Introduction
Heart failure (HF), also known as chronic HF, is one
of the major causes of mortality affecting approximately 4% of the
world's population, while the prevalence of this condition is
currently increasing (1,2). In addition to the widely known risk
factor of glucose abnormalities (observed in ~43% of HF patients)
(3), HF can also result from certain
other factors, which is classified as nondiabetic HF (ND-HF) and is
based on a complicated pathological mechanism (4,5). Since
HF greatly affects human health and has an unclear pathogenesis,
numerous studies have investigated this condition.
Previous studies have proposed certain markers
associated with HF. For instance, hyperuricemia and elevated levels
of circulating markers of inflammation are common in HF (6,7), and
thus the inflammatory biomarker YKL-40 was investigated and found
to be significantly associated with all-cause mortality in patients
with HF (8). In addition, as a
marker of cardiomyocyte injury, cardiac troponin T is a predictor
of adverse outcomes for patients with chronic HF (9). Troughton et al (10) observed that patients with impaired
systolic function or symptomatic HF could be treated under
N-terminal brain natriuretic peptide (N-BNP) guidance to partly
reduce the total number of cardiovascular events. Despite vast
efforts to predict and prevent HF in order to decrease the
morbidity and mortality associated with this condition, there is no
clear division between ND-HF and diabetic HF. Furthermore, simple
and reliable measurements to diagnose this disease earlier and to
effectively predict the prognosis remain insufficient.
In the current study, the gene expression profiles
generated from healthy controls and ND-HF patients were analyzed.
Biopsy tissues were collected during the surgical ventricular
restoration in patients with dilated hypokinetic ischemic
cardiomyopathy. Differentially expressed genes (DEGs) were screened
and their possible roles in the pathogenesis of HF were explored
using multiple bioinformatics methods. The main aim of the present
study was to identify better markers for the diagnosis and
treatment of ND-HF.
Materials and methods
Microarray dataset
The microarray dataset under the accession number
GSE26887 (11) were obtained from
the Gene Expression Omnibus (12)
database (http://www.ncbi.nlm.nih.gov/geo/) of the National
Center for Biotechnology Information (Bethesda, MD, USA). The gene
expression profile was generated based on the platform GPL6244
(Affymetrix Human Gene 1.0 ST Array; Affymetrix, Inc., Santa Clara,
CA, USA). This dataset was derived from RNA samples extracted from
12 ND-HF patients (12 males) and 5 healthy controls (2 males, 3
females). Myocardial biopsy samples were collected from the vital,
non-infarcted zone of left ventricular of patients with dilated
ischemic hypokinetic cardiomyopathy during surgical ventricular
restoration procedures (11). In
addition, left ventricle cardiac biopsy samples were collected by
Greco et al (11) from the
vital, non-infarcted zone of control patients who had succumbed to
mortality (as a result of non-cardiac associated causes), within
<24 h.
Data preparation and DEGs
screening
Robust multichip average (RMA) (13), which contained three steps including
background adjustment, quantile normalization and summarization,
was used as a probe set algorithm. The original dataset and the
annotation file of the platform were preprocessed using the RMA
method of the BioConductor Oligo package (version 2.12; www.bioconductor.org). Probe set IDs were transformed
into gene symbols, and the gene expression matrix was
constructed.
Statistically significant differences in the
expression levels of the various genes were first calculated by the
unequal variance t-test, and was then adjusted for multiple testing
using the Benjamini and Hochberg procedure (14). After comparing the expression of
these genes in the control and HF tissues, the adjusted P-value was
obtained, and DEGs with an adjusted P-value of <0.05 and a
|log2 fold change (FC)| of >1 were screened and were
considered as potential HF-associated genes.
Protein-protein interaction (PPI)
network
The Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING) (15) is a
widely used database that includes the known and predicted protein
interactions. PPI network analysis of the upregulated and
downregulated DEGs was performed utilizing the STRING online tool.
A confidence (combined) score of >0.4 was selected as the
threshold of PPIs.
The PPI network was constructed using the Cytoscape
software platform (16) based on the
PPI associations identified. Since the vast majority of biological
networks are subject to the scale-free (without scale) properties
of the network, the connectivity degree was used for the analysis
of important nodes (hub proteins) in the PPI network (17,18).
Gene ontology (GO) and pathway
analysis
The Kyoto Encyclopedia of Genes and Genomes (KEGG)
(19) is an authoritative database
containing a variety of biochemical pathways. In addition, the
Database for Annotation, Visualization and Integration Discovery
(DAVID) (20) is a gene functional
classification tool that organizes and condenses abundant
heterogeneous annotation content. Functional enrichment analysis
was conducted in order to recognize the DEG enriched biochemical
pathways using KEGG database and GO-associated biological
functions. Furthermore, DAVID online tools were applied for the GO
and KEGG pathway enrichment analyses with a P-value set to
<0.05.
Results
DEG screening
A significant gender difference of the sample source
existed between the control and ND-HF subjects; thus,
gender-correlated results were carefully considered or abandoned. A
total of 255 DEGs were obtained in the ND-HF patients, including
122 upregulated and 133 downregulated genes. As shown in Table I, the EIF1AY, NPPA and
DSC1 were the three most upregulated genes. Similarly, the
three most downregulated genes were SERPINE1,
SERPINA3 and TNC, and their respective
log2 FC values were −3.182, −2.904 and −2.223 (Table I).
 | Table I.Top 10 upregulated and downregulated
genes. |
Table I.
Top 10 upregulated and downregulated
genes.
| Gene |
log2FC | Adjusted
P-value |
|---|
| Upregulated |
|
|---|
|
EIF1AY |
3.412 | 0.00281 |
|
NPPA |
3.115 | 0.00038 |
|
DSC1 |
2.445 | 0.00031 |
|
NEB |
2.418 | 0.00545 |
|
MYL4 |
2.346 | 0.00204 |
|
UTY |
2.254 | 0.00414 |
|
FRZB |
2.171 | 0.00165 |
|
USP9Y |
2.095 | 0.00550 |
|
SLN |
2.092 | 0.00712 |
|
RBMY1A1 |
1.993 | 0.00038 |
| Downregulated |
|
|
SERPINE1 | −3.182 | 0.00301 |
|
SERPINA3 | −2.904 | 0.00037 |
|
TNC | −2.223 | 0.01926 |
|
SPP1 | −2.129 | 0.01003 |
|
CYP1B1 | −2.027 | 0.00858 |
|
S100A8 | −1.955 | 0.00130 |
|
ANKRD2 | −1.903 | 0.00079 |
|
GFPT2 | −1.824 | 0.00020 |
|
MYC | −1.821 | 0.00117 |
|
CD163 | −1.805 | 0.00022 |
PPI network
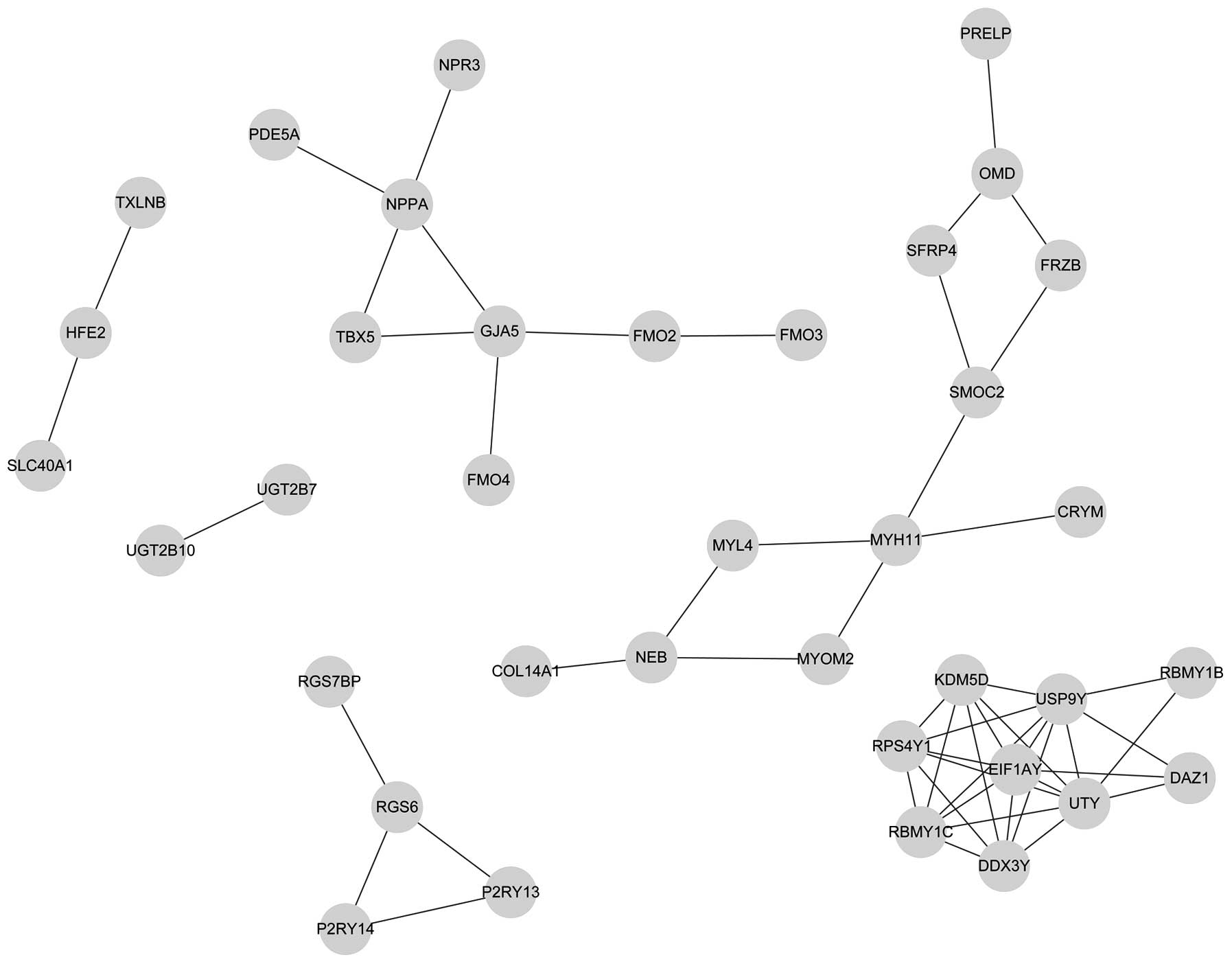
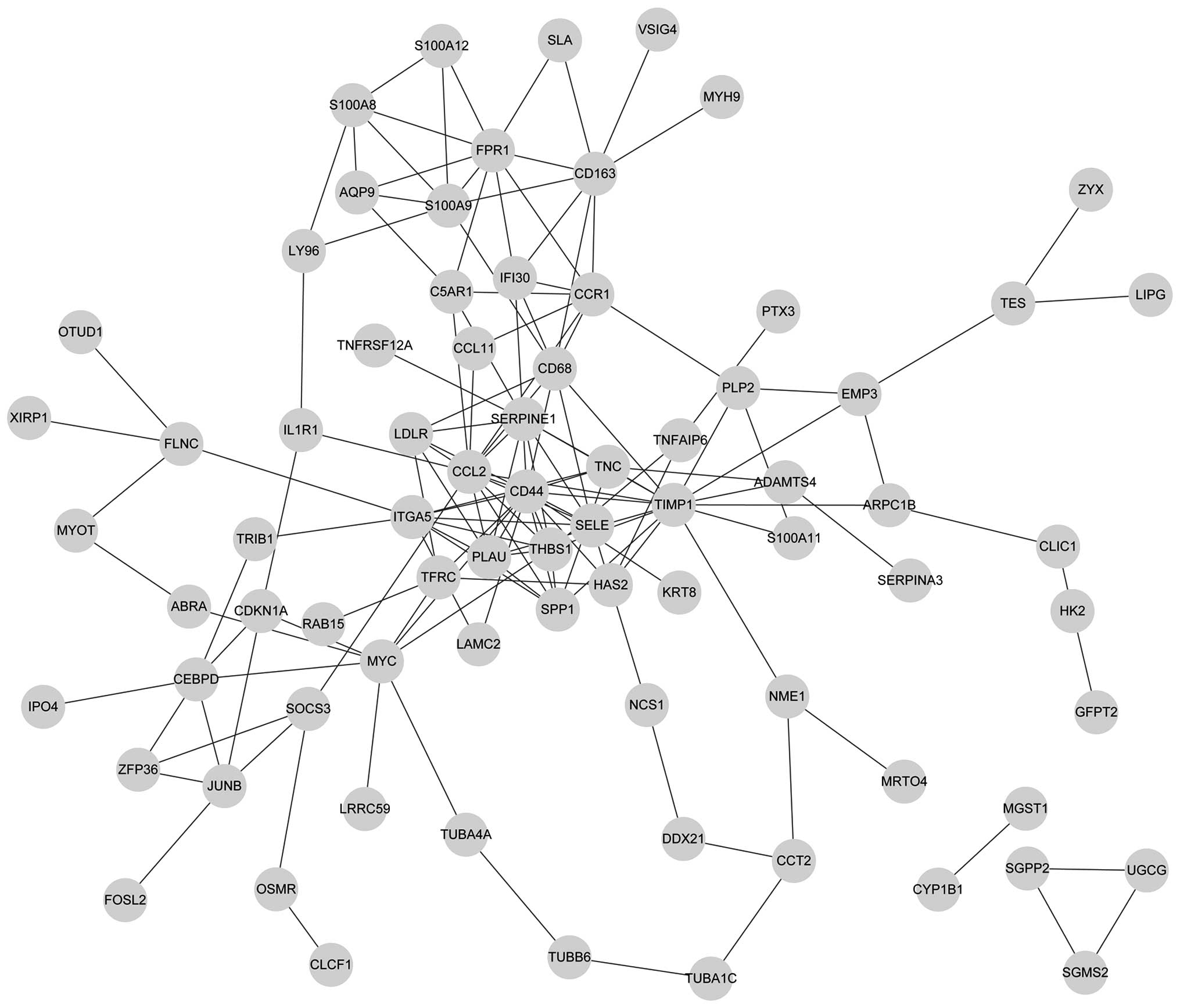
In total, 38 nodes and 53 node-pairs were identified
in the PPI network of the upregulated DEGs. Furthermore, 77 nodes
and 149 node-pairs were obtained in the PPI network of
downregulated DEGs. Subsequent to filtering out the low-degree
nodes and nodes without connections, the up- and downregulated PPI
networks were constructed, as shown in Figs. 1 and 2, respectively.
Tables II and
III exhibited the connectivity
degree of the top 30% nodes in the PPI network of upregulated and
downregulated DEGs, respectively. According to the calculation
results, the connectivity degree of each node was >4 in the
upregulated and downregulated networks. The connectivity degree of
NPPA was 4, without any connections with the USP9Y,
UTY and EIF1AY genes. In the downregulated network,
the top five nodes with a high connectivity degree were
CD44, TIMP1, CCL2, THBS1 and
SERPINE1.
 | Table II.Top 30% of the node connections in
the upregulated protein-protein interaction network. |
Table II.
Top 30% of the node connections in
the upregulated protein-protein interaction network.
| Gene | Degree |
|---|
| USP9Y | 8 |
| UTY | 8 |
| EIF1AY | 7 |
| DDX3Y | 6 |
| KDM5D | 6 |
| RBMY1C | 6 |
| RPS4Y1 | 6 |
| GJA5 | 4 |
| MYH11 | 4 |
| NPPA | 4 |
 | Table III.Top 30% of the node connections in
the downregulated protein-protein interaction network. |
Table III.
Top 30% of the node connections in
the downregulated protein-protein interaction network.
| Gene | Degree |
|---|
| CD44 | 16 |
| TIMP1 | 15 |
| CCL2 | 14 |
| THBS1 | 9 |
|
SERPINE1 | 9 |
| FPR1 | 9 |
| CD68 | 9 |
| ITGA5 | 9 |
| CCR1 | 8 |
| CD163 | 8 |
| MYC | 8 |
| PLAU | 8 |
| SPP1 | 7 |
| S100A9 | 7 |
| CEBPD | 6 |
| SELE | 6 |
| TNC | 6 |
| LDLR | 6 |
| C5AR1 | 5 |
| IFI30 | 5 |
| TFRC | 5 |
| S100A8 | 5 |
| JUNB | 5 |
GO and KEGG pathway analyses of
DEGs
GO analysis revealed that the significantly-enriched
GO terms of upregulated DEGs included muscle contraction, muscle
system process, circulatory system process (involving NPPA),
blood circulation, muscular organ development, male gamete
generation, spermatogenesis, cGMP metabolic process and skeletal
system development. In addition, the significantly enriched GO
terms of downregulated DEGs were mainly associated with the
stimulus response, response to bacterium and response to nutrient.
NPPA was also involved in the GO term of regulation of cell
growth (Table IV).
 | Table IV.Gene ontology term enrichment
analyses of the differentially expressed genes. |
Table IV.
Gene ontology term enrichment
analyses of the differentially expressed genes.
| Category | GO-BP Term | Count | P-value |
|---|
| Upregulated |
|
|
|
|
GO:0006936 | Muscle
contraction | 4 | 0.004604 |
|
GO:0003012 | Muscle system
process | 4 | 0.005971 |
|
GO:0003013 | Circulatory system
process | 4 | 0.007904 |
|
GO:0008015 | Blood
circulation | 4 | 0.007904 |
|
GO:0007517 | Muscle organ
development | 4 | 0.011139 |
|
GO:0048232 | Male gamete
generation | 4 | 0.030149 |
|
GO:0007283 |
Spermatogenesis | 4 | 0.030149 |
|
GO:0046068 | cGMP metabolic
process | 2 | 0.030618 |
|
GO:0001501 | Skeletal system
development | 4 | 0.032968 |
| Downregulated |
|
|
|
|
GO:0009611 | Response to
wounding | 21 |
5.31×10−13 |
|
GO:0006954 | Inflammatory
response | 17 |
3.16×10−12 |
|
GO:0006952 | Defense
response | 20 |
7.41×10−11 |
|
GO:0032496 | Response to
lipopolysaccharide | 6 |
3.84×10−5 |
|
GO:0002237 | Response to
molecule of bacterial origin | 6 |
6.54×10−5 |
|
GO:0009617 | Response to
bacterium | 7 |
3.78×10−4 |
|
GO:0009991 | Response to
extracellular stimulus | 8 |
1.04×10−4 |
|
GO:0031667 | Response to
nutrient levels | 7 |
4.22×10−4 |
|
GO:0007584 | Response to
nutrients | 6 |
6.38×10−4 |
|
GO:0033273 | Response to
vitamins | 4 | 0.004253 |
KEGG pathway analysis revealed that the
significantly enriched pathways of upregulated DEGs were drug
metabolism, ascorbate and aldarate metabolism, and pentose and
glucuronate interconversions (Table
V). By contrast, the significantly enriched pathways of the
downregulated DEGs were extracellular matrix (ECM)-receptor
interactions (involving the genes THBS1, CD44 and
TNC), pathogenic Escherichia coli infection, focal
adhesion (involving TNC and THBS1), cytokine-cytokine
receptor interaction (involving CCL11 and CCL2),
hematopoietic cell lineage (involving CD44), sphingolipid
metabolism, and bladder cancer (involving THBS1; Table V).
 | Table V.Kyoto Encyclopedia of Genes and
Genomes pathway analysis of the differentially expressed genes. |
Table V.
Kyoto Encyclopedia of Genes and
Genomes pathway analysis of the differentially expressed genes.
| Pathway term | Pathway
description | Count | P-value | Associated
genes |
|---|
| Upregulated |
|
|
|
|
|
hsa00982 | Drug
metabolism | 5 |
6.20×10−6 | FMO4,
FMO2, FMO3, UGT2B10, UGT2B7 |
|
hsa00053 | Ascorbate and
aldarate metabolism | 2 | 0.036201 | UGT2B10,
UGT2B7 |
|
hsa00040 | Pentose and
glucuronate interconversions | 2 | 0.038293 | UGT2B10,
UGT2B7 |
| Downregulated |
|
|
|
|
|
hsa04512 | Extracellular
matrix-receptor interaction | 6 |
5.13×10−4 | CD44,
ITGA5, TNC, LAMC2, THBS1, SPP1,
ARPC1B, LY96, |
|
hsa05130 | Pathogenic
Escherichia coli infection | 5 | 0.001056 | TUBB6,
TUBA4A, TUBA1C |
|
hsa04510 | Focal adhesion | 7 | 0.005006 | ITGA5,
TNC, LAMC2, ZYX, FLNC, THBS1,
SPP1 |
|
hsa04060 | Cytokine-cytokine
receptor interaction | 7 | 0.017379 | CCL11,
IL1R1, CCL2, TNFRSF12A, OSMR,
CLCF1, CCR1 |
|
hsa04640 | Hematopoietic cell
lineage | 4 | 0.031377 | IL1R1,
TFRC, CD44, ITGA5 |
|
hsa00600 | Sphingolipid
metabolism | 3 | 0.038951 | SGMS2,
SGPP2, UGCG |
|
hsa05219 | Bladder cancer | 3 | 0.044581 | CDKN1A,
THBS1, MYC |
Discussion
HF with fairly high morbidity and mortality
(21), is increasing in prevalence
with the aging of the worldwide population (22). In order to improve the understanding
on the underlying mechanisms and identify molecular markers of HF,
particularly in dilated ischemic cardiomyopathy-associated HF, the
present study screened the DEGs between control and ND-HF patients.
In addition, these DEGs were used for PPI network construction,
while GO and KEGG pathway analyses were also performed. A total of
122 upregulated and 133 downregulated genes were detected. The most
significantly upregulated and downregulated genes were NPPA
and SERPINE1, respectively. Furthermore, NPPA and
SERPINE1 were not only differentially expressed in ND-HF
patients, but were also found to be hub nodes in the PPI network.
Certain GO terms and KEGG pathways enriched by DEGs were obtained.
Therefore, these hub genes and functional terms may be closely
associated with ND-HF.
The protein encoded by the upregulated NPPA
gene is the atrial natriuretic peptide (ANP), which is a member of
the natriuretic peptide family that is involved in the control of
the extracellular fluid volume and electrolyte homeostasis
(23,24). The GO term of circulatory system
process, in which NPPA is involved, is vital for
homeostasis. In addition, mutations in NPPA gene are linked
to atrial fibrillation (25). In
1998, Maeda et al (26)
stated that brain natriuretic peptide (BNP) levels were correlated
with the left ventricular end-diastolic pressure. However, a more
recent study by Seronde et al (27) found that the mid-regional sequence of
pro-ANP (MR-proANP) has a more long term prognostic value when
compared with BNP in patients with acute HF. Furthermore, Potocki
et al (28) suggested that
MR-proANP appears to provide incremental information superior to
BNP in certain subgroups of patients. Notably, GO terms and KEGG
pathways enriched by NPPA or other genes are essential in
cardiac failure. Therefore, due to these advantages of ANP when
compared with BNP, NPPA may also be an essential gene
associated with ND-HF and may be used as a potential therapeutic
target in ND-HF.
In addition to the poor contractility and low
cardiac output, patients with HF also present with abnormal
manifestations of platelets and endothelial dysfunction (29), while HF patients in sinus rhythm
still present a higher thromboembolic risk (30). SERPINE1, also known as
plasminogen activator inhibitor 1 (PAI-1) precursor, which
was downregulated and pertained to the serine proteinase inhibitor
superfamily, has a core effect in the regulation of fibrinolysis,
coagulation, inflammation and neuromuscular patterning (31). Askari et al (32) hypothesized that genetic disruption of
PAI-1 is essential in order to suppress ventricular remodeling in
null mice with myocardial infarction; furthermore, PAI-1 is
essential in microvascular integrity and cardiac homeostasis
(33). Based on the results of the
present and previous studies (31,33), the
plasma level of SERPINE1 is associated with thrombophilia
and an increased risk of coronary artery disease (34). Therefore, SERPINE1 may be a
useful marker for the diagnosis and treatment of ND-HF.
HF is accompanied by degradation of the collagen
network of the ECM (35), and may
subsequently cause heart dysfunction (36). Zheng et al (37) reported an overall decrease in the
ECM-associated genes which are indispensable to the overall ECM
structure and collagen assembly. Therefore, it is no surprise that
CD44, which is involved in the ECM-receptor interaction, was
found to be downregulated in the current study. Chatila et
al (38) also found that certain
compositions of the infarct border zone may slow down left
ventricular remodeling by suppressing inflammation. In the current
study, GO terms enriched by downregulated genes were mainly
associated with the stimulus and immune response. Considering these
findings, the connections between ECM and inflammation
participating in HF require further investigation, particularly in
ND-HF.
In conclusion, based on the bioinformatics methods
used in the current study, a number of DEGs were highlighted,
particularly NPPA and SERPINE1, although the results
were interfered by certain Y-linked genes to some extent. These two
genes may be potential therapeutic targets and molecular markers
contributing to improved prevention and treatment of cardiogenic
disease. Additionally, the complicated correlation between
ECM-protein expression and inflammation was further investigated.
However, further comparison of these genes and those obtained from
diabetic HF patients with dilated ischemic cardiomyopathy and
controls is required to verify these results. Furthermore,
gender-matched studies are needed, with a sufficiently large sample
size. Future research should focus on these areas and verify these
DEGs based on serum sample analysis.
References
|
1
|
Van Diepen S, Hellkamp AS, Patel MR,
Becker RC, Breithardt G, Hacke W, Halperin JL, Hankey GJ, Nessel
CC, Singer DE, et al: Efficacy and safety of rivaroxaban in
patients with heart failure and nonvalvular atrial fibrillation:
Insights from ROCKET AF. Circ Heart Fail. 6:740–747. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carr HJ, McDermott A, Tadbiri H, Uebbing
A-M and Londrigan M: The effectiveness of computer-based learning
in hospitalized adults with heart failure on knowledge,
re-admission, self-care, quality of life and patient satisfaction:
A systematic review protocol. The JBI Database of Systematic
Reviews and Implementation Reports. 11:129–145. 2013. View Article : Google Scholar
|
|
3
|
Suskin N, McKelvie RS, Burns RJ, Latini R,
Pericak D, Probstfield J, Rouleau JL, Sigouin C, Solymoss CB,
Tsuyuki R, et al: Glucose and insulin abnormalities relate to
functional capacity in patients with congestive heart failure. Eur
Heart J. 21:1368–1375. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diercks G, Van Boven A, Hillege H, Janssen
W, Kors J, De Jong P, Grobbee D, Crijns H and Van Gilst W:
Microalbuminuria is independently associated with ischaemic
electrocardiographic abnormalities in a large non-diabetic
population. The PREVEND (Prevention of REnal and Vascular ENdstage
Disease) study. Eur Heart J. 21:1922–1927. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klamann A, Sarfert P, Launhardt V, Schulte
G, Schmiegel WH and Nauck MA: Myocardial infarction in diabetic vs
non-diabetic subjects. Survival and infarct size following therapy
with sulfonylureas (glibenclamide). Eur Heart J. 21:220–229. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anker SD, Egerer KR, Volk H-D, Kox WJ,
Poole-Wilson PA and Coats AJ: Elevated soluble CD14 receptors and
altered cytokines in chronic heart failure. Am J Cardiol.
79:1426–1430. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krishnan E: Hyperuricemia and incident
heart failure. Circ Heart Fail. 2:556–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harutyunyan M, Christiansen M, Johansen
JS, Køber L, Torp-Petersen C and Kastrup J: The inflammatory
biomarker YKL-40 as a new prognostic marker for all-cause mortality
in patients with heart failure. Immunobiology. 217:652–656. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kusumoto A, Miyata M, Kubozono T, Ikeda Y,
Shinsato T, Kuwahata S, Fujita S, Takasaki K, Yuasa T and Hamasaki
S: Highly sensitive cardiac troponin T in heart failure: Comparison
with echocardiographic parameters and natriuretic peptides. J
Cardiol. 59:202–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Troughton RW, Frampton CM, Yandle TG,
Espiner EA, Nicholls MG and Richards AM: Treatment of heart failure
guided by plasma aminoterminal brain natriuretic peptide (N-BNP)
concentrations. The Lancet. 355:1126–1130. 2000. View Article : Google Scholar
|
|
11
|
Greco S, Fasanaro P, Castelvecchio S,
D'Alessandra Y, Arcelli D, Di Donato M, Malavazos A, Capogrossi MC,
Menicanti L and Martelli F: MicroRNA dysregulation in diabetic
ischemic heart failure patients. Diabetes. 61:1633–1641. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kapoun AM, Liang F, O'Young G, Damm DL,
Quon D, White RT, Munson K, Lam A, Schreiner GF and Protter AA:
B-type natriuretic peptide exerts broad functional opposition to
transforming growth factor-beta in primary human cardiac
fibroblasts: Fibrosis, myofibroblast conversion, proliferation and
inflammation. Circ Res. 94:453–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: A practical and powerful approach to
multiple testing. Journal of the Royal Statistical Society. Series
B (Methodological). 289–300. 1995.
|
|
15
|
Von Mering C, Huynen M, Jaeggi D, Schmidt
S, Bork P and Snel B: STRING: A database of predicted functional
associations between proteins. Nucleic Acids Res. 31:258–261. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smoot ME, Ono K, Ruscheinski J, Wang PL
and Ideker T: Cytoscape 2.8: New features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He X and Zhang J: Why do hubs tend to be
essential in protein networks? PLoS Genet. 2:e882006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC Bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alvord WG, Roayaei J, Stephens R, Baseler
MW, Lane HC, Lempicki RA, Huang DW, Sherman BT, Tan Q and Collins
JR: The DAVID Gene Functional Classification Tool: A novel
biological module-centric algorithm to functionally analyze large
gene lists. Genome Biol. 8:R1832007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bottomley PA, Panjrath GS, Lai S, Hirsch
GA, Wu K, Najjar SS, Steinberg A, Gerstenblith G and Weiss RG:
Metabolic rates of ATP transfer through creatine kinase (CK Flux)
predict clinical heart failure events and death. Sci Transl Med.
5:215re32013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burchell AE, Sobotka PA, Hart EC,
Nightingale AK and Dunlap ME: Chemohypersensitivity and autonomic
modulation of venous capacitance in the pathophysiology of acute
decompensated heart failure. Curr Heart Fail Rep. 10:139–146. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tulassay T, Seri I and Rascher W: Atrial
natriuretic peptide and extracellular volume contraction after
birth. Acta Paediatr Scand. 76:444–446. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Samson WK: Atrial natriuretic factor
inhibits dehydration and hemorrhage-induced vasopressin release.
Neuroendocrinology. 40:277–279. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren X, Xu C, Zhan C, Yang Y, Shi L, Wang
F, Wang C, Xia Y, Yang B, Wu G, et al: Identification of NPPA
variants associated with atrial fibrillation in a Chinese GeneID
population. Clin Chim Acta. 411:481–485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maeda K, Tsutamoto T, Wada A, Hisanaga T
and Kinoshita M: Plasma brain natriuretic peptide as a biochemical
marker of high left ventricular end-diastolic pressure in patients
with symptomatic left ventricular dysfunction. Am Heart J.
135:825–832. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seronde MF, Gayat E, Logeart D, Lassus J,
Laribi S, Boukef R, Sibellas F, Launay JM, Manivet P, Sadoune M, et
al: Comparison of the diagnostic and prognostic values of B-type
and atrial-type natriuretic peptides in acute heart failure. Int J
cardiol. 168:3404–3411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Potocki M, Breidthardt T, Reichlin T,
Hartwiger S, Morgenthaler NG, Bergmann A, Noveanu M, Freidank H,
Taegtmeyer AB, Wetzel K, et al: Comparison of midregional
pro-atrial natriuretic peptide with N-terminal pro-B-type
natriuretic peptide in the diagnosis of heart failure. J Intern
Med. 267:119–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lip GY and Gibbs CR: Does heart failure
confer a hypercoagulable state? Virchow's triad revisited. J Am
Coll Cardiol. 33:1424–1426. 1999.PubMed/NCBI
|
|
30
|
Rengo G, Pagano G, Squizzato A, Moja L,
Femminella GD, de Lucia C, Komici K, Parisi V, Savarese G, Ferrara
N, et al: Oral anticoagulation therapy in heart failure patients in
sinus rhythm: A systematic review and meta-analysis. PLoS One.
8:e529522013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Camp NP, Jones SD, Liebeschuetz JW, et al:
Serine protease inhibitors. Journal. 2005.
|
|
32
|
Askari AT, Brennan ML, Zhou X, Drinko J,
Morehead A, Thomas JD, Topol EJ, Hazen SL and Penn MS:
Myeloperoxidase and plasminogen activator inhibitor 1 play a
central role in ventricular remodeling after myocardial infarction.
J Exp Med. 197:615–624. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Z, Castellino FJ and Ploplis VA:
Plasminogen activator inhibitor-1 (PAI-1) is cardioprotective in
mice by maintaining microvascular integrity and cardiac
architecture. Blood. 115:2038–2047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Spiroski I, Kedev S, Antov S, Trajkov D,
Petlichkovski A, Dzhekova-Stojkova S, Kostovska S and Spiroski M:
Investigation of SERPINE1 genetic polymorphism in Macedonian
patients with occlusive artery disease and deep vein thrombosis.
Kardiol Pol. 67:1088–1094. 2009.PubMed/NCBI
|
|
35
|
Iwanaga Y, Aoyama T, Kihara Y, Onozawa Y,
Yoneda T and Sasayama S: Excessive activation of matrix
metalloproteinases coincides with left ventricular remodeling
during transition from hypertrophy to heart failure in hypertensive
rats. J Am Coll Cardiol. 39:1384–1391. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Janicki JS and Brower GL: The role of
myocardial fibrillar collagen in ventricular remodeling and
function. J Card Fail. 8(Suppl 6): S319–S325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng J, Chen Y, Pat B, Dell'italia LA,
Tillson M, Dillon AR, Powell PC, Shi K, Shah N, Denney T, et al:
Microarray identifies extensive downregulation of noncollagen
extracellular matrix and profibrotic growth factor genes in chronic
isolated mitral regurgitation in the dog. Circulation.
119:2086–2095. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chatila K, Ren G, Xia Y, Huebener P, Bujak
M and Frangogiannis NG: The role of the thrombospondins in healing
myocardial infarcts. Cardiovasc Hematol Agents Med Chem. 5:21–27.
2007. View Article : Google Scholar : PubMed/NCBI
|