Introduction
Typically, genetic mutations are closely associated
with retinal degenerative diseases in humans, such as retinitis
pigmentosa (RP) which are a group of inherited retinal dystrophic
diseases that cause retinal cell apoptosis and irreversible loss of
vision (1,2). Although it is known that >50 million
individuals worldwide have been affected by retinal degenerative
diseases since the 1980's (3), there
is currently no effective treatment method, due to the lack of
understanding of their complex genetic mechanisms (4). As has been reported in a mouse model,
photoreceptor cell apoptosis is a common end stage in retinal
degeneration, although the detailed mechanisms of this process
remain unclear (5).
N-methyl-N-nitrosourea (MNU) is a strong alkylating agent that
belongs to the nitrose compounds of nitrosamine, and may induce
photoreceptor cell apoptosis with high selectivity and
repeatability. As a result, it is frequently used to construct
animal models of RP (6,7).
Donepezil is one of the second generation specific
reversible central acetylcholinesterase (AChE) inhibitors, and is
widely used for improving cognitive performance and global function
in patients with moderate Alzheimers disease (8). Numerous studies have demonstrated that
donepezil serves a protective role in cortical neurons by acting on
nicotinic acetylcholine receptors (AChR) or the toadstool
cholinergic receptor pathway (9,10). In
addition, research has suggested that donepezil exerts a protective
effect on retinal ganglion cells (RGCs) in vitro and in
vivo (11). However, Miki et
al (12) reported that a
nicotinic AChR (nAChR) inhibitor and a toadstool cholinergic
receptor inhibitor did not offset the neuroprotective effect of
donepezil. This implies that the precise neuroprotective mechanisms
of donepezil are complex and remain controversial. Therefore, more
research is required to determine the effect and mechanisms of
donepezil acting on on photoreceptor cells (13). It has been demonstrated that AChE
inhibitors lead to increased mRNA expression levels of heat shock
protein 70 (Hsp70), resulting in enhanced cellular defenses in
neurons (14). Therefore, it can be
hypothesized that one of the putative neuroprotective mechanisms of
AChE inhibitors is mediated through Hsp70.
HSPs are highly conserved within their family, and
are stress activated proteins that participate in protein folding
and repair (15). The biological
functions of HSPs include acting as a molecular chaperone,
providing cell protection, anti-inflammatory properties, and
preventing apoptosis and cell damage. In particular, the 70-kDa
HSP, Hsp70, a molecular chaperone, serves a pivotal role in
protecting cells against the stresses of various types and origins.
Tytell et al (16) observed
that Hsp70 mRNA and protein levels were significantly increased in
rat retinal photoreceptor cell layers following whole body
hyperthermia. The work by Tytell et al (16), for the first time, identified that
the photoreceptor cell layer was an important part of the retina
for the expression of Hsp70, and that Hsp70 induced by hyperthermia
serves a protective role by applying its anti-apoptotic properties
to photoreceptors against heat-induced damage. At present, the
anti-apoptotic mechanisms of Hsp70 have been drawing increasing
attention, since Hsp70 was shown to interfere with apoptosis by
affecting cytochrome c release from mitochondria and
initiator caspase activation (17–19). In
addition, it has been determined that the release of cytochrome
c can be blocked by the anti-apoptotic protein Bcl-2, which
is heavily involved in cell survival (20). Kelly et al (21) observed that viral vector-mediated
Hsp70 gene transfer can increase B-cell lymphoma 2 (Bcl-2)
expression in neurons in rat brains. These studies suggest that the
anti-apoptotic effects of HSP 70 are closely associated with the
expression of Bcl-2. In the present study, the effect of donepezil
on MNU-induced photoreceptor cell apoptosis in mice was
investigated and the mechanisms behind this process were
explored.
Materials and methods
Materials and reagents
MNU and donepezil were purchased from Sigma-Aldrich
(St. Louis, MO, USA). HSP inhibitor I was purchased from Merck
Millipore (Darmstadt, Germany), ABT-199 (a Bcl-2 inhibitor) was
purchased from Selleck Chemicals (Houston, TX, USA) and the
Apoptosis Detection kit was provided by BD Biosciences (San Jose,
CA, USA).
Animals
A total of 168 male C57BL/6 mice (age, 7–9 weeks;
weight, 20–22 g; Hunan Laboratory Animal Co., Ltd, Changsha, China)
were housed under standard laboratory conditions, and provided with
standard rodent chow and free access to water with a 12 h
light-dark cycle at 23°C. The present study was approved by the
ethics committee of the Affiliated Eye Hospital of Nanchang
University (Nanchang, China).
Morphological observation
In order to investigate the loss of photoreceptor
cells induced by MNU, 30 mice were intraperitoneally injected with
MNU (60 mg/kg in saline) and 6 mice per day were sacrificed by
cervical dislocation after 0, 1, 3, 5 and 7 days. In addition, the
ability of donepezil to protect against MNU-induced photoreceptor
loss was investigated. Briefly, 24 mice were divided into four
groups, as follows (6 mice/group): i) Control; ii) MNU; iii)
donepezil plus MNU; and iv) Hsp70 inhibitor plus donepezil plus
MNU. The mice in the Hsp70 inhibitor plus donepezil plus MNU group
were anesthetized by intraperitoneal injection with sodium
pentobarbital (30–40 mg/kg body weight; Shanghai Qiao Xing Trading,
Co., Ltd., Shanghai, China), prior to intravitreal injection with 5
µl HSP inhibitor I using a Hamilton microsyringe (Hamilton
Robotics, Reno, NV, USA) with a 33 G needle. At 1 day following HSP
inhibitor I administration, the mice underwent oral gavage with
donepezil (10 mg/kg body weight) for 3 consecutive days, followed
by intraperitoneal injection with 60 mg/kg MNU.
Following sacrifice, the mouse eyes were harvested
and fixed in 4% paraformaldehyde overnight at 4°C, then dehydrated
using alcohol, made transparent using xylene (Shanghai Sheng Jun
Industrial Investment, Co., Ltd., Shanghai, China) and embedded in
paraffin (Shanghai Yu Jie Trade, Co., Ltd., Shanghai, China).
Retinal sections were cut along the optic nerve at 12 µm thickness
and mounted on Superfrost Plus glass slides (Yancheng Hongda
Medical Instrument Co., Ltd., Jiangsu, China). Tissue sections were
then dehydrated with 75, 95 and 100% alcohol for 1 min each, and
then with xylene for 5 min. Hematoxylin and eosin (H&E;
Shanghai Blue Skies Biological Technology, Co., Ltd., Shanghai,
China) staining of transverse sections was used to evaluate the
thickness of the retina [photoreceptor inner segment (IS), outer
nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear
layer (INL) or ganglion cell layer (GCL)] under a microscope
(BX-53; Olympus Corporation, Tokyo, Japan).
Immunofluorescence
In order to investigate the effects of donepezil on
the expression of Hsp70, 18 mice were randomly divided into three
groups, as follows (6 mice/group): i) Control; ii) donepezil; and
iii) donepezil plus Hsp70 inhibitor groups. The mice were
anesthetized by intraperitoneal injection with sodium pentobarbital
(30–40 mg/kg body weight) prior to intravitreal injection with HSP
inhibitor I (5 µl) and oral gavage with donepezil (10 mg/kg body
weight) for 3 consecutive days. Subsequent to washing and blocking
with fetal bovine serum (Sigma-Aldrich), retinal sections were
incubated with rabbit anti-mouse Hsp70 monoclonal antibodies (1:50;
ab45133; Abcam, Cambridge, MA, USA) at 4°C overnight. Following
incubation, the sections were washed three times for 5 min each in
the dark with phosphate-buffered saline. Subsequently, the sections
were incubated with biotin-conjugated mouse anti-IgG-B monoclonal
antibody (1:200; sc-2491; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) for 1 h at 37°C in the dark. A fluorescence microscope
(BX-53; Olympus Corporation) was used to capture and measure the
retinal sections two to three disc diameters from the optic nerve.
Two areas per section of ONL were randomly selected and the
fluorescence intensity was measured using ImageJ software 1.46
(National Institutes of Health, Bethesda, MD, USA).
Western blot analysis
In order to investigate the concentration-dependent
effect of donepezil on the protein expression levels of Hsp70, 24
mice were randomly divided into four groups, according to the dose
of donepezil administered, as follows (6 mice/group): i) Control;
ii) 1 mg/kg donepezil; iii) 5 mg/kg donepezil; and iv) 10 mg/kg
donepezil. The mice were treated with donepezil for 3 consecutive
days. Furthermore, the time-dependent effect of donepezil treatment
on the protein expression of Hsp70 was investigated by oral gavage
with 10 mg/kg body weight donepezil for 1, 3 or 5 days. In
addition, the effect of donepezil-induced Hsp70 expression on the
protein expression levels of Bcl-2 were examined. Briefly, 24 mice
were divided into four groups, as follows (6 mice/group): i)
Control; ii) donepezil (10 mg/kg body weight); iii) donepezil plus
Hsp70 inhibitor; and iv) donepezil plus Bcl-2 inhibitor. The mice
in the donepezil plus Bcl-2 inhibitor group were orally gavaged
with 100 mg/kg body weight ABT-199 for 3 consecutive days prior to
treatment with 10 mg/kg donepezil. All mice were sacrificed by
cervical dislocation.
The protein concentration in each sample was
determined using a Bradford Protein Assay. The retinas were
isolated and equal quantities of total protein from each sample
were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and then transferred onto a polyvinylidene fluoride
membrane (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Subsequent to blocking of nonspecific binding sites with 5% skimmed
milk in Tris-buffered saline (TBS; pH 7.6) for 1 h, membranes were
incubated with primary antibodies, as follows: Rabbit anti-Hsp70
monoclonal antibody (1:1,000; Abcam), rabbit anti-Bcl-2 polyclonal
antibody (1:1,000; ab59348; Abcam) and rabbit anti-β-actin
monoclonal antibody (1:1,000; ab8226; Abcam) overnight at 4°C.
Next, the membranes were washed with 0.15% Tween 20 in TBS and
incubated with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit (bs-0295G; BIOSS, Beijing, China) or biotin-conjugated
rabbit anti-mouse (sc-358943; Santa Cruz Biotechnology, Inc.)
secondary antibodies for 1 h at room temperature. After four
washes, the proteins were detected using enhanced chemiluminescence
(ECL kit, Thermo Fisher Scientific, Inc.). The bands on the gel
were quantified using Quantity One software, version 4.6.2 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). β-actin served as the
internal control. All experiments were repeated a minimum of three
times.
Flow cytometry
The ability of donepezil to protect against
MNU-induced apoptosis of photoreceptor cells was investigated by
flow cytometry. Following the morphological analysis, mouse eyes
were enucleated, retinas were separated from retinal pigment
epithelium and choroid and then rinsed immediately in D-Hank liquid
(Beijing TransGen Biotech Co., Ltd., Beijing, China) twice. Tissue
was cut using eye scissors, softly ground, and all large blood
vessels were removed. The dispersed cells were filtered through a
300-mesh stainless steel sieve, and the cell suspension was
cultured in an incubator at 37°C with 25% CO2 for 1 day.
Next, 10 µl Annexin V phycoerythrin/7-aminoactinomycin (PE/7-AAD;
BD Biosciences) was added into the cell suspension liquid at room
temperature in the dark for 10 min. Finally, samples were analyzed
using flow cytometry (FACSCalibur; Bio-Rad Laboratories, Inc.)
within 1 h of staining.
Statistical analysis
Data are expressed as the mean ± standard deviation
of three to five experiments. Statistical differences were analyzed
by Students t-test using SPSS Software version 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
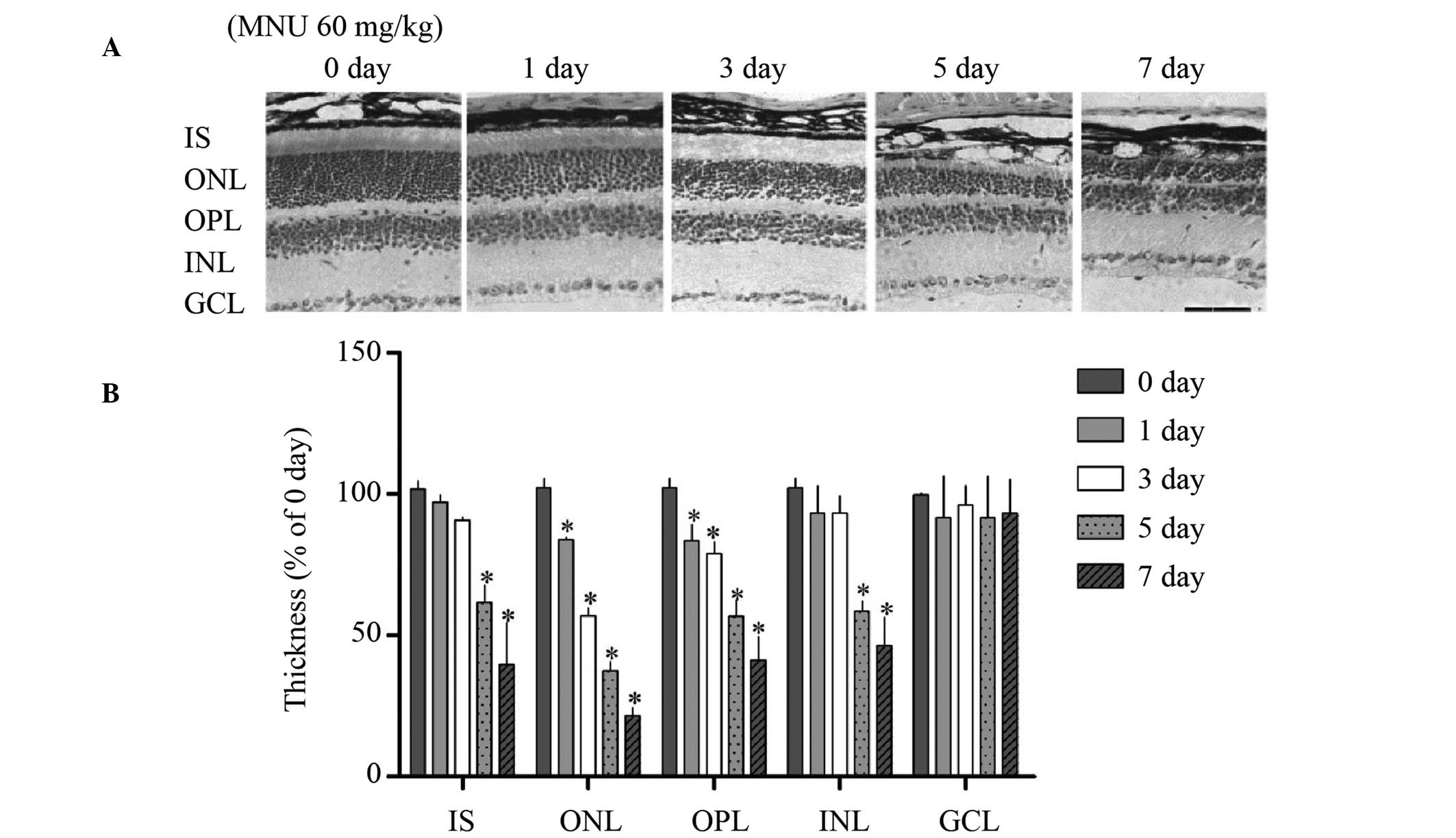
Intraperitoneal treatment with MNU
induces loss of photoreceptor cells
Typical photomicrographs of mouse retinal sections
treated with MNU (60 mg/kg) and stained with H&E are presented
in Fig. 1A. Results demonstrated
that the overall retinal thickness significantly reduced subsequent
to MNU treatment as a result of the marked degeneration of ONL
(P<0.01). The ONL was significantly reduced in thickness by day
3 and was further reduced by day 7 when compared with control
retinas (control, day 0; P<0.01). The OPL was significantly
thinner on days 1, 3, 5 and 7, whereas the INL became significantly
thinner between 5 and 7 days (Fig.
1B; P<0.01), however no alterations in the ganglion cell
layer were observed following MNU treatment for 7 days.
Donepezil increases the expression
levels of Hsp70, as determined using immunofluorescence
staining
It has been reported that Hsp70, induced by valproic
acid, can protect photoreceptors against MNU-induced cell loss
(19). In addition, one report
demonstrated that donepezil can induce the expression of Hsp70 in
neurons, creating a neuroprotective effect (22). To determine whether donepezil
treatment can increase the promoter activity and the distribution
pattern of Hsp70 within the retina, immunofluorescence was
performed. In the control group, the expression of Hsp70 was
faintly observed in the retinal IS and ONL. However, strong Hsp70
immunoreactivity was observed in the IS and ONL after donepezil
pretreatment for 3 days (Fig.
2Aa-i), and the expression of Hsp70 was significantly reduced
subsequent to administration of HSP inhibitor I when compared with
the donepezil and control groups (P<0.01; Fig. 2A and B).
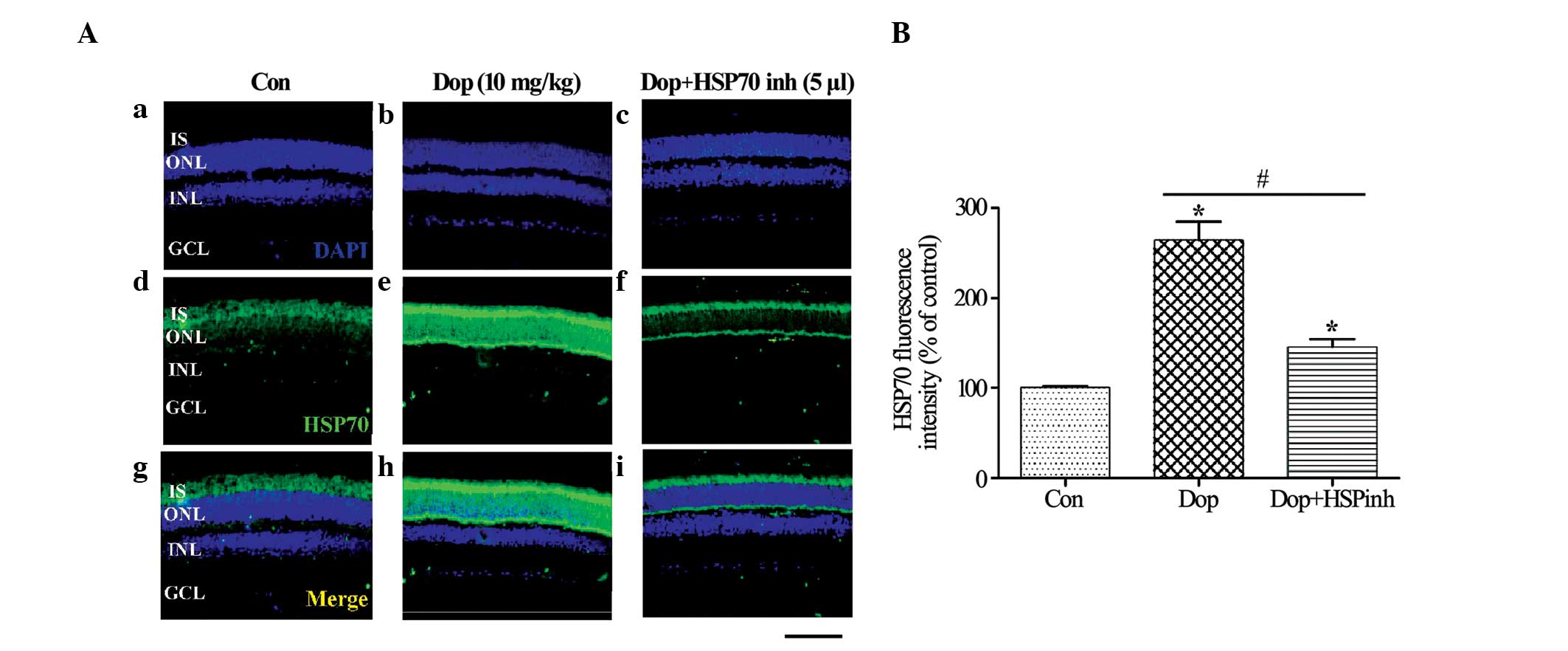 | Figure 2.Influence of Dop on the expression
levels of Hsp70 by immunofluorescence staining. (A)
Immunohistochemistry for Hsp70 in the mouse retina. (Aa-c) DAPI
(blue) staining, (Ad-f) Hsp70 (green) staining, (Ag-i) merged image
of DAPI and Hsp70 [(a, d, g) vehicle Con, (b, e, h) pretreatment
with 10 mg/kg body weight Dop for 3 days and (c, f, i) HSP inh +
pretreatment with Dop for 3 days]. (B) Hsp70 expression quantified
by analysis of fluorescence intensity. Scale bar, 50 µm, *P<0.01
vs. vehicle Con, #P<0.01 vs. donepezil. Con, control;
Dop, donepezil; HSP inh, HSP inhibitor. HSP, heat shock protein;
IS, photoreceptor inner segment; ONL, outer nuclear layer; OPL,
outer plexiform layer; INL, inner nuclear layer; GCL, ganglion cell
layer. |
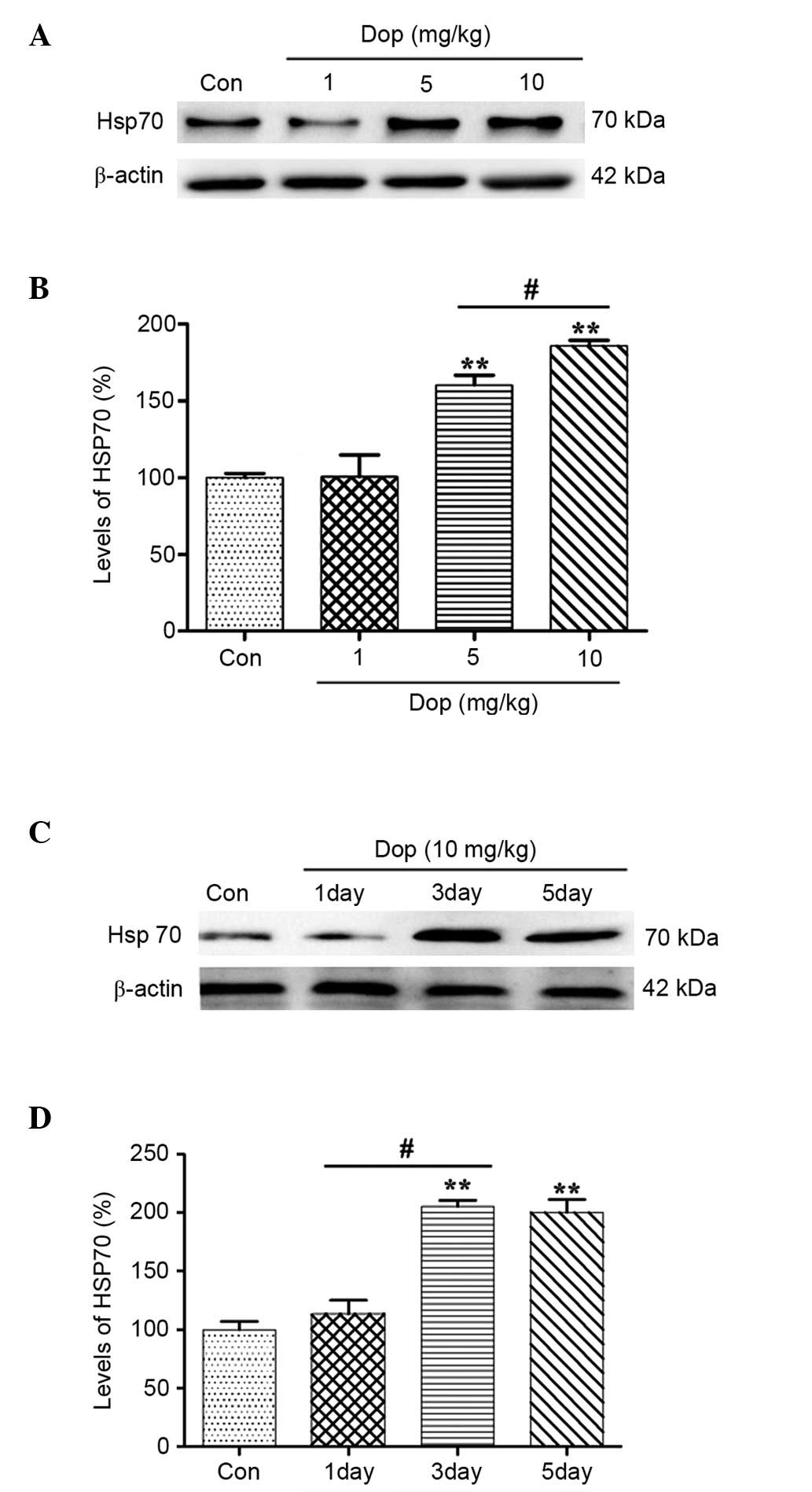
Donepezil induces Hsp 70 expression in
a time- and dose-dependent manner, as determined by western blot
analysis
A previous study demonstrated that an AChE inhibitor
was able to induce the expression of Hsp70 in neurons, thus
exerting neuroprotective effects (14). In order to determine whether
administration of donepezil induces an increase in Hsp70 expression
levels in the mouse retina, western blot analysis was performed.
Mice were orally gavaged with donepezil at 1, 5 and 10 mg/kg for 3
consecutive days prior to MNU treatment. Results demonstrated that
donepezil treatment induces an increase in Hsp70 protein expression
in a dose-dependent manner. The expression of Hsp70 was
significantly increased following 5 and 10 mg/kg donepezil
treatment, as compared with the control (Fig. 3A and B; control, 0-fold; 5 mg/kg,
0.2-fold; 10 mg/kg, 1.9-fold; P<0.01). In addition, pretreatment
with donepezil for 3 days significantly increased Hsp70 protein
levels 2.1-fold in comparison with the control group (Fig. 3C and D; control, 0-fold; 3 days,
2.1-fold; 5 days, 2.0-fold; P<0.01). The observations of the
current study therefore demonstrate that donepezil can induce Hsp70
expression in a dose- and time-dependent manner.
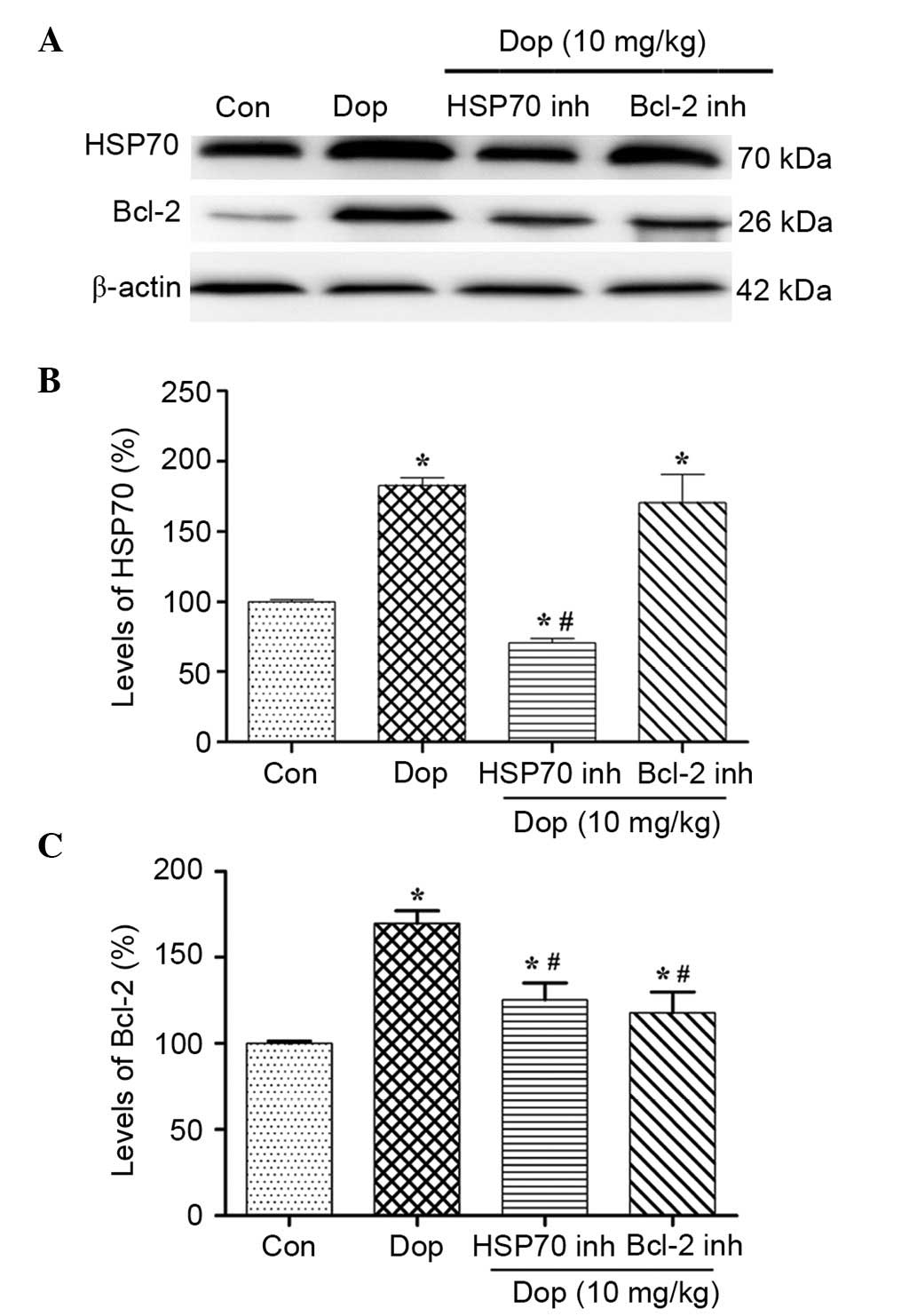
Expression levels of Bcl-2 are
associated with donepezil-induced Hsp70
Although numerous studies have reported that Hsp70
serves a vital role in protecting retinal cells (23,24), the
precise mechanisms of this process remain unclear. Western blotting
demonstrated that the protein expression levels of Hsp70 and Bcl-2
were increased following 3 days pretreatment with 10 mg/kg
donepezil (Fig. 4A), and this was
shown to be significant by densitometric analyses (P<0.01;
Fig. 4B and C). Furthermore,
pretreatment with HSP inhibitor I resulted in a significant
reduction in the expression levels of Hsp70 and Bcl-2, as compared
with the donepezil group (P<0.01; Fig. 4). In addition, the Bcl-2 inhibitor
could only reduce the expression levels of Bcl-2 (Fig. 4A and C), whereas the expression
levels of Hsp70 were not significantly altered (Fig. 4A and B). These results suggested that
Hsp70 induced by donepezil may upregulate the expression levels of
Bcl-2, and that pretreatment with an HSP inhibitor was able to
reduce the interaction between Hsp70 and Bcl-2.
Donepezil prevents photoreceptor cell
death caused by MNU by increasing Hsp70 expression in mice
To further elucidate the role of donepezil in
photoreceptor apoptosis and the possible mechanisms underlying this
process, H&E staining was performed to evaluate the effect of
donepezil on MNU-induced alterations in ONL thickness. Typical
photomicrographs with H&E staining demonstrated that the ONL
was significantly thinner in MNU-treated retinas, as compared with
the control (P<0.01; Fig. 5A–C).
Furthermore, pretreatment with donepezil for 3 days significantly
increased the thickness of the ONL, as compared with the MNU group
(P<0.01; Fig. 5A, C and D).
Conversely, pretreatment with the HSP inhibitor I significantly
attenuated the protective effects of donepezil (P<0.01; Fig. 5A, D and E).
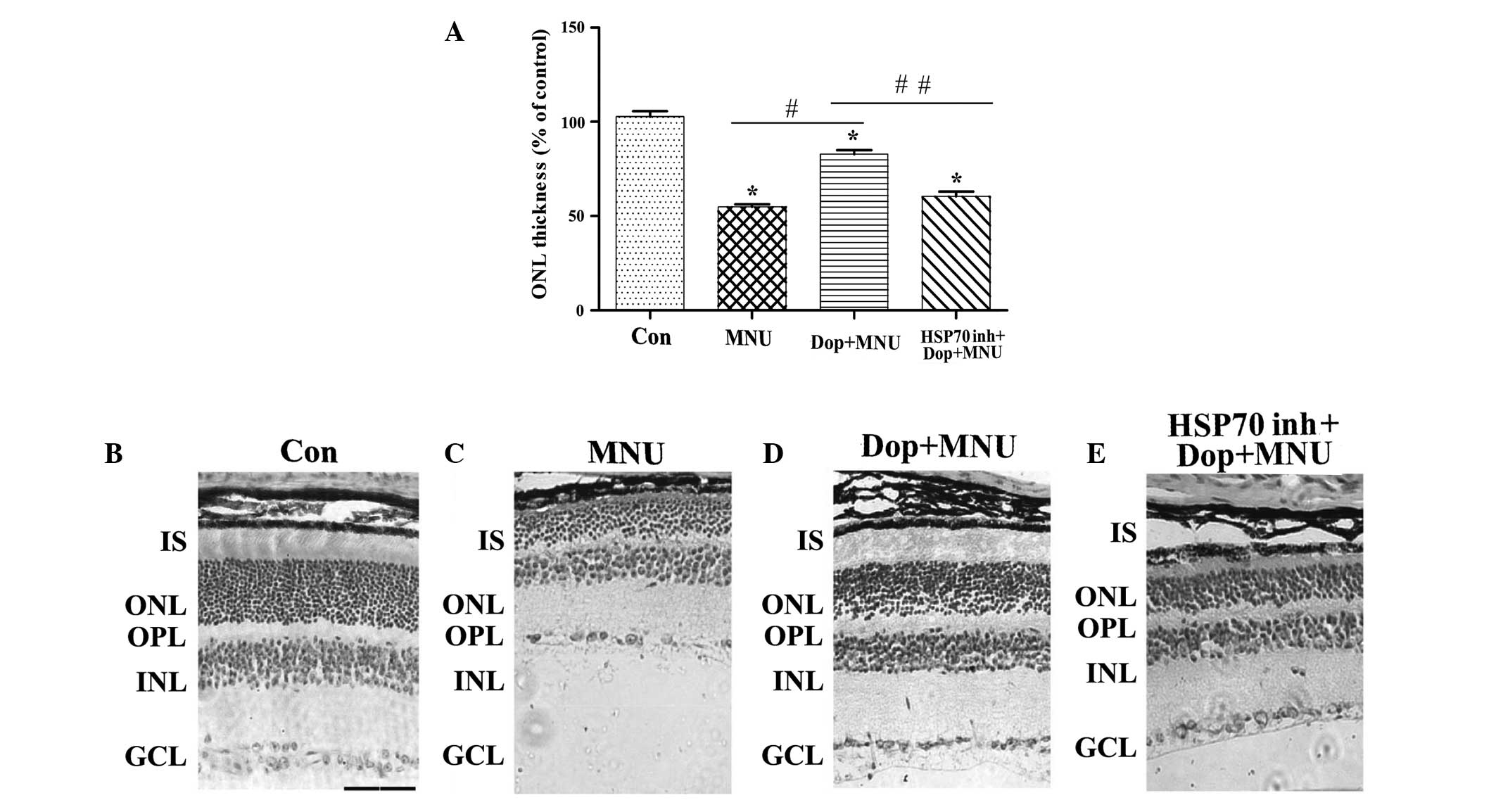 | Figure 5.Dop protects photoreceptor cells
against MNU in mice. (A) The thickness of the ONL in the retina of
the mice. Data are presented as the mean ± standard deviation.
*P<0.01 vs. con, #P<0.01 vs. MNU,
##P<0.01 vs. Dop + MNU (n=6). Microscopic images of
the retina of the mice treated with (B) Con, (C) MNU (pretreatment
for 3 days), (D) Dop + MNU (pretreatment for 3 days) and (E) Hsp70
inhibitor + Dop + MNU (pretreatment for 3 days). Scale bar, 50 µm.
Hematoxylin and eosin staining. Con, control; MNU,
N-methyl-N-nitrosourea; Dop, dopenezil; HSP70 inh, heat shock
protein 70 inhibitor; IS, photoreceptor inner segment; ONL, outer
nuclear layer; OPL, outer plexiform layer; INL, inner nuclear
layer; GCL, ganglion cell layer. |
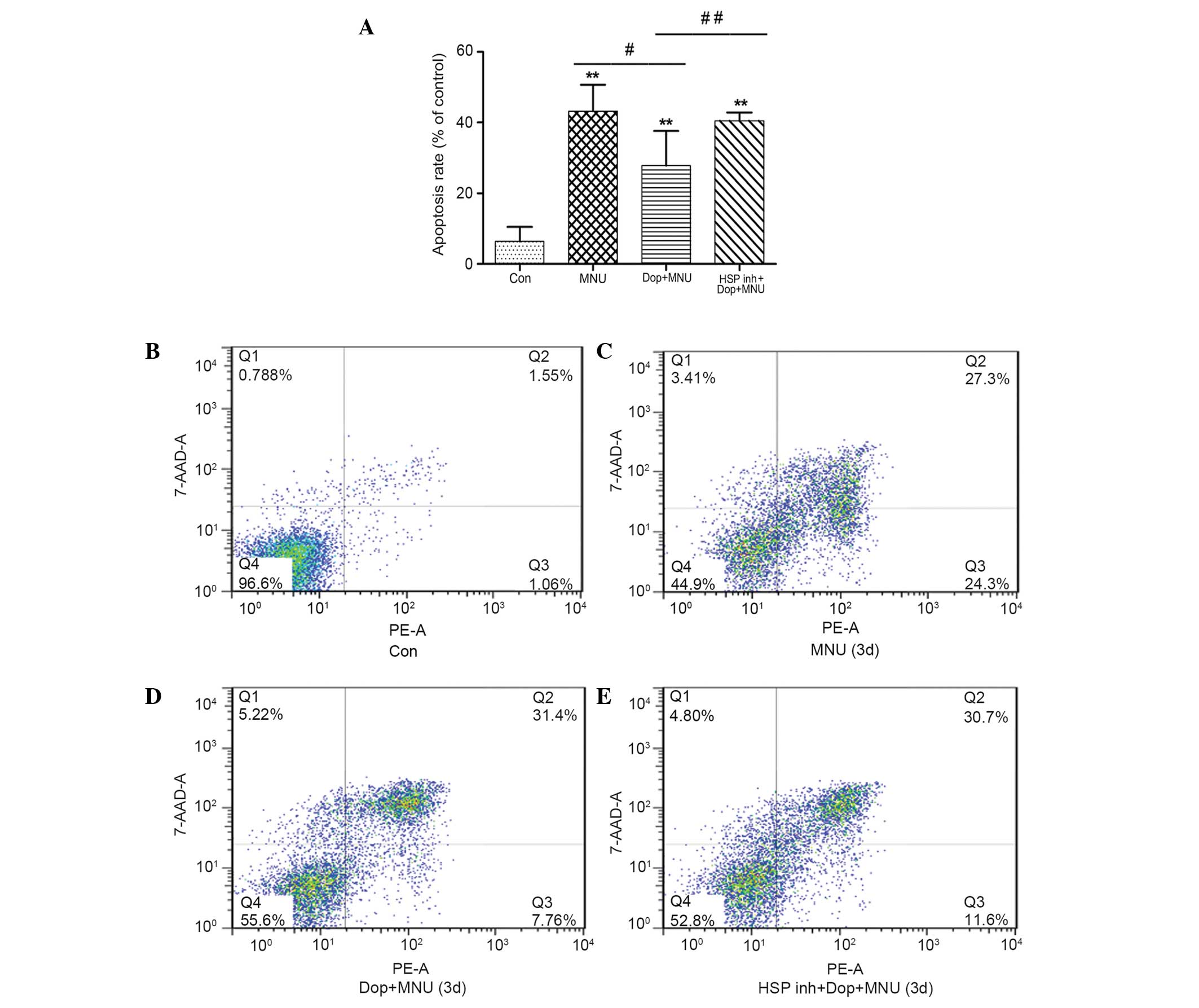
The rate of photoreceptor cell apoptosis was
detected by double labeling the cells with Annexin V-PE/7-AAD. The
apoptotic rate in the MNU group (43.2±7.4%) was significantly
increased, as compared with the control group (6.4±4.1%; P<0.01;
Fig. 6A–C). Furthermore, the
apoptotic rate in the donepezil plus MNU group was 29.4±4.1%, which
was significantly reduced, as compared with the MNU group
(P<0.05; Fig. 6A, C and D).
However, the protective effects of donepezil were partially
attenuated by the administration of HSP inhibitor I; the apoptotic
rate in the HSP inhibitor group was significantly increased
(40.6±2.3%), as compared with the donepezil plus MNU group
(P<0.05; Fig. 6A, D and E).
Notably, donepezil did not affect the number of the cells in the
late apoptotic stage; this may be a result of mechanical damage in
the process of cell separation which led to an increased number of
cells in the late apoptotic stage.
Discussion
Retinal degenerative diseases, such as inherited RP,
remain a challenge for investigators and clinicians in the field of
ophthalmology. Currently, it is evident that retinal degeneration
is an apoptotic event involving complex crosstalk and
interconnected signals (25),
however, improved animal models that fully mimic human retinal
degenerative diseases are required in order to thoroughly
understand the mechanism. Numerous researchers have summarized that
MNU causes photoreceptor cell apoptosis in golden hamsters in 7
days (7,26,27), and
that injection of MNU at 60 mg/kg into mice and rats results in
photoreceptor cell loss and exhaustion within one week (19). In the present study, the data
indicated a similar time course of photoreceptor cell apoptosis
subsequent to MNU treatment. It was observed that the thickness of
photoreceptor IS and ONL in the retina were significantly reduced
following intraperitoneal injection of MNU (60 mg/kg) after 3 days,
with no alterations observed in the ganglion cell layer after 7
days. It can therefore be concluded that MNU selectively damages
photoreceptor cells.
Donepezil is a potent AChE inhibitor that is widely
used in the treatment of Alzheimers disease, and has been reported
to exert a number of beneficial functions. In previous studies,
donepezil was reported to protect against cell damage induced by
oxygen-glucose deprivation in rat pheochromocytoma cells (28), and increase glutathione and reduce
malondialdehyde levels in a rat model of dementia (29). In addition, one study indicated that
donepezil exhibits neuroprotective effects on cerebral and optic
nerves by improving the blood flow in patients with normal-tension
glaucoma (22). However, the
mechanisms underlying the neuroprotective effects of donepezil
remain unclear. Sakamoto et al (30) observed that the activation of AChRs
and a mechanism unrelated to AChE inhibition contributes to the
protective effect of donepezil. In experiments using rat cortical
neurons, Takada et al (9)
showed that the neuroprotection afforded by donepezil was prevented
by methyllycaconitine (MLA), an α7-selective nAChR antagonist, but
not by scopolamine, a muscarinic (m)AChR antagonist. These results
were consistent with a previous study (31), which observed that the
neuroprotective effect of donepezil was reversed by MLA in human
neuroblastoma cells and that donepezil exerted neuroprotective
effects via nAChRs. Conversely, Miki et al (12) reported that both mecamylamine, a
nAChR antagonist, and scopolamine, were unable to affect the
neuroprotective effect of donepezil on RGCs in vitro. These
findings suggested that the activation of mAChRs or nAChRs may not
be the key mechanism underlying neuroprotection in RGCs. Narimatsu
et al (32) suggested that
donepezil may improve cognitive function in mice by increasing the
hippocampal production of insulin-like growth factor-I via sensory
neuron stimulation. Therefore, the mechanism underlying the
neuroprotective effects of donepezil is currently unclear, and
remains to be clarified. An additional study demonstrated that the
AChE inhibitor rivastigmine was able to enhance cellular defenses
in neurons by upregulating Hsp70 mRNA levels (14). Therefore, it can be hypothesized that
the mechanisms underlying the neuroprotective effects of AChE
inhibitors may be mediated via the expression of Hsp70. In the
present study, an increase in Hsp70 protein expression was observed
following donepezil pretreatment in MNU-induced photoreceptor cell
apoptosis mice.
The present study demonstrated that donepezil was
able to induce the protein expression of Hsp70, which is strongly
associated with cell survival. A previous study indicated that
Hsp70 is critical for the photoreceptor stress response after
retinal detachment (13), however
further investigation is required to determine the mechanisms of
interaction between Hsp70 and photoreceptor cell death. Hsp70
assists in denatured protein folding to repair DNA damage,
transfers irreversibly damaged proteins to protein degradation
system and allows cellular adaptation, which is necessary for cell
survival (33), and numerous reports
have demonstrated that Hsp70 has manifold anti-apoptotic effects
both upstream and downstream of caspase activation (13,34,35). For
example, upregulation of Hsp70 can increase the expression of the
major anti-apoptotic protein Bcl-2 (36), which interferes with the release of
cytochrome c, the oligomerization of apoptotic protease
activating factor-1 and the activation of caspase-9, thereby
preventing the aggregation of the apoptosome (37). Several studies have highlighted that
the overexpression of Hsp70 is associated with reduced apoptotic
cell death and a high expression level of the anti-apoptotic
protein Bcl-2 (21,38,39).
Proteins of the Bcl-2 family are identified as being
fundamental in the execution of the mitochondrial pathway of
apoptosis (40,41). The regulation of active anti- and
pro-apoptotic Bcl-2 family member proteins is critical for
determining the fate of cells (40,42).
Therefore, aberrant expression of these proteins can cause various
diseases associated with apoptosis, such as retinal dystrophy
(43,44). It is widely accepted that the
Bcl-2-associated signaling pathway is involved in the apoptosis of
photoreceptors in the Rpe65−/− murine model of Leber's
congenital amaurosis (45).
Eversole-Cire et al (46)
observed that overexpression of the Bcl-2 associated X protein
(Bax) protein can aggravate retinal photoreceptor cell apoptosis,
however that Bcl-2 overexpression alone can inhibit apoptosis.
However, the definite anti-apoptotic mechanism of the Bcl-2 family
of proteins in retinal degeneration remains unclear. Further
investigation has confirmed that donepezil reduces the Bax/Bcl-2
ratio during ischemia/reperfusion (47), and that Hsp70 inhibits heat-induced
apoptosis upstream of mitochondria by restraining Bax translocation
(39).
In the present study, a photoreceptor apoptosis
mouse model was established with an intraperitoneal injection of
MNU. Immunofluorescence results demonstrated that donepezil
increased the expression levels of Hsp70 in the IS and ONL.
Furthermore, a western blot analysis investigated the time- and
concentration-dependent effects of donepezil on the expression
levels of Hsp70, and determined that donepezil (10 mg/kg) treatment
results in a marked increase in the expression levels of Hsp70,
when compared with lower dosage groups, and that donepezil
pretreatment for 3 days leads to a significant increase in Hsp70
expression levels. In addition, it was observed that a Bcl-2
inhibitor could not significantly reduce the expression of Hsp70
induced by donepezil, but was able to reduce the expression of
Bcl-2. Subsequent to binding of the Bcl-2 inhibitor (ABT-199) to
Bcl-2, the sensitivity of Bcl-2 bound to primary antibodies was
reduced. This may be the reason why the Bcl-2 inhibitor reduced the
expression level of Bcl-2 when the western blot analysis was
performed. To conclude, the results from the present study suggest
that Hsp70 can promote photoreceptor cell survival which is closely
associated with the expression levels of Bcl-2. Therefore, it is
suggested that Hsp70, induced by donepezil, can resist MNU-induced
photoreceptor cell apoptosis, which may be partially offset by an
HSP inhibitor. To the best of our knowledge, this is the first
study that demonstrates the key role of donepezil in inhibiting
MNU-induced photoreceptor cell apoptosis associated with Hsp70.
In conclusion, the present study demonstrated that
donepezil can prevent photoreceptor cell apoptosis, induced by MNU
treatment, via upregulating the expression of Hsp70 and Bcl-2.
Further studies are required in order to clarify the possible
mechanisms of interaction between donepezil, Hsp70 and Bcl-2. The
results from the present study indicate that donepezil has the
potential to be used as a novel therapeutic agent for the treatment
of RP.
References
|
1
|
van Soest S, Westerveld A, de Jong PT,
Bleeker-Wagemaker EM and Bergen AA: Retinitis pigmentosa: Defined
from a molecular point of view. Surv Ophthalmol. 43:321–334. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hartong DT, Berson EL and Dryja TP:
Retinitis pigmentosa. Lancet. 368:1795–1809. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pagon RA: Retinitis pigmentosa. Surv
Ophthalmol. 33:137–177. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rossmiller B, Mao H and Lewin AS: Gene
therapy in animal models of autosomal dominant retinitis
pigmentosa. Mol Vis. 18:2479–2496. 2012.PubMed/NCBI
|
|
5
|
Emoto Y, Yoshizawa K, Kinoshita Y, Yuri T,
Yuki M, Sayama K, Shikata N and Tsubura A: Green tea extract
suppresses N-methyl-N-nitrosourea-induced photoreceptor apoptosis
in Sprague-Dawley rats. Graefes Arch Clin Exp Ophthalmol.
252:1377–1384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshizawa K, Nambu H, Yang J, Oishi Y,
Senzaki H, Shikata N, Miki H and Tsubura A: Mechanisms of
photoreceptor cell apoptosis induced by N-methyl-N-nitrosourea in
Sprague-Dawley rats. Lab Invest. 79:1359–1367. 1999.PubMed/NCBI
|
|
7
|
Tsubura A, Yoshizawa K, Kuwata M and
Uehara N: Animal models for retinitis pigmentosa induced by MNU;
disease progression, mechanisms and therapeutic trials. Histol
Histopathol. 25:933–944. 2010.PubMed/NCBI
|
|
8
|
Rogers SL and Friedhoff LT: Long-term
efficacy and safety of donepezil in the treatment of Alzheimers
disease: An interim analysis of the results of a US multicentre
open label extension study. Eur Neuropsychopharmacol. 8:67–75.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takada Y, Yonezawa A, Kume T, Katsuki H,
Kaneko S, Sugimoto H and Akaike A: Nicotinic acetylcholine
receptor-mediated neuroprotection by donepezil against glutamate
neurotoxicity in rat cortical neurons. J Pharmacol Exp Ther.
306:772–777. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pereira SP, Medina SV and Araujo EG:
Cholinergic activity modulates the survival of retinal ganglion
cells in culture: The role of M1 muscarinic receptors. Int J Dev
Neurosci. 19:559–567. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akasofu S, Kosasa T, Kimura M and Kubota
A: Protective effect of donepezil in a primary culture of rat
cortical neurons exposed to oxygen-glucose deprivation. Eur J
Pharmacol. 472:57–63. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miki A, Otori Y, Morimoto T, Okada M and
Tano Y: Protective effect of donepezil on retinal ganglion cells
in vitro and in vivo. Curr Eye Res. 31:69–77. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kayama M, Nakazawa T, Thanos A, Morizane
Y, Murakami Y, Theodoropoulou S, Abe T, Vavvas D and Miller JW:
Heat shock protein 70 (HSP70) is critical for the photoreceptor
stress response after retinal detachment via modulating
anti-apoptotic Akt kinase. Am J Pathol. 178:1080–1091. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou X, Patel AR, Perez F and Jurivich DA:
Acteylcholinesterase inhibitor rivastigmine enhances cellular
defenses in neuronal and macrophage-like cell lines. Transl Res.
153:132–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Snoeckx LH, Cornelussen RN, Van
Nieuwenhoven FA, Reneman RS and Van Der Vusse GJ: Heat shock
proteins and cardiovascular pathophysiology. Physiol Rev.
81:1461–1497. 2001.PubMed/NCBI
|
|
16
|
Tytell M, Barbe MF and Brown IR: Induction
of heat shock (stress) protein 70 and its mRNA in the normal and
light-damaged rat retina after whole body hyperthermia. J Neurosci
Res. 38:19–31. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lanneau D, de Thonel A, Maurel S, Didelot
C and Garrido C: Apoptosis Versus Cell Differentiation: Role of
heat shock proteins HSP90, HSP70 and HSP27. Prion. 1:53–60. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Banerjee Mustafi S, Chakraborty PK, Dey RS
and Raha S: Heat stress upregulates chaperone heat shock protein 70
and antioxidant manganese superoxide dismutase through reactive
oxygen species (ROS), p38MAPK, and Akt. Cell Stress Chaperones.
14:579–589. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koriyama Y, Sugitani K, Ogai Km and Kato
S: Heat shock protein 70 induction by valproic acid delays
photoreceptor cell death by N-methyl-N-nitrosourea in mice. J
Neurochem. 130:707–719. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mosser DD, Caron AW, Bourget L, Meriin AB,
Sherman MY, Morimoto RI and Massie B: The chaperone function of
hsp70 is required for protection against stress-induced apoptosis.
Mol Cell Biol. 20:7146–7159. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kelly S, Zhang ZJ, Zhao H, Xu L, Giffard
RG, Sapolsky RM, Yenari MA and Steinberg GK: Gene transfer of HSP72
protects cornu ammonis 1 region of the hippocampus neurons from
global ischemia: Influence of Bcl-2. Ann Neurol. 52:160–167. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshida Y, Sugiyama T, Utsunomiya K, Ogura
Y and Ikeda T: A pilot study for the effects of donepezil therapy
on cerebral and optic nerve head blood flow, visual field defect in
normal-tension glaucoma. J Ocul Pharmacol Ther. 26:187–192. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwong JM, Lam TT and Caprioli J:
Hyperthermic pre-conditioning protects retinal neurons from
N-methyl-D-aspartate(NMDA)-induced apoptosis in rat. Brain Res.
970:119–130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barbe MF, Tytell M, Gower DJ and Welch WJ:
Hyperthermia protects against light damage in the rat retina.
Science. 241:1817–1820. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cottet S and Schorderet DF: Mechanisms of
apoptosis in retinitis pigmentosa. Curr Mol Med. 9:375–383. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Herrold KM: Pigmentary degeneration of the
retina induced by N-methyl-N-nitrosourea. An experimental study in
syrian hamsters. Arch Ophthalmol. 78:650–653. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshizawa K and Tsubura A: Characteristics
of N-methyl-N-nitrosourea-induced retinal degeneration in animals
and application for the therapy of human retinitis pigmentosa.
Nippon Ganka Gakkai Zasshi. 109:327–337. 2005.(In Japanese).
PubMed/NCBI
|
|
28
|
Zhou J Fu and Tang XC: Huperzine A and
donepezil protect rat pheochromocytoma cells against oxygen-glucose
deprivation. Neurosci Lett. 306:53–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saxena G, Singh SP, Agrawal R and Nath C:
Effect of donepezil and tacrine on oxidative stress in
intracerebral streptozotocin-induced model of dementia in mice. Eur
J Pharmacol. 581:283–289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sakamoto K, Ohki K, Saito M, Nakahara T
and Ishii K: Histological protection by donepezil against
neurodegeneration induced by ischemia-reperfusion in the rat
retina. J Pharmacol Sci. 112:327–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arias E, Gallego-Sandín S, Villarroya M,
García AG and López MG: Unequal neuroprotection afforded by the
acetylcholinesterase inhibitors galantamine, donepezil, and
rivastigmine in SH-SY5Y neuroblastoma cells: Role of nicotinic
receptors. J Pharmacol Exp Ther. 315:1346–1353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Narimatsu N, Harada N, Kurihara H,
Nakagata N, Sobue K and Okajima K: Donepezil improves cognitive
function in mice by increasing the production of insulin-like
growth factor-I in the hippocampus. J Pharmacol Exp Ther. 330:2–12.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Týč J, Klingbeil MM and Lukeš J:
Mitochondrial heat shock protein machinery hsp70/hsp40 is
indispensable for proper mitochondrial DNA maintenance and
replication. MBio. 6:e02414–e02425. 2015. View Article : Google Scholar
|
|
34
|
Garrido C, Schmitt E, Candé C, Vahsen N,
Parcellier A and Kroemer G: HSP27 and HSP70: Potentially oncogenic
apoptosis inhibitors. Cell Cycle. 2:579–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rashmi R, Kumar S and Karunagaran D:
Ectopic expression of Hsp70 confers resistance and silencing its
expression sensitizes human colon cancer cells to curcumin-induced
apoptosis. Carcinogenesis. 25:179–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee
JE and Giffard RG: Antiapoptotic and anti-inflammatory mechanisms
of heat-shock protein protection. Ann N Y Acad Sci. 1053:74–83.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saleh A, Srinivasula SM, Balkir L, Robbins
PD and Alnemri ES: Negative regulation of the Apaf-1 apoptosome by
Hsp70. Nat Cell Biol. 2:476–483. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang B, Liang P, Deng G, Tu Z, Liu M and
Xiao X: Increased stability of Bcl-2 in HSP70-mediated protection
against apoptosis induced by oxidative stress. Cell Stress
Chaperones. 16:143–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stankiewicz AR, Lachapelle G, Foo CP,
Radicioni SM and Mosser DD: Hsp70 inhibits heat-induced apoptosis
upstream of mitochondria by preventing Bax translocation. J Biol
Chem. 280:38729–38739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Levin LA, Schlamp CL, Spieldoch RL,
Geszvain KM and Nickells RW: Identification of the bcl-2 family of
genes in the rat retina. Invest Ophthalmol Vis Sci. 38:2545–2553.
1997.PubMed/NCBI
|
|
42
|
Chipuk JE and Green DR: How do BCL-2
proteins induce mitochondrial outer membrane permeabilization?
Trends Cell Biol. 18:157–164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen J, Flannery JG, LaVail MM, Steinberg
RH, Xu J and Simon MI: bcl-2 overexpression reduces apoptotic
photoreceptor cell death in three different retinal degenerations.
Proc Natl Acad Sci USA. 93:7042–7047. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Quiambao AB, Tan E, Chang S, Komori N,
Naash MI, Peachey NS, Matsumoto H, Ucker DS and Al-Ubaidi MR:
Transgenic Bcl-2 expressed in photoreceptor cells confers both
death-sparing and death-inducing effects. Exp Eye Res. 73:711–721.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cottet S and Schorderet DF: Triggering of
Bcl-2-related pathway is associated with apoptosis of
photoreceptors in Rpe65-/- mouse model of Lebers congenital
amaurosis. Apoptosis. 13:329–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eversole-Cire P, Chen J and Simon MI: Bax
is not the heterodimerization partner necessary for sustained
anti-photoreceptor-cell-death activity of Bcl-2. Invest Ophthalmol
Vis Sci. 43:1636–1644. 2002.PubMed/NCBI
|
|
47
|
Ye W, Gong X, Xie J, Wu J and Zhang X,
Ouyang Q, Zhao X, Shi Y and Zhang X: AChE deficiency or inhibition
decreases apoptosis and p53 expression and protects renal function
after ischemia/reperfusion. Apoptosis. 15:474–487. 2010. View Article : Google Scholar : PubMed/NCBI
|