdipoR1, are upregulated in
malignancies such as endometrial cancer. Furthermore, there is a
possibility that APN affects cancer cell proliferation and tumor
formation and progression directly by receptor-mediated stimulation
of signaling pathways and indirectly by moderating insulin
sensitivity. APN binding to its receptors (AdipoR1/AdipoR2) may
exert direct effects on these malignancy signaling pathways, mainly
5′ adenosine monophosphate-activated protein kinase (AMPK), but
also PPAR-α, mitogen-activated protein kinase and nuclear factor
(NF)-κB. AMPK activation regulates cell proliferation, decreases
the expression of transcriptional regulators and positively
regulates important proteins associated with controlling cell cycle
arrest and apoptosis. It is suggested that APN may affect tumor
cells by directly inhibiting pro-angiogenic factors, including
basic fibroblast growth factor and interleukin-8 produced by
tumors, or platelet-derived growth factor BB produced by
endothelial cells. APN also exerts an indirect action by
insulin-sensitizing, anti-inflammatory and anti-angiogenic effects.
Hyperinsulinemia, obesity and physical inactivity result in higher
concentrations of circulating sex hormone-binding globulin and
insulin-like growth factor 1, which leads to increased cell
proliferation, decreased apoptosis and increased inflammation.
In vitro studies to date reveal that APN decreases cell
viability and proliferation in endometrial cancer. Although these
results are promising, further studies are required in animals and
humans to elucidate the effects of circulating APN and its
association with endometrial cancer.
To the best of our knowledge, the present study was
the first to perform a meta-analysis that adds to the current
understanding of the association between circulating APN levels and
endometrial cancer. The present study included a large number of
cases and controls that significantly increased its statistical
power. However, several limitations in the study should be
addressed. The study is based on observational studies
(case-control) and is particularly vulnerable to potential biases
(information or selection bias) and bias in the original studies.
The significant heterogeneity observed across the results of the
study reduces the reliability of the observations, suggesting
different outcomes across studies with different patients,
controls, methods and measurements. All eligible studies were
published in English and Chinese and a number of relevant studies
in other languages were not included. Additionally, the conversion
of non-normally distributed statistics maybe have been less
accurate than if individual data had been available. Finally, the
sample sizes for certain subgroup analyses were small. Therefore,
the conclusions drawn from the results of this meta-analysis should
be regarded cautiously.
In summary, the present study indicated that low
circulating APN levels may increase the risk of endometrial cancer,
although there was a significant heterogeneity across the studies,
and that the high APN level may decrease the risk of endometrial
cancer in postmenopausal women. As APN is theorized to affect cell
proliferation, tumor formation and progression of endometrial
cancer, it may be a promising tool in endometrial cancer
prevention, diagnosis and therapy as a simple biomarker. However,
further studies are required in order to elucidate the mechanisms
of APN.
Acknowledgements
The authors are very grateful to Dr Petridou for
providing the additional data requested. This present research was
supported by the Graduate Innovation Fund of Jilin University
(grant no. 2014113).
References
|
1
|
Burzawa JK, Schmeler KM, Soliman PT, Meyer
LA, Bevers MW, Pustilnik TL, Anderson ML, Ramondetta LM,
Tortolero-Luna G, Urbauer DL, et al: Prospective evaluation of
insulin resistance among endometrial cancer patients. Am J Obstet
Gynecol. 204:355.e1–355.e7. 2011. View Article : Google Scholar
|
|
2
|
Lucenteforte E, Bosetti C, Talamini R,
Montella M, Zucchetto A, Pelucchi C, Franceschi S, Negri E, Levi F
and La Vecchia C: Diabetes and endometrial cancer: Effect
modification by body weight, physical activity and hypertension. Br
J Cancer. 97:995–998. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamauchi N, Takazawa Y, Maeda D, Hibiya T,
Tanaka M, Iwabu M, Okada-Iwabu M, Yamauchi T, Kadowaki T and
Fukayama M: Expression levels of adiponectin receptors are
decreased in human endometrial adenocarcinoma tissues. Int J
Gynecol Pathol. 31:352–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin XH, Jia HY, Xue XR, Yang SZ and Wang
ZQ: Clinical analysis of endometrial cancer patients with obesity,
diabetes and hypertension. Int J Clin Exp Med. 7:736–743.
2014.PubMed/NCBI
|
|
5
|
Fader AN, Arriba LN, Frasure HE and von
Gruenigen VE: Endometrial cancer and obesity: Epidemiology,
biomarker, prevention and survivorship. Gynecol Oncol. 114:121–127.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Linkov F, Edwards R, Balk J, Yurkovetsky
Z, Stadterman B, Lokshin A and Taioli E: Endometrial hyperplasia,
endometrial cancer and prevention: Gaps in existing research of
modifiable risk factors. Eur J Cancer. 44:1632–1644. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu XT, Xu Q, Tong JL, Zhu MM, Huang ML,
Ran ZH and Xiao SD: Meta-analysis: Circulating adiponectin levels
and risk of colorectal cancer and adenoma. J Dig Dis. 12:234–244.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cust AE, Armstrong BK, Friedenreich CM,
Slimani N and Bauman A: Physical activity and endometrial cancer
risk: A review of the current evidence, biologic mechanisms and the
quality of physical activity assessment methods. Cancer Causes
Control. 18:243–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schouten LJ, Goldbohm RA and van den
Brandt PA: Anthropometry, physical activity, and endometrial cancer
risk: Results from the Netherlands Cohort Study. J Natl Cancer
Inst. 96:1635–1638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barb D, Pazaitou-Panayiotou K and
Mantzoros CS: Adiponectin: A link between obesity and cancer.
Expert Opin Investig Drugs. 15:917–931. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calle EE and Kaaks R: Overweight, obesity
and cancer: Epidemiological evidence and proposed mechanisms. Nat
Rev Cancer. 4:579–591. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Joshi RK and Lee SA: Obesity related
adipokines and colorectal cancer: A review and meta-analysis. Asian
Pac J Cancer Prev. 15:397–405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Renehan AG, Roberts DL and Dive C: Obesity
and cancer: Pathophysiological and biological mechanisms. Arch
Physiol Biochem. 114:71–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dalamaga M, Diakopoulos KN and Mantzoros
CS: The role of adiponectin in cancer: A review of current
evidence. Endocr Rev. 33:547–594. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kelesidis I, Kelesidis T and Mantzoros CS:
Adiponectin and cancer: A systematic review. Br J Cancer.
94:1221–1225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Housa D, Housová J, Vernerová Z and
Haluzík M: Adipocytokines and Cancer. Physiol Res. 55:233–244.
2006.PubMed/NCBI
|
|
17
|
Roberts DL, Dive C and Renehan AG:
Biological mechanisms linking obesity and cancer risk: New
perspectives. Annu Rev Med. 61:301–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Friedenreich CM, Langley AR, Speidel TP,
Lau DC, Courneya KS, Csizmadi I, Magliocco AM, Yasui Y and Cook LS:
Case-control study of markers of insulin resistance and endometrial
cancer risk. Endocr Relat Cancer. 19:785–792. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ashizawa N, Yahata T, Quan J, Adachi S,
Yoshihara K and Tanaka K: Serum leptin-adiponectin ratio and
endometrial cancer risk in postmenopausal female subjects. Gynecol
Oncol. 119:65–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Er LX and Liu FQ: Roles of VEGF,
adiponectin and EGFR in endometrial adenocarcinoma. Yi Xue Zong
Shu. 17:1417–1418. 2011.(In Chinese).
|
|
21
|
Friedenreich CM, Biel RK, Lau DC, Csizmadi
I, Courneya KS, Magliocco AM, Yasui Y and Cook LS: Case-control
study of the metabolic syndrome and metabolic risk factors for
endometrial cancer. Cancer Epidemiol Biomarkers Prev. 20:2384–2395.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu JR, Lu LN, Yu LP, Wang DY and Ding KS:
Serum changes of adiponectin, insulin resistance and their
correlation in endometrial cancer patients. Zhonghua Fu Chan Ke Za
Zhi. 47:672–675. 2012.(In Chinese). PubMed/NCBI
|
|
23
|
Wang G: Analysis of relationship between
serum adiponectin level and insulin resisitance index in endomerial
carcinoma. Shi Yong Ai Zheng Za Zhi She. 28:599–601. 2013.(In
Chinese).
|
|
24
|
Li SP, Huang XH and Zhang AL: Expression
of serum adiponectin in patients with endometrial cancer and its
clinical significance. J Clin Res. 28:1252–1253. 2011.
|
|
25
|
Ma Y, Liu Z, Zhang Y and Lu B: Serum
leptin, adiponectin and endometrial cancer risk in Chinese women. J
Gynecol Oncol. 24:336–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mihu D, Ciortea R and Mihu CM: Abdominal
adiposity through adipocyte secretion products, a risk factor for
endometrial cancer. Gynecol Endocrinol. 29:448–451. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Petridou E, Mantzoros C, Dessypris N,
Koukoulomatis P, Addy C, Voulgaris Z, Chrousos G and Trichopoulos
D: Plasma adiponectin concentrations in relation to endometrial
cancer: A case-control study in Greece. J Clin Endocrinol Metab.
88:993–997. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rzepka-Górska I, Bedner R, Cymbaluk-Płoska
A and Chudecka-Głaz A: Serum adiponectin in relation to endometrial
cancer and endometrial hyperplasia with atypia in obese women. Eur
J Gynaecol Oncol. 29:594–597. 2008.PubMed/NCBI
|
|
29
|
Soliman PT, Wu D, Tortolero-Luna G,
Schmeler KM, Slomovitz BM, Bray MS, Gershenson DM and Lu KH:
Association between adiponectin, insulin resistance, and
endometrial cancer. Cancer. 106:2376–2381. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tan ZQ, Liu FX and Long D: Expression and
clinical significance of serum adiponectin in patients with
endometrial cancer. Guangdong Med J. 33:1757–1759. 2012.
|
|
31
|
Tang JM, Xiao Q and Fang Y: Study on the
risk ofactors related to sign the comprehensive endometrial cancer
and metabolism. Zhejiang Prev Med. 24:69–70. 2012.
|
|
32
|
Xu L: Relationship between the expression
of adiponectin in serum and type-I Endometrial Carcinoma. Hua Xi Yi
Xue. 29:736–737. 2014.(In Chinese).
|
|
33
|
Yurkovetsky Z, Ta'asan S, Skates S, Rand
A, Lomakin A, Linkov F, Marrangoni A, Velikokhatnaya L, Winans M,
Gorelik E, et al: Development of multimarker panel for early
detection of endometrial cancer. High diagnostic power of
prolactin. Gynecol Oncol. 107:58–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang LZ, Wen K and Liu R: Change and
significance of serum adiponectin level in patients with
endometrial carcinoma. Shangdong Med. 50:4–5. 2010.
|
|
35
|
Zhou MN: Research on expression of
adiponectin in serum of the patients with endometrial cancer and
its clinical significance. Zhong Guo Wei Sheng Chan Ye Za Zhi She.
10:156–158. 2013.(In Chinese).
|
|
36
|
Wells GA, Shea B, O'connell D, et al: The
Newcastle-Ottawa Scale (NOS) for assessing the quality of
nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspACCESS
DATE month, day, year.
|
|
37
|
Stroup DF, Berlin JA, Morton SC, Olkin I,
Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA and Thacker
SB: Meta-analysis of observational studies in epidemiology: A
proposal for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pinthus JH, Kleinmann N, Tisdale B,
Chatterjee S, Lu JP, Gillis A, Hamlet T, Singh G, Farrokhyar F and
Kapoor A: Lower plasma adiponectin levels are associated with
larger tumor size and metastasis in clear-cell carcinoma of the
kidney. Eur Urol. 54:866–873. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hozo SP, Djulbegovic B and Hozo I:
Estimating the mean and variance from the median, range, and the
size of a sample. BMC Med Res Methodol. 5:132005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
DerSimonian R: Meta-analysis in the design
and monitoring of clinical trials. Stat Med. 15:1237–1252. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leimu R and Koricheva J: Cumulative
meta-analysis: A new tool for detection of temporal trends and
publication bias in ecology. Proc Biol Sci. 271:1961–1966. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dallal CM, Brinton LA, Bauer DC, Buist DS,
Cauley JA, Hue TF, Lacroix A, Tice JA, Chia VM, Falk R, et al:
B~FIT Research Group: Obesity-related hormones and endometrial
cancer among postmenopausal women: A nested case-control study
within the B~FIT cohort. Endocr Relat Cancer. 20:151–160. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Modesitt SC, Geffel DL, Via JL and Weltman
A: Morbidly obese women with and without endometrial cancer: Are
there differences in measured physical fitness, body composition,
or hormones? Gynecol Oncol. 124:431–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cust AE, Kaaks R, Friedenreich C, Bonnet
F, Laville M, Lukanova A, Rinaldi S, Dossus L, Slimani N, Lundin E,
et al: Plasma adiponectin levels and endometrial cancer risk in
pre- and postmenopausal women. J Clin Endocrinol Metab. 92:255–263.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dal Maso L, Augustin LS, Karalis A,
Talamini R, Franceschi S, Trichopoulos D, Mantzoros CS and La
Vecchia C: Circulating adiponectin and endometrial cancer risk. J
Clin Endocrinol Metab. 89:1160–1163. 2004. View Article : Google Scholar : PubMed/NCBI
|