Introduction
Acute myocardial infarction is a common medical
emergency of the cardiovascular system. The clinical treatments for
myocardial infarction mainly focus on interventional or
thrombolytic therapy. Although interventional or thrombolytic
therapy is able to clear the coronary artery and restore blood flow
(1), it can also cause reperfusion
injury (2). This means that blood
reperfusion may occasionally further aggravate the damage (3), and can even cause respiratory
dysfunction, with patients with severe cases succumbing to
respiratory failure (4). Therefore,
the development of drugs that effectively control or reduce
ischemia/reperfusion (I/R)-induced lung injury is required. A
previous study has found that an imbalance of inflammatory
mediators is an important cause of myocardial I/R injury (5). Thus, redressing the balance of
inflammatory mediators is an important target for reducing or
preventing I/R injury.
Xuebijing (XBJ) is a traditional Chinese medicine
preparation (6). In clinical
settings, XBJ is combined with antibiotics to treat severe acute
lung diseases, including acute lung injury (ALI) (7), sepsis (8) and multiple organ dysfunction syndrome
(9). However, to the best of our
knowledge, no previous studies have investigated the protective
effects of XBJ injection against left ventricular I/R-induced ALI.
Therefore, the present study established a rabbit model of left
ventricular I/R-induced ALI in order to investigate the underlying
pathogenic mechanisms and the protective role of XBJ on respiratory
function. The following parameters were assessed: The partial
pressure of oxygen (PaO2) and carbon dioxide
(PaCO2); the expression levels of tumor necrosis factor
(TNF)-α in peripheral blood and bronchoalveolar lavage (BAL) fluid
(BALF); the lung wet/dry weight ratio (W/D); and the protein
expression levels of intercellular adhesion molecule-1 (ICAM-1) and
TNF-α in the rabbit lung tissue samples.
Materials and methods
Animals and grouping
A total of 120 healthy adult (6–7 months old)
mixed-gender New Zealand rabbits weighing 200–250 g were obtained
from the Experimental Animal Center of Zhengzhou University
(Zhengzhou, China). Each rabbit was housed in an individual cage
(at 20 ± 5°C) under a 12:12 h light and dark cycle for 1 week prior
to experimentation. All rabbits had free access to water, and food
was removed 8 h prior to the study. The rabbits were randomly
divided into four groups (n=30/group), as follows: i) The normal
control group (CG); ii) the ischemia group (IG); iii) the I/R group
(I/RG); and iv) the I/R + XBJ treatment group (TG). In addition,
each group was further divided into three subgroups (n=10/subgroup)
as follows: i) 30 min pre-ischemia (T1); ii) 30 min post-ischemia
(T2); and iii) 30 m in post-reperfusion (T3). For 12 h prior to the
experiment, the rabbits were fasted but had ad libitum
access to water. All experimental procedures were approved by the
Ethics Committee of Xinxiang Medical University (Xinxiang, China).
The present study was conducted in accordance with internationally
recognized guidelines on animal welfare (10), as well as local and national
regulations.
Reagents and instruments
The instruments included a Leica RM2235 microtome
(Leica Microsystems GmbH, Wetzlar, Germany), a CR-21G High-Speed
Refrigerated Centrifuge (Hitachi, Ltd., Tokyo, Japan), a DG5033A
Enzyme-linked Immunosorbent Assay (ELISA) Analyzer (Shanghai Bogoo
Biotechnology, Co., Ltd., Shanghai, China), a fully automated M248
Blood Gas Analyzer (Bayer China Ltd., Shanghai, China) and a BL-420
Biological Signal Collecting and Processing system (Chengdu TME
Technology, Co., Ltd., Chengdu, China). The main reagents were as
follows: XBJ (10 ml ampoules; Tianjin Chase Sun Pharmaceutical,
Co., Ltd., Tianjin, China), 20% urethane (Shanghai Yunqiang
Chemical, Co., Ltd., Shanghai, China), and ICAM-1 and TNF-α
polyclonal antibodies (Beijing Biosynthesis Biotechnology Co.,
Ltd., Beijing, China). Surgical instruments and general laboratory
supplies were provided by the Functional Laboratory of Xinxiang
Medical University.
Surgical procedure
The rabbits underwent general anesthesia with 20%
urethane (5 ml/kg) via the ear vein, followed by isolation and
intubation of the trachea, right jugularis externa and left carotis
communis. Following a thoracotomy, the left anterior descending
coronary artery was separated and ligated, with the ST segment
elevation arched upward, and with regional myocardial darkening
serving as an effective indicator of ischemia. In the CG, the left
anterior descending coronary artery was separated but not ligated,
and the rabbits were observed for 4 h. In the IG, myocardial
ischemia was established by making a slipknot using a 6–0 silk
suture around the left anterior descending coronary artery for 1.5
h. In the I/RG, following ligation of the left anterior descending
coronary artery for 30 min, the ligature was loosened and the blood
supply was restored. The TG underwent the same procedures as the
I/RG, and were also given XBJ (10 ml/kg) at two time points: 10 min
prior to ischemia and immediately following reperfusion. Prior to
the thoracotomy, needle electrodes were inserted into the limbs and
chest, and connected to the BL-420 Biological Signal Collecting and
Processing system. The electrocardiogram (ECG; paper speed, 50
mm/sec; gain, l mV=20 ram) and diaphragm discharge curve were
monitored continuously. One end of a lead was connected to a hook
positioned on the skin at the point of strongest respiratory
movement, and the other end of the lead was connected to a pressure
transducer chip connected to the BL-420 system for the recording of
respiratory curves.
Samples
At the T1, T2 and T3 time points, 5-ml peripheral
blood samples were collected from the left common carotid artery,
and the PaO2 and PaCO2 were measured using
the M248 Blood Gas Analyzer. Also, 2-ml venous blood samples were
collected from the right external jugular vein for measurement of
the level of TNF-α. Subsequently, all rabbits were sacrificed by
aeroembolism and the lungs were harvested. BALF samples (5 ml) were
collected using Y-type tracheal intubation with one inlet connected
to a 50-ml syringe of air, and another connected a 10-ml syringe
containing 10 ml 0.9% NaCl. Air and 0.9% NaCl were concurrently
introduced into the lungs, and then the 10-ml syringe was pumped
back and forth several times to collect the BALF. The lower lobe of
the right lung was removed, fixed with paraformaldehyde solution (1
ml/100 g; Qingzhou Hengxing Chemical Co., Ltd., Qingzhou, China),
embedded in paraffin (Dekang Medical Instrument Co., Ltd.,
Xinxiang, China) and cut into 5-µm sections using the Leica RM2235
microtome, prior to being fixed to glass slides coated with
(3-aminopropyl)triethoxysilane (Dekang Medical Instrument Co.,
Ltd.).
ELISA
The level of TNF-α in the venous blood and BALF was
measured by a double-antigen sandwich ELISA (EIA-1126; Beijing
Zhongshan Golden Bridge Biological Technology, Co., Ltd.),
according to the manufacturer's protocol. Briefly, each sample
(sera; diluted with coating buffer) was applied in triplicate to
96-well plates pre-coated with anti-TNF-α antibody and incubated
overnight at 4°C. Following this, the plates were washed three
times with phosphate-buffered saline (PBS) and then incubated with
biotinylated goat anti-rabbit IgG (1:20,000) for 2 h at 37°C. The
plates were then incubated with 0.01%
3,3′,5,5′-tetramethylbenzidine (Beijing CellChip Biotechnology Co.,
Ltd., Beijing, China), after which 2 M H2SO4
(Nanjing Taiye Chemical Industry Co., Ltd., Nanjing, China) was
added to terminate the reaction. The optical density was recorded
at 450 nm using a DG5033A ELISA Analyzer. The experiments were
performed in triplicate. The sensitivity and specificity of the
ELISA were 99 and 92%, respectively.
Lung weight and water content
Lungs were weighed, and then dried in a vacuum
drying oven (cat. no. DHG-9420; Shanghai Haixiang Equipment
Factory, Shanghai, China) at 80°C for 48 h to obtain the dry
weight. The lung wet weight/dry weight (W/D) ratios of left lung
tissue samples were determined, and the water content of the lung
tissue was calculated using the following formula: Water content
(%) = (wet weight - dry weight)/wet weight × 100.
Immunohistochemistry
Lung tissue sections, fixed in formalin (Shanghai
Fengshou Biotechnology Co., Ltd., Shanghai, China) and embedded in
paraffin, were deparaffinized, routinely rehydrated using graded
ethanol, peroxidase-quenched with 3% H2O2,
blocked with 5% normal goat serum (Amyjet Scientific, Inc., Wuhan,
China) and probed with rabbit anti-rabbit ICAM-1 antibody (final
concentration:0.1%; bs-0608R) or rabbit anti-rabbit TNF-α antibody
(final concentration:0.1%; bs-2081R) overnight at 4°C. The sections
were then thoroughly washed with PBS for 5 min, repeated three
times. Subsequently, the tissue sections were incubated with goat
anti-rabbit IgG secondary antibody (bs-0295Gs; Beijing Biosynthesis
Biotechnology Co., Ltd.) at room temperature for 1 h. The sections
were thoroughly washed with PBS for 5 min, repeated three times.
Following this, sections were incubated with horseradish peroxidase
(HRP)-conjugated rabbit anti-goat antibody (bs-0294R-HRP; Beijing
Biosynthesis Biotechnology Co., Ltd.) at 4°C for 30 min.
Eventually, reaction products were visualized following
diaminobenzidine (DAB) and hematoxylin (Shanghai Yantuo
Biotechnology Co., Ltd., Shanghai, China) counterstaining.
The protein expression levels of ICAM-1 and TNF-α in
the lung tissue samples of the rabbits at each of the T1, T2 and T3
time points were measured. These experiments were performed in
triplicate. According to the results of a semi-quantitative method
(11), 10 random fields were
selected under a Nikon microscope (80i; Nikon Instruments Co., Ltd,
Shanghai China), in which 100 cells were counted. The tissue
sections were scored as follows: No staining, ‘-’; <25% positive
cells, ‘+’; 25–75% positive cells, ‘++’; and >75% positive
cells, ‘+++’.
Statistical analysis
Statistical analyses were conducted using SPSS
software, version 11.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. The data were analyzed
using an Independent Samples t-test and categorical data were
analyzed using a χ2 test. P<0.05 was considered to
indicate a statistically significant difference.
Results
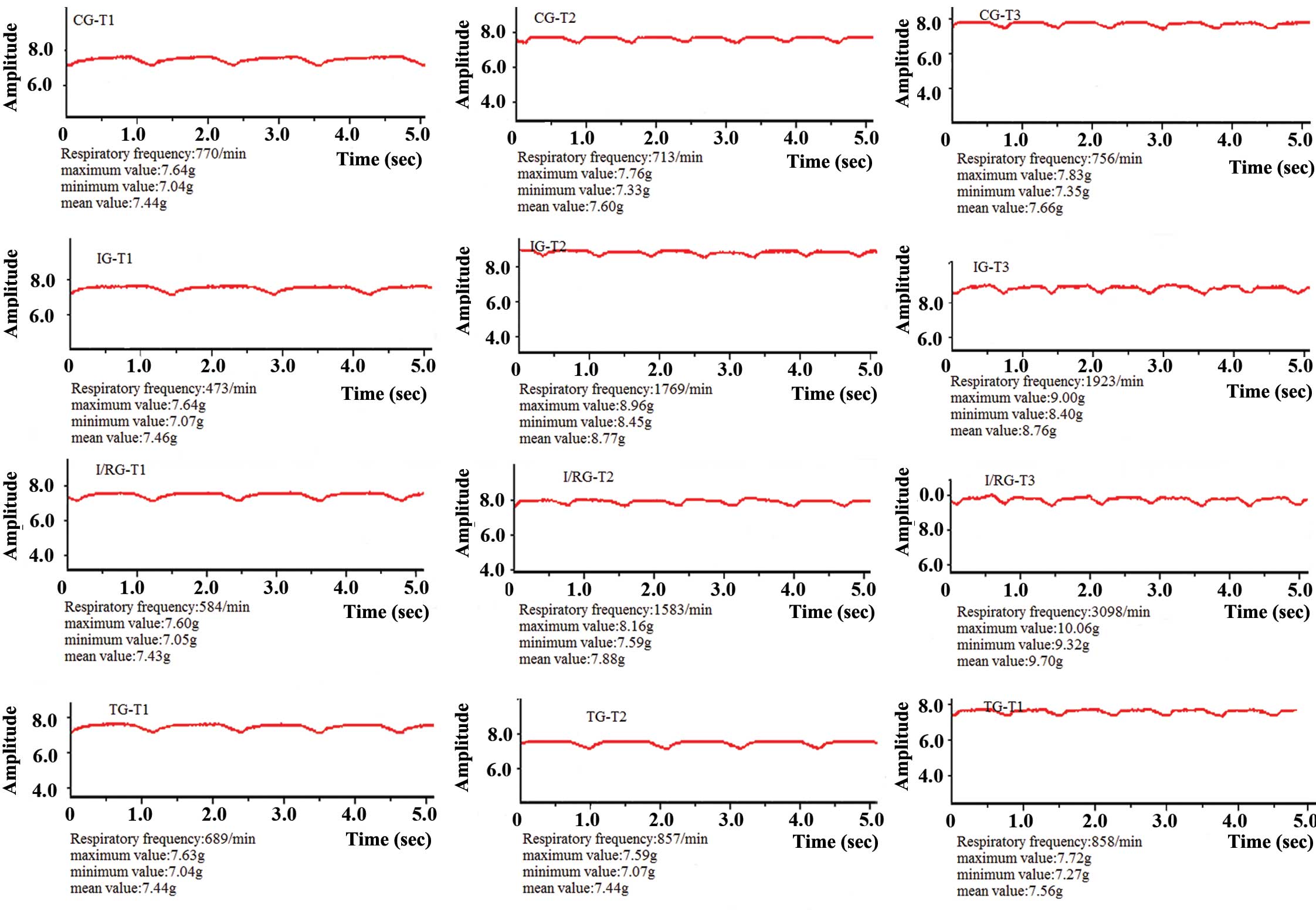
Comparison of respiratory curves
Respiratory curves (Fig.
1) were collected and recorded using the BL-420 Biological
Signal Collecting and Processing system. At the T1, T2 and T3 time
points, the breathing of the rabbits in the CG was steady and the
respiratory amplitude and duration did not significantly fluctuate.
Conversely, the respiratory amplitude for the rabbits in the IG was
shallow and the respiratory duration was short. In addition,
respiratory fluctuations were observed between the three time
points. In comparison with the IG, the respiratory amplitude for
the rabbits in the I/RG was more shallow and the respiratory
duration was shorter. The respiratory curves for the rabbits in the
TG resembled those of the rabbits in the CG (Fig. 1).
Comparison of PaO2 and
PaCO2
The PaO2 and PaCO2 were
measured using the fully automated M248 Blood Gas Analyzer. The
PaO2 and PaCO2 of rabbits in the TG were not
significantly different from those in the CG at the T1, T2 and T3
time points (P>0.05). The PaO2 values of the rabbits
in the IG and I/RG were significantly decreased compared with those
in the CG, at the T2 and T3 time points (P<0.001), and
particularly decreased at T3 in the I/RG. Furthermore, the
PaCO2 values of the rabbits in the IG and I/RG were
significantly increased compared with those in the CG, at the T2
and T3 time points (P<0.001; Fig.
2).
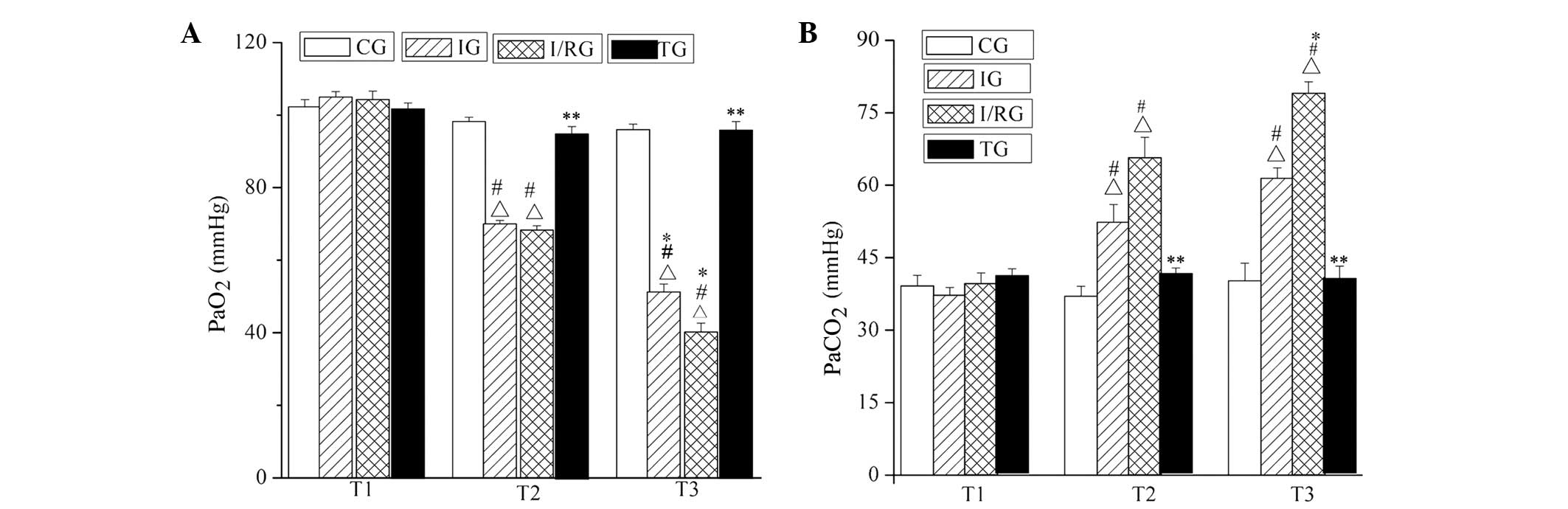 | Figure 2.Comparison of PaO2 and
PaCO2 among the groups at the T1, T2 and T3 time points.
The (A) PaO2 and (B) PaCO2 in the CG, IG,
I/RG and TG groups. Data are presented as the mean ± standard
deviation. ∆P<0.001 vs. the CG at the same time;
#P<0.001 vs. T1 in the same group; *P<0.001 vs. T2
in the same group; **P<0.001 vs. the IG and I/RG at the same
time. CG, normal-control group; IG, ischemia group; I/RG,
ischemia/reperfusion group; TG, treatment group; T1, 30 min
pre-ischemia; T2, 30 min post-ischemia; T3, 30 min
post-reperfusion; PaO2, partial pressure of oxygen;
PaCO2, partial pressure of carbon dioxide. |
Comparison of W/D value and water
content in the left lung tissue samples of the rabbits
At the T1, T2 and T3 time points, the W/D weight
ratio and water content of the left lung tissue from the CG did not
significantly differ (P>0.05). Conversely, those of the tissue
from the IG and I/RG were significantly increased (P<0.001)
compared with those in the CG, with the greatest increases being
observed for the I/RG. The W/D weight ratio and water content of
the left lung tissue from the TG were not significantly different
from those in the CG at any of the three time points (P>0.05;
Fig. 3).
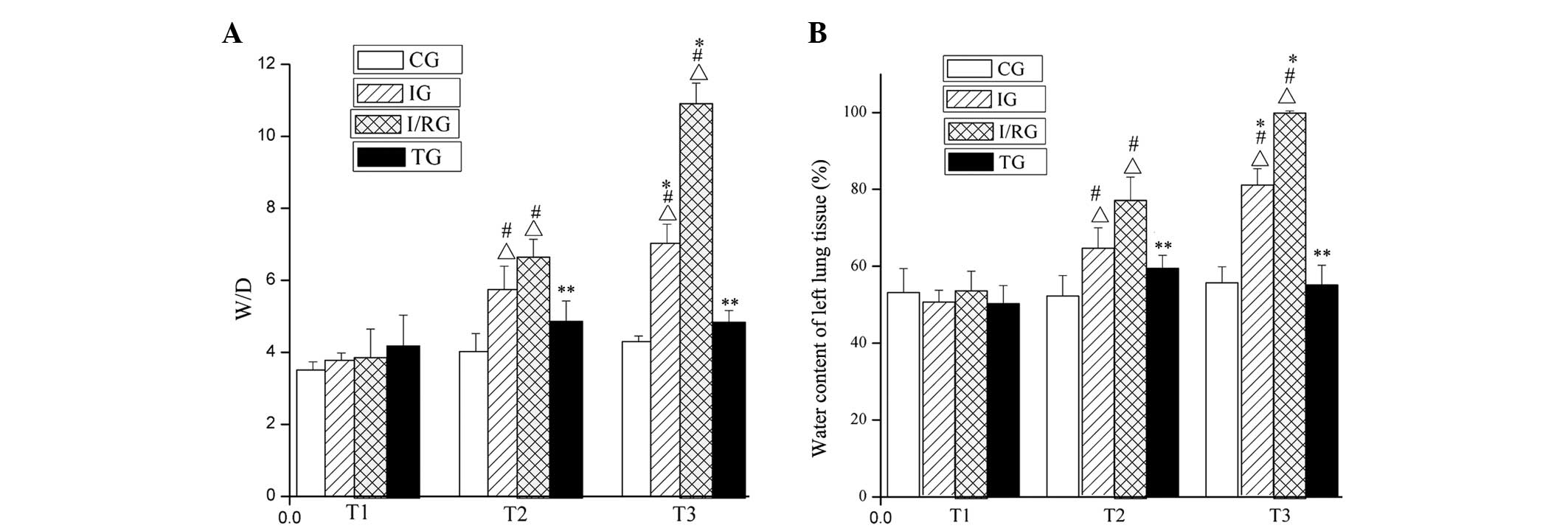 | Figure 3.Comparison of the W/D and water
content of left lung tissue from rabbits in the the four groups at
the T1, T2 and T3 time points. The (A) W/D and (B) water content of
the left lung tissue from rabbits in the CG, IG, I/RG and TG. Data
are presented as the mean ± standard deviation.
∆P<0.001 vs. the CG at the same time;
#P<0.001 vs. T1 in the same group; *P<0.001 vs. T2
in the same group; **P<0.001 vs. the IG and I/RG at the same
time. CG, normal-control group; IG, ischemia group; I/RG,
ischemia/reperfusion group; TG, treatment group; T1, 30 min
pre-ischemia; T2, 30 min post-ischemia; T3, 30 min
post-reperfusion; W/D, wet/dry weight ratio. |
General observation of the rabbit
lungs at T3
At the T3 time point, the lungs of rabbits in the CG
were light pink and small in size. In the IG, the lungs appeared
dark red and small, with an uneven surface and visible hemorrhages.
Similarly, congestion and pulmonary edema were apparent in the
lungs of the I/RG, and the volume was larger, the margins of the
lungs were blunt, the color was uneven and clear spot bleeding and
bruising was observed in the lower lobe of the lungs. In addition,
white foamy/bloody liquid was overflowing from the lung section.
Conversely, the TG lungs exhibited only mild edema, appeared red in
color and had a smooth surface.
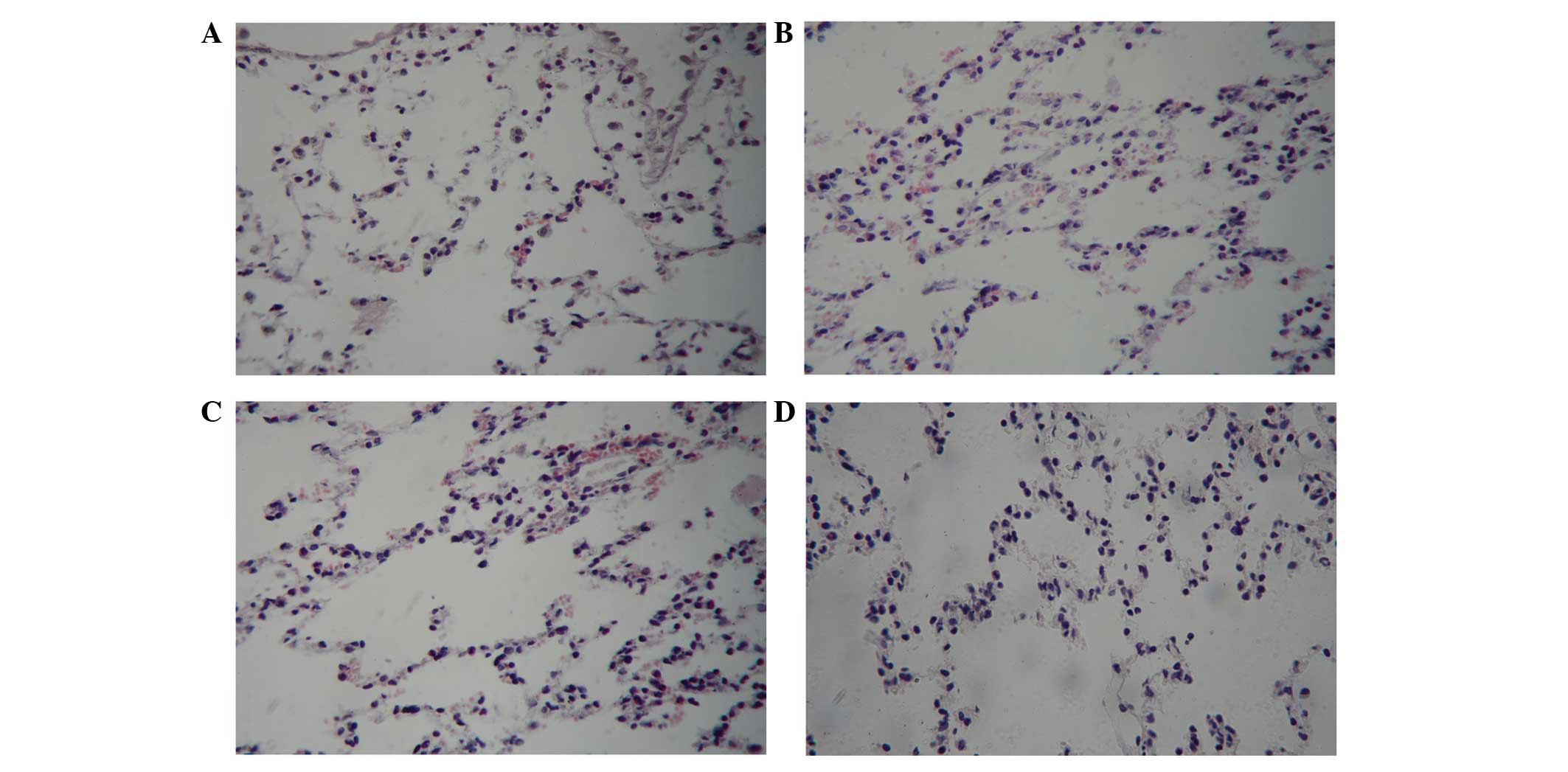
Light microscopy observations of the
rabbit lungs at T3
At the T3 time point, the alveolar structure of the
lungs in the CG group was clear, the walls of the alveolae were
thin and no inflammatory cells had infiltrated into the alveolar
space and lung interstitium. In the IG, pulmonary vascular
congestion, focal alveolar hemorrhages and small focal alveolar
collapse/atelectasis were observed in some areas of the lung. In
the I/RG, dilatation and congestion was observed in the pulmonary
capillaries, the alveolar septum was thickened, a large number of
leukocytes had infiltrated and aggregated and hemorrhage and
exudation were detected in the alveolar cavity. In addition,
atelectasis was observed in some alveolae. Conversely, in the TG,
the alveolar structure was relatively complete and hemorrhage and
exudation of the alveolar was markedly reduced, as compared with
the I/RG lungs, although alveolar atrophy and infiltrated
inflammatory cells were infrequently observed (Fig. 4).
Expression levels of TNF-α in the BALF
and the peripheral blood
The expression levels of TNF-α in the BALF and
peripheral blood from the four groups were detected by performing
an ELISA. At the T1, T2 and T3 time points, there was no
significant difference in the expression levels of TNF-α in the
peripheral blood and BALF between the CG and TG (P>0.05). In the
IG, the expression levels of TNF-α in the peripheral blood and BALF
were increased at T2, and reached a maximum at T3 (P<0.001),
these changes were more pronounced in the I/RG (P<0.001). At T2
and T3, the expression levels of TNF-α in the peripheral blood and
BALF in the TG were significantly lower compared with those in the
IG and I/RG (P<0.001; Fig.
5).
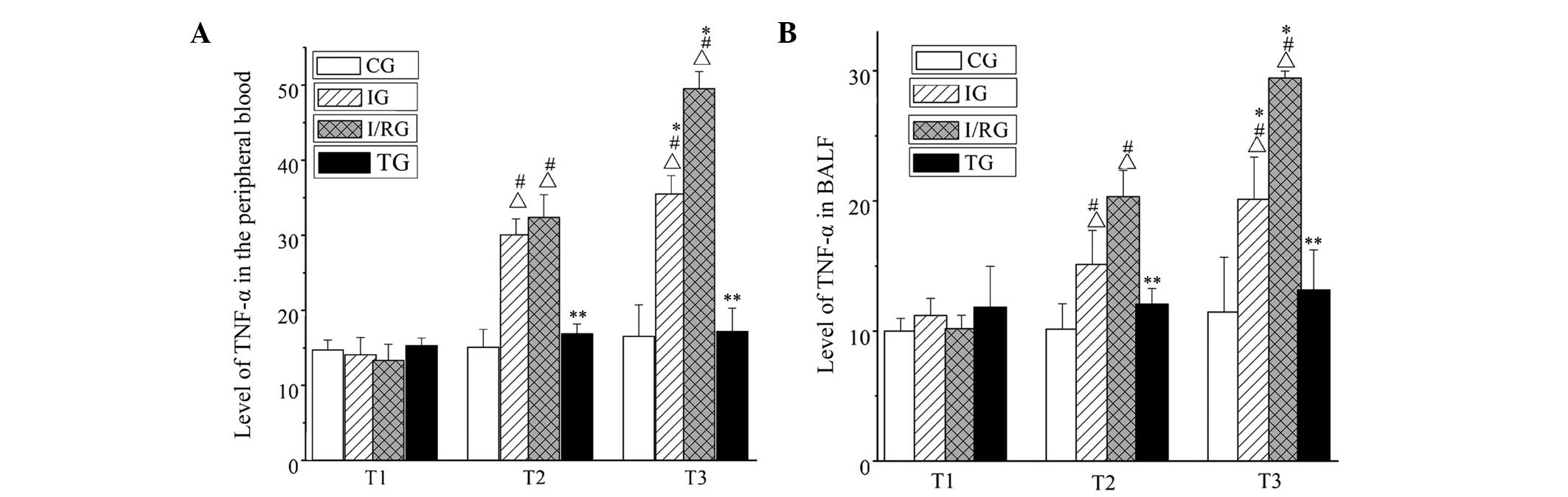 | Figure 5.Expression levels of TNF-α in the
peripheral blood and BALF in the four groups at the T1, T2 and T3
time points. The expression levels of TNF-α in the (A) peripheral
blood and (B) BALF. Data are presented as the mean ± standard
deviation. ΔP<0.001, at the same time vs. the CG;
#P<0.001, the same group vs. T1; *P<0.001, the
same group vs. T2; **P<0.001, at the same time vs. the IG and
I/RG. CG, normal-control group; IG, ischemia group; I/RG,
ischemia/reperfusion group; TG, treatment group; T1, 30 min
pre-ischemia; T2, 30 min post-ischemia; T3, 30 min
post-reperfusion; BALF, brochoalveolar lavage fluid. |
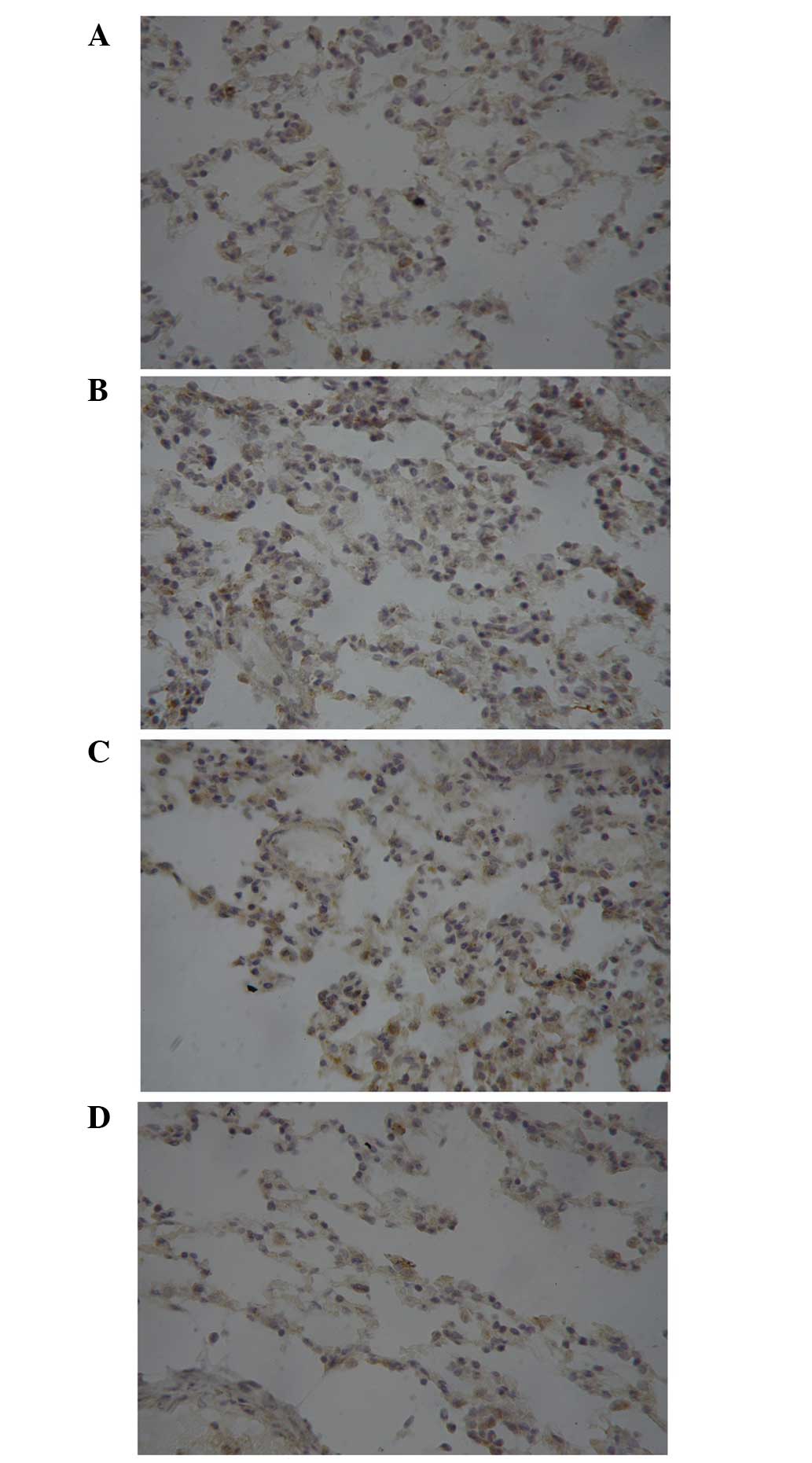
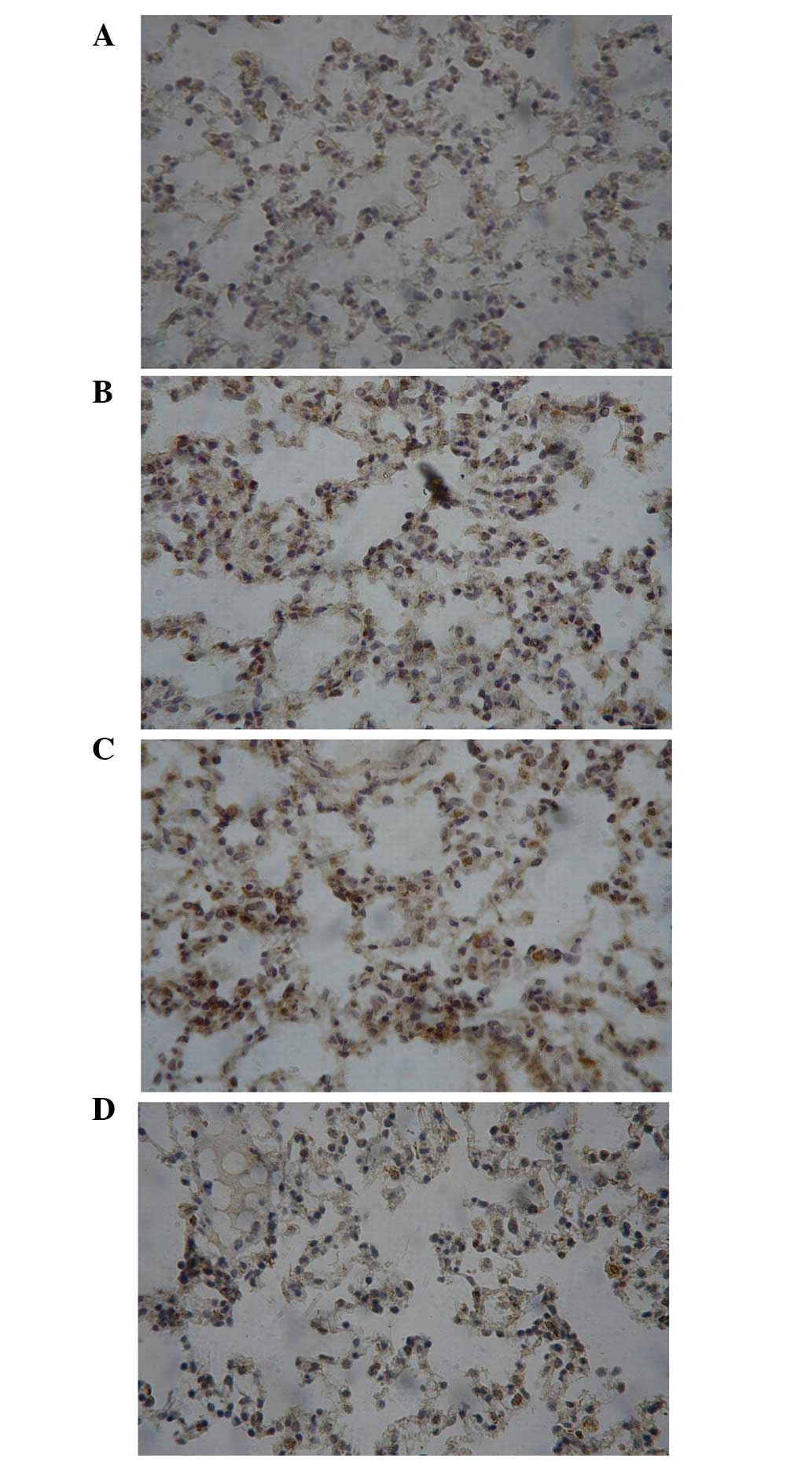
Protein expression of ICAM-1 and TNF-α
in the rabbit lung tissue samples at T3
The immunoreactive product of ICAM-1 was brown and
was observed in the membrane and cytoplasm. TNF-α formed dark
brown-to-yellow granules that were observed in the cytoplasm. As
shown Figs. 6 and 7, there was an evident increasing trend in
the expression of ICAM-1 and TNF-α from CG and IG to I/RG at T3,
and the levels of ICAM-1 and TNF-α in the TG were comparable to
those in the CG and markedly lower than those in the IG and
I/RG.
Comparison of the association between
the protein expression levels of ICAM-1 and TNF-α in the rabbit
lung tissue samples at T3
The association between the protein expression
levels of ICAM-1 and TNF-α in the rabbit lung tissues samples at T3
was investigated. A positive correlation between the protein
expression levels of ICAM-1 and TNF-α was observed (Table I).
 | Table I.Comparison of the association between
the protein expression levels of ICAM-1 and TNF-α in the lung
tissue samples of rabbits in the 4 groups at the T3 time point. |
Table I.
Comparison of the association between
the protein expression levels of ICAM-1 and TNF-α in the lung
tissue samples of rabbits in the 4 groups at the T3 time point.
|
| ICAM-1 |
|---|
|
|
|
|---|
| TNF-α | − | + | ++ | +++ |
|---|
| CG |
|
|
|
|
| − | 6 | 1 | 0 | 0 |
| + | 1 | 2 | 0 | 0 |
| ++ | 0 | 0 | 0 | 0 |
|
+++ | 0 | 0 | 0 | 0 |
| IG |
|
|
|
|
| − | 2 | 1 | 0 | 0 |
| + | 0 | 3 | 1 | 0 |
| ++ | 0 | 2 | 0 | 0 |
|
+++ | 0 | 0 | 1 | 0 |
| I/RG |
|
|
|
|
| − | 0 | 1 | 0 | 0 |
| + | 0 | 3 | 2 | 0 |
| ++ | 0 | 1 | 0 | 0 |
|
+++ | 0 | 0 | 2 | 1 |
| TG |
|
|
|
|
| − | 4 | 1 | 1 | 0 |
| + | 1 | 2 | 0 | 0 |
| ++ | 0 | 1 | 0 | 0 |
|
+++ | 0 | 0 | 0 | 0 |
Discussion
Due to the structural characteristics of the left
anterior descending coronary artery, the blood supply of the heart
is prone to ischemia (12–14), and the recovery of blood perfusion to
the ischemic myocardium is an important therapeutic strategy that
reduces the occurrence of ischemic injury. However, in some cases
reperfusion may further aggravate damage or induce irreversible
damage; this phenomenon is called reperfusion injury. As an
important organ with roles in immunity and metabolism, the lung has
a high probability of being affected by reperfusion injury and is
prone to inflammation (15).
Previous studies have demonstrated that the
activation of a large number of neutrophils occurs in the early
stages of ischemia (16), and that
these neutrophils enhance the process of reperfusion (17). In addition, activated neutrophils
promote the release of large amounts of inflammatory cytokines,
including TNF-α (18,19), interleukin (IL)-6 (20,21),
IL-10 (22–24) and ICAM-1 (25), in order to expand the inflammatory
response. ICAM-1 has a key role in neutrophil aggregation,
activation and the release of inflammatory mediators (26–28).
Under normal circumstances, the protein expression levels of ICAM-1
are negligible, and this prevents pathological damage to the body
(29). However, during I/R and other
pathological conditions, the expression of proinflammatory
mediators is stimulated and the expression levels of ICAM-1 are
increased significantly (30). This
in turn promotes the adherence of neutrophils to endothelial cells
and their migration across the endothelial barrier, resulting in
inflammation and various pathological changes that characterize
reperfusion injury (7).
XBJ is a traditional Chinese medicine preparation
with anti-bacterial, anti-endotoxin and anti-inflammatory
properties (31). XBJ is a herbal
preparation consisting mainly of red peony root, chuanxiong,
Salvia divinorum and Angelica sinensis. XBJ has a
critical role in inhibiting the generation of free oxygen radicals
and endotoxins, as well as the uncontrolled release of inflammatory
mediators (32).
The present study established a rabbit model of left
ventricular I/R-induced ALI, in order to investigate the protective
effects of XBJ against lung reperfusion injury. The results of the
present study demonstrated that, as compared with the CG and IG,
the respiratory amplitude of rabbits in the I/RG was shallow, the
respiratory frequency was increased, the PaO2 and
PaCO2 were decreased and the lung volume was enlarged at
the T3 time point, thus suggesting that ALI occurred in the rabbits
of the I/RG. Conversely, for the rabbits in the TG, the
PaO2 and PaCO2 increased, edema and hyperemia
of the lung tissue was reduced and the W/D weight ratio was
decreased, suggesting that XBJ was able to improve respiratory
function. A quantitative analysis revealed that, as compared with
that in the CG, the levels of TNF-α in the peripheral blood and
BALF were significantly increased in the IG at T2, and reached a
peak at T3. Similarly, the TNF-α levels were significantly
increased in the I/RG at T2 and T3, as compared with the CG, and at
T3, as compared with IG. Conversely, there was no significant
difference between CG and TG at the T1, T2 and T3 time points.
A gross observation of the lung tissue indicated
that lung tissue injury occurred in the IG, and was aggravated in
the I/RG at T3. Conversely, lung tissue damage in the TG was
markedly attenuated, as compared with that in the I/RG.
Immunohistochemical analyses demonstrated that the expression
levels of ICAM-1 and TNF-α exhibited an increasing trend from CG
and IG to I/RG at T3, whereas the expression levels of ICAM-1 and
TNF-α in the TG were markedly lower, as compared with the IG and
I/RG. A correlation analysis demonstrated that the expression
levels of ICAM-1 were positively correlated with the expression
levels of TNF-α in the IG, I/RG and TG at T3. These results
suggested that, during the pathogenesis of left ventricular
I/R-induced ALI, a large number of neutrophils were activated,
promoting the release of proinflammatory mediators, including
TNF-α. The excessive release of TNF-α in turn induced the
overexpression of ICAM-1, which led to the adhesion of neutrophils
to endothelial cells, eventually leading to lung inflammatory
injury. XBJ exhibited a protective effect in the rabbit lung tissue
samples, inhibited the excessive release of early inflammatory
cytokines, including TNF-α, and decreased the expression levels of
ICAM-1.
In conclusion, the present study investigated the
effect of XBJ on left ventricular I/R-induced ALI by performing a
blood gas analysis and measuring the expression levels of ICAM-1
and TNF-α. The results demonstrated that, following treatment with
XBJ, the release of the inflammatory mediators ICAM-1 and TNF-α was
effectively inhibited, and the PaO2 was improved.
Understanding the molecular mechanism underlying the
anti-inflammatory effect of XBJ will provide a theoretical basis
and experimental evidence for its clinical application.
Acknowledgements
The present study was supported by grants from the
Foundation of Henan Educational Commission (grant no. 2011GGJS-127)
and the Henan Science and Technology Bureau (grant no.
132300410160). The authors would like to thank Professor Zhibin
Qian for providing the facilities to carry out the study.
Glossary
Abbreviations
Abbreviations:
|
ICAM-1
|
intercellular adhesion molecule-1
|
|
TNF-α
|
tumor necrosis factor-α
|
|
XBJ
|
Xuebijing injection
|
|
CG
|
control group
|
|
IG
|
ischemia group
|
|
I/RG
|
ischemia/reperfusion group
|
|
TG
|
treatment group
|
|
T1
|
30 min pre-ischemia
|
|
T2
|
30 min post-ischemia
|
|
T3
|
30 min post-reperfusion
|
|
PaO2
|
partial pressure of oxygen
|
|
PaCO2
|
partial pressure of carbon dioxide
|
|
BALF
|
bronchoalveolar lavage fluid
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
ECG
|
electrocardiogram
|
|
ALI
|
acute lung injury
|
References
|
1
|
Waldo SW, Brenner DA, Li S, Alexander K
and Ganz P: Reperfusion times and in-hospital outcomes among
patients with an isolated posterior myocardial infarction: Insights
from the national cardiovascular data registry (NCDR). Am Heart J.
167:350–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boersma E, Maas AC, Deckers JW and Simoons
ML: Early thrombolytic treatment in acute myocardial infarction:
Reappraisal of the golden hour. Lancet. 348:771–775. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buja LM: Myocardial ischemia and
reperfusion injury. Cardiovasc Pathol. 14:170–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guido BC, Zanatelli M, Tavares-de-Lima W,
Oliani SM and Damazo AS: Annexin-A1 peptide down-regulates the
leukocyte recruitment and up-regulates interleukin-10 release into
lung after intestinal ischemia-reperfusion in mice. J Inflamm
(Lond). 10:102013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X: Investigational anti-inflammatory
agents for the treatment of ischaemic brain injury. Expert Opin
Investig Drugs. 14:393–409. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gong P, Lu Z, Xing J, Wang N and Zhang Y:
Traditional Chinese medicine Xuebijing treatment is associated with
decreased mortality risk of patients with moderate paraquat
poisoning. PLoS One. 10:e01235042015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang YX, Ji ML, Chen LP, Wu XJ and Wang L:
Breviscapine reduces acute lung injury induced by left heart
ischemic reperfusion in rats by inhibiting the expression of ICAM-1
and IL-18. Exp Ther Med. 6:1322–1326. 2013.PubMed/NCBI
|
|
8
|
Hou SY, Feng XH, Lin CL and Tan YF:
Efficacy of Xuebijing for coagulopathy in patients with sepsis.
Saudi Med J. 36:164–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu Q, Liu J, Guo X, Tang Y, Zhou G, Liu Y,
Huang Q, Geng Y, Liu Z and Su L: Xuebijing injection reduces organ
injuries and improves survival by attenuating inflammatory
responses and endothelial injury in heatstroke mice. BMC Complement
Altern Med. 15:42015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
National Institutes of Health: Guide for
the Care and Use of Laboratory Animals (8th). National Academies
Press. Washington (DC): 2011.
|
|
11
|
Yu P, Bu H, Wang H, Zhao GP, Zhang J and
Zhou Q: Comparative study on image analysis and manual counting of
immunohistochemistry. J Biomed Eng. 20:288–290. 2003.(In
Chinese).
|
|
12
|
Zhao Q, Sun C, Xu X, Zhou J, Wu Y, Tian Y,
Yuan Z and Liu Z: CD34+ cell mobilization and
upregulation of myocardial cytokines in a rabbit model of
myocardial ischemia. Int J Cardiol. 152:18–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang CH, Tsai SK, Chiang SC, Wang YY,
Chih CL, Weng ZC and Lai ST: Brief pressure overload preconditions
rabbit myocardium independent of adenosine receptor activation. Ann
Thorac Surg. 92:1727–1732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun ZH: Coronary CT angiography in
coronary artery disease: Correlation between virtual intravascular
endoscopic appearances and left bifurcation angulation and coronary
plaques. Biomed Res Int. 2013:7320592013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arun Prakash, Kailin R. Mesa, Kevin
Wilhelmsen, Fengyun Xu, Jeffrey M. Dodd-o and Judith Hellman:
Alveolar Macrophages and Toll-like Receptor 4 Mediate Ventilated
Lung Ischemia Reperfusion Injury in Mice.
|
|
16
|
Hughes SF, Hendricks BD, Edwards DR,
Bastawrous SS, Roberts GE and Middleton JF: Mild episodes of
tourniquet-induced forearm ischaemia-reperfusion injury results in
leukocyte activation and changes in inflammatory and coagulation
markers. J Inflamm (Lond). 4:122007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ranganathan PV, Jayakumar C, Mohamed R,
Dong Z and Ramesh G: Netrin-1 regulates the inflammatory response
of neutrophils and macrophages and suppresses ischemic acute kidney
injury by inhibiting COX-2 mediated PGE2 production. Kidney Int.
83:1087–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dziodzio T, Biebl M and Pratschke J:
Impact of brain death on ischemia/reperfusion injury in liver
transplantation. Curr Opin Organ Transplant. 19:108–114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zygner W, Gójska-Zygner O, Bąska P and
Długosz E: Increased concentration of serum TNF alpha and its
correlations with arterial blood pressure and indices of renal
damage in dogs infected with Babesia canis. Parasitol Res.
113:1499–1503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JW, Kim SC, Ko YS, Lee HY, Cho E, Kim
MG, Jo SK, Cho WY and Kim HK: Renoprotective effect of paricalcitol
via a modulation of the TLR4-NF-κB pathway in
ischemia/reperfusion-induced acute kidney injury. Biochem Biophys
Res Commun. 444:121–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
la Guzmán-de Garza FJ, Ibarra-Hernández
JM, Cordero-Pérez P, Villegas-Quintero P, Villarreal-Ovalle CI,
Torres-González L, Oliva-Sosa NE, Alarcón-Galván G, Fernández-Garza
NE, Muñoz-Espinosa LE, et al: Temporal relationship of serum
markers and tissue damage during acute intestinal
ischemia/reperfusion. Clinics (Sao Paulo). 68:1034–1038. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salim SY, Young PY, Lukowski CM, Madsen
KL, Sis B, Churchill TA and Khadaroo RG: VSL#3 probiotics provide
protection against acute intestinal ischaemia/reperfusion injury.
Benef Microbes. 4:357–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pérez-de Puig I, Miró F, Salas-Perdomo A,
Bonfill-Teixidor E, Ferrer-Ferrer M, Márquez-Kisinousky L and
Planas AM: IL-10 deficiency exacerbates the brain inflammatory
response to permanent ischemia without preventing resolution of the
lesion. J Cereb Blood Flow Metab. 33:1955–1966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bodhankar S, Chen Y, Vandenbark AA, Murphy
SJ and Offner H: IL-10-producing B-cells limit CNS inflammation and
infarct volume in experimental stroke. Metab Brain Dis. 28:375–386.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Volin MV and Koch AE: Interleukin-18: A
mediator of inflammation and angiogenesis in rheumatoid arthritis.
J Interferon Cytokine Res. 31:745–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Celik E, Faridi MH, Kumar V, Deep S, Moy
VT and Gupta V: Agonist leukadherin-1 increases
CD11b/CD18-dependent adhesion via membrane tethers. Biophys J.
105:2517–2527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He P, Srikrishna G and Freeze HH:
N-glycosylation deficiency reduces ICAM-1 induction and impairs
inflammatory response. Glycobiology. 24:392–398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pillay J, Kamp VM, Pennings M, Oudijk EJ,
Leenen LP, Ulfman LH and Koenderman L: Acute-phase concentrations
of soluble fibrinogen inhibit neutrophil adhesion under flow
conditions in vitro through interactions with ICAM-1 and MAC-1
(CD11b/CD18). J Thromb Haemos. 11:1172–1182. 2013. View Article : Google Scholar
|
|
29
|
Wu R, Zhou Q, Lin S, Ao X, Chen X and Yang
J: Effect of cordceps sinensis on the expression of ICAM-1 and
VCAM-1 in the kidney of spontaneously hypertensive rats. Zhong Nan
Da Xue Xue Bao Yi Xue Ban. 35:152–158. 2010.(In Chinese).
PubMed/NCBI
|
|
30
|
Sakai N, Shin T, Schuster R, Blanchard J,
Lentsch AB, Johnson WT and Schuschke DA: Marginal copper deficiency
increases liver neutrophil accumulation after ischemia/reperfusion
in rats. Biol Trace Elem Res. 142:47–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu MW, Wang YH, Qian CY and Li H:
Xuebijing exerts protective effects on lung permeability leakage
and lung injury by upregulating Toll-interacting protein expression
in rats with sepsis. Int J Mol Med. 34:1492–1504. 2014.PubMed/NCBI
|
|
32
|
Gong P, Lu Z, Xing J, Wang N and Zhang Y:
Traditional Chinese medicine Xuebijing treatment is associated with
decreased mortality risk of patients with moderate paraquat
poisoning. PLoS One. 10:e01235042015. View Article : Google Scholar : PubMed/NCBI
|