Introduction
Hericium erinaceus (H. erinaceus), a
wood-rotting fungus, is a wild edible delicacy in China. Due to its
edibility and functionality, the mushroom has attracted
considerable attention. H. erinaceus is able to alleviate
complex neurodegeneration, decrease gastric mucosal damage and
significantly reduce the levels of plasma triglycerides (1). In addition, H. erinaceus
possesses antioxidant activities, immunoregulatory activities and
anti-cancer roles (1). H.
erinaceus contains proteins, unsaturated fatty acids,
carbohydrates and a variety of trace elements, including
phosphorus, sulfur, calcium, magnesium, zinc, iron and copper
(1). All the nutrients serve
different important roles in the multiple physiological systems of
the organism, including the nervous, digestive, circulatory and
immune systems (1).
The physiological systems in the body should be
balanced to maintain whole-body homeostasis. Once the balance is
disturbed, a disease can occur (2).
There is increasing evidence to indicate that oxidative stress is
one of the causes of complicated diseases, including cancer and
Alzheimer's disease in humans (3).
For example, oxidative stress can contribute to tissue and cell
injury and accelerate the proliferation of colorectal cancer cells
(4). Reactive oxygen species (ROS)
and lipid peroxides are damaging compounds involved in the
oxidative stress process. An imbalance between ROS levels and
antioxidant defenses occurs in various different diseases, such as
Crohn's disease (5). Intake of
antioxidants is important to sustain an adequate level in order to
balance the ROS in vivo. A number of studies have
demonstrated the close association between the prevention of
ROS-associated diseases and intake of food rich in antioxidants,
including mushrooms (6).
As a medicinal mushroom, H. erinaceus has
abundant active substances that perform antioxidative roles. The
ethanol and hot water extracts of H. erinaceus have
antioxidant activities, including reducing ability, chelating
effects on ferrous ions, 1-diphenyl-2-picrylhydrazyl (DPPH) free
radical scavenging activity and inhibition of lipid peroxidation in
emulsified corn oil or in emulsified egg yolk buffer (6,7).
Polysaccharides (water extracts) have a strong antioxidant activity
in vitro, and they have been demonstrated to significantly
increase the activity of antioxidant enzymes in vivo in a
dose-dependent manner (8,9). Therefore, H. erinaceus is a
plentiful source of exogenous antioxidants, which may be considered
important remedies for ameliorating pathological alterations in
oxidative stress-associated disease. There is great diversity of
active molecules in the fruit-body with different biological
activities (10). The active
ingredients in H. erinaceus can not be 100% extracted by a
single reagent. Furthermore, different bioactive molecules have
different solubilities in various solvents (11). Therefore, it is essential to evaluate
the antioxidant activities of extracts produced by multiple
representative solvents with different polarities.
The majority of the reports on antioxidant
properties of H. erinaceus utilize ethanol, methanol and hot
water for preparation of extracts, individually. However, there are
various extraction conditions available in the literature.
Therefore, it is rather difficult to compare the activities of
different extracts when using different extraction conditions.
Additionally, there are various other representative solvents,
which are usually used to extract bioactive compounds.
Nevertheless, few previous studies have investigated them. Hence,
the objective of the present study was to analyze the in
vitro antioxidant properties of H. erinaceus extracts
produced by eight reagents, including n-hexane, xylene, chloroform,
anhydrous ether, ethyl acetate, acetone, anhydrous ethanol and
distilled water.
Materials and methods
Material preparation
Fresh H. erinaceus mushrooms (Houza 19
strain) were obtained from the Institute of Edible Fungus at Anhui
Science and Technology University (Fengyang, China) at a mature
stage. The mushrooms were sorted, cleaned, washed in cold
sterilized water and drained. The fresh mushrooms were then
lyophilized and powdered with a mixer (Zhejiang Ronghao Industry
and Trade Co., Ltd., Zhejiang, China). The extractions and analyses
were immediately conducted following lyophilization. All chemicals
and reagents used in the present study were purchased from
Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) unless
otherwise stated.
Preparation of crude extracts
The powder was then subjected to extraction by the
following eight solvents: n-Hexane, xylene, chloroform, anhydrous
ether, ethyl acetate, acetone, anhydrous ethanol and distilled
water. The optimized extraction conditions were used as follows: 20
g powder and 100 ml reagent solution (100%) added into a flask and
extracted at room temperature for 24 h. This process was repeated
twice more. The liquid was collected by centrifugation (2,500 × g,
5 min, room temperature), and then condensed by rotary evaporation
at 50°C. The extracts were put into an evaporating dish and dried
in a water bath at 50°C. The crude extracts were dissolved in
methanol, yielding a series of sample solutions with different
concentrations.
Determination of reducing power
The reducing power was determined by the method of
Jiang et al (12). The
K4Fe(CN)6 was generated after the
antioxidants reacted with K3Fe(CN)6. Then
K4Fe(CN)6 reacted with FeCl3 to
produce Perl's Prussian blue
(Fe4[Fe(CN)6]3) which had the
maximum absorbance at 700 nm. The reaction mixture containing 2.5
ml different concentrations of samples in phosphate-buffered saline
(0.2 M, pH 6.6) was incubated with 2.5 ml K3Fe
(CN)6 (1%, w/v) at 50°C for 20 min. The reaction was
terminated by adding 2.5 ml trichloroacetic acid (10%) and the
mixture was centrifuged at 1,400 × g for 10 min. The supernatant
(2.5 ml) was mixed gently with 2.5 ml distilled water and 0.5 ml
FeCl3 (0.1%, w/v) and the absorbance was measured at 700
nm against a blank sample (V-1200 spectrophotometer; Shanghai
Meipuda Instrument Co., Ltd., Shanghai, China). A higher absorbance
indicated a higher reducing power. The experiments were performed
in triplicate and the data was the average of all three.
DPPH radical scavenging activity
The DPPH radical scavenging activity of the samples
was measured according to the procedure described by Negro et
al (13). The solution of DPPH
(0.2 mM; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in
methanol and was freshly prepared prior to the measurements. In
brief, the extracted solutions (2 ml) were thoroughly mixed with 2
ml DPPH solution and allowed to stand for 30 min in the dark. The
blank control solution contained an equal volume of distilled water
instead of the sample solution. The absorbance was measured at 517
nm (V-1200 spectrophotometer). Lower absorbance of the reaction
mixture indicated higher free radical scavenging activity. The
experiments were performed in triplicate and the data were
averaged. The scavenging rate was calculated according to the
following equation: DPPH scavenging rate (%) =
(Ac-As) × 100/Ac, in which
Ac was the absorbance of the control, and As
was the absorbance of the sample.
Superoxide anion radical scavenging
activity
The superoxide radical scavenging activity of the
samples was evaluated according to the method reported by Chen
et al (14) with a slight
modification. The extract produced by different solvents was
dissolved in 2 ml methanol to form varying concentrations. The 2 ml
extracts and 2 ml Tris-HCl buffer (50 mM, pH 8.2) were blended and
incubated at 37°C for 20 min. Next, the solution was mixed with 1
ml 50 mM pyrogallol solution (preheated to 37°C) and the absorbance
variation was detected within 2 min at 325 nm (UV762 ultraviolet
and visible spectrophotometer; Shanghai Jingke Scientific
Instrument Co., Ltd., Shanghai, China). For the blank control, the
sample was replaced by Tris-HCl buffer. The scavenging rate was
calculated within 2 min by the following equation: Scavenging rate
(%) = (∆Ac-∆As) × 100/∆Ac, in
which ∆As and ∆Ac were the absorbance
variations of the extractions and the control.
Inhibition of lipid peroxidation
The peroxide value (POV) was used to assess the
inhibition ability of extracts against lipid peroxidation. POV was
measured as described in the Standard Methods for the Analysis of
Fats and Oils established by China (GB/T5538-2005) (15). Autoxidation caused the formation of
peroxides, which in turn resulted in the release of iodine. The
free iodine was then titrated with a sodium thiosulfate solution
(15). To artificially induce a
quick oxidation of the corn oil, all samples were marked and stored
in an oven at 60°C for 120 h. During this process of oxidation, the
POVs of samples were determined every 24 h using the Standard
Methods for the Analysis of Fats and Oils established by China
(GB/T5538-2005) (15). In brief, 2 g
oil sample (the minimal effective concentration of extracts was 5%)
was mixed with 50 ml isooctane and acetic acid mixture (2:3, v/v).
Next, 0.5 ml saturated potassium iodide solution was added to the
reaction system. The mixture was left in the darkness for 3 min and
the supernatant was titrated with sodium thiosulfate solution (2
mM). The POV was expressed as milliequivalents of active oxygen per
kilogram of oil (meq/kg). The experiment was performed in
triplicate. The POV was calculated as follows: POV (meq/kg) = 1,000
× ∆V xc/w, in which the c was the molar concentration of sodium
thiosulfate solution, and w was the mass of the oil sample. ∆V was
the volume variation of the sodium thiosulfate solution that was
consumed by the sample and the reagent blank control.
Statistical analysis
The antioxidant data were analyzed using analysis of
variance in the SPSS statistics software version 18.0 (SPSS, Inc.,
Chicago, IL, USA) for significance. The data are expressed as the
mean ± standard deviation. P<0.05 and P<0.01 were considered
to indicate a statistical significant difference. All the graphs
were prepared using Microsoft Excel 2003 (Microsoft, Redmond, WA,
USA).
Results
Reducing power
The antioxidants donate electrons and convert the
oxidized form of iron (Fe3+) to the ferrous form
(Fe2+), which can be monitored by measuring the
formation of Perl's Prussian blue at 700 nm (16). The efficacy of certain antioxidants
is associated with their reducing power (17). In the present assay, the results
demonstrated that the absorbance, which reflects the reducing
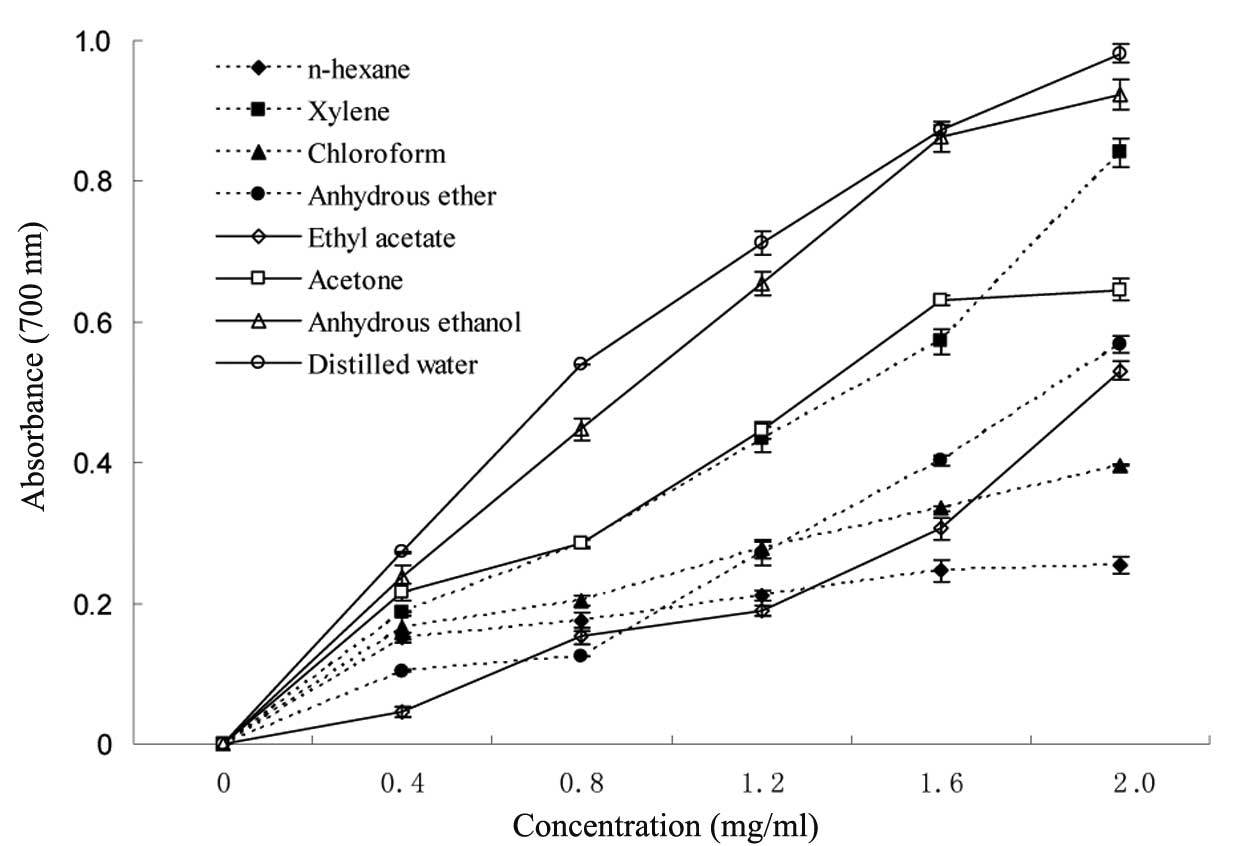
power, increased with the concentration of extractions (Fig. 1). The reducing powers at a maximum
concentration of 2 mg/ml ranged between 0.254 for n-Hexane and
0.982 for distilled water. The reducing powers of the extractions
that were produced by distilled water and anhydrous ethanol yielded
the higehst absorbance levels. Thus, it may be deduced that the
aqueous and alcoholic extracts possess significant levels of
antioxidant compounds. The reducing power of the compounds after
n-hexane extraction was the lowest.
DPPH radical scavenging activity
The stable DPPH free radical has an absorption of
517 nm in methanol (18). It can be
widely used to evaluate the free radical scavenging ability of
natural compounds with the hydrogen-donating ability (16). The antioxidant donates protons to the
DPPH radical and the absorption decreases with the discoloration of
the initial purple compound. The radical scavenging activity
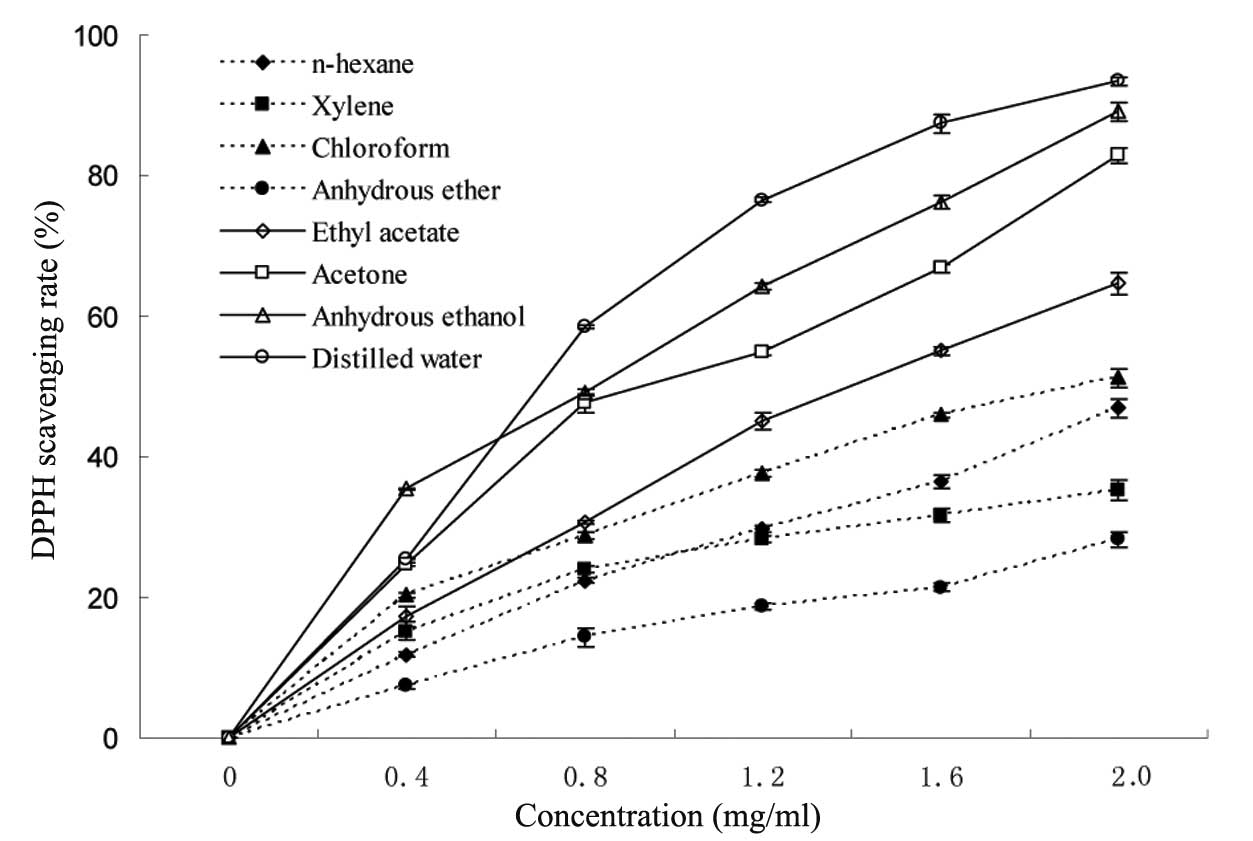
increases as the absorption at 517 nm is decreased (19). The results demonstrated that the DPPH
radical scavenging activities of all types of extractions were
concentration-dependent (Fig. 2).
The extractions at high doses exhibited significant scavenging
effects on DPPH free radicals. The scavenging activity of different
extracts at 2 mg/ml ranged from 28.2 to 93.4%. The distilled water
and anhydrous ethanol presented the highest DPPH scavenging
activities, whereas, the anhydrous ether extracts had the lowest
DPPH scavenging activities, whereas, the extractions obtained from
the four solvents with the higher polarity had a stronger
scavenging rate than those from the four solvents with the lower
polarity. The effective components in H. erinaceus with
different polarities could be extracted by the solvents with the
corresponding polarities. Furthermore, the biological activities of
the effective components were different when the polarities were
different. The results indicated that the scavenging activity of
the extractions was associated with the polarities of the used
reagents, and, the stronger the polarity of the solvent was, the
higher the DPPH scavenging activity of its extraction.
Superoxide anion radical scavenging
activity
Superoxide anions are the most common free radicals
in vivo generated in a variety of biological systems. Higher
levels of superoxide anion radicals are regarded as the beginning
of ROS accumulation in cells. In addition, they have been
implicated in a number of human diseases, including cancer and
diabetes (20). In the present
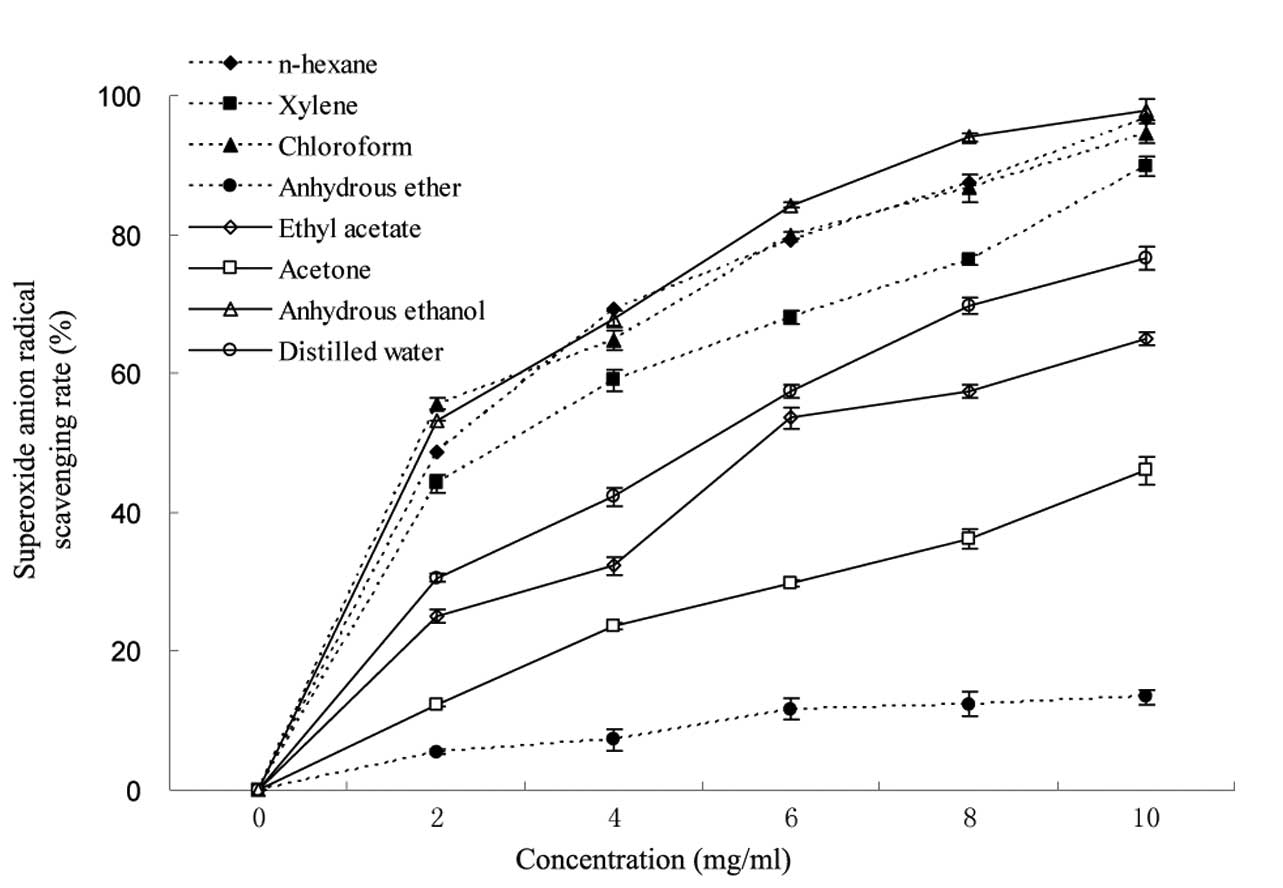
study, the superoxide anion radical scavenging effects of different
extractions exhibited a dose-dependent association (Fig. 3). The scavenging rate of different
extractions at 10 mg/ml was in the range of 13.4–97.9%.
Furthermore, the extractions from anhydrous ethanol, n-hexane and
chloroform had strong scavenging activities against superoxide
anion radicals, whilst that of anhydrous ether was the weakest.
Lipid peroxidation
Edible oils can be autoxidized in the air. The
peroxidation of lipids is a free radical driven chain reaction and
various toxic secondary products are generated (21). In addition, these products are known
to damage DNA and cell and organelle membranes, or cause cancers,
such as liver cancer. Antioxidants terminate the lipid peroxidation
chain reactions by removing free radical intermediates or
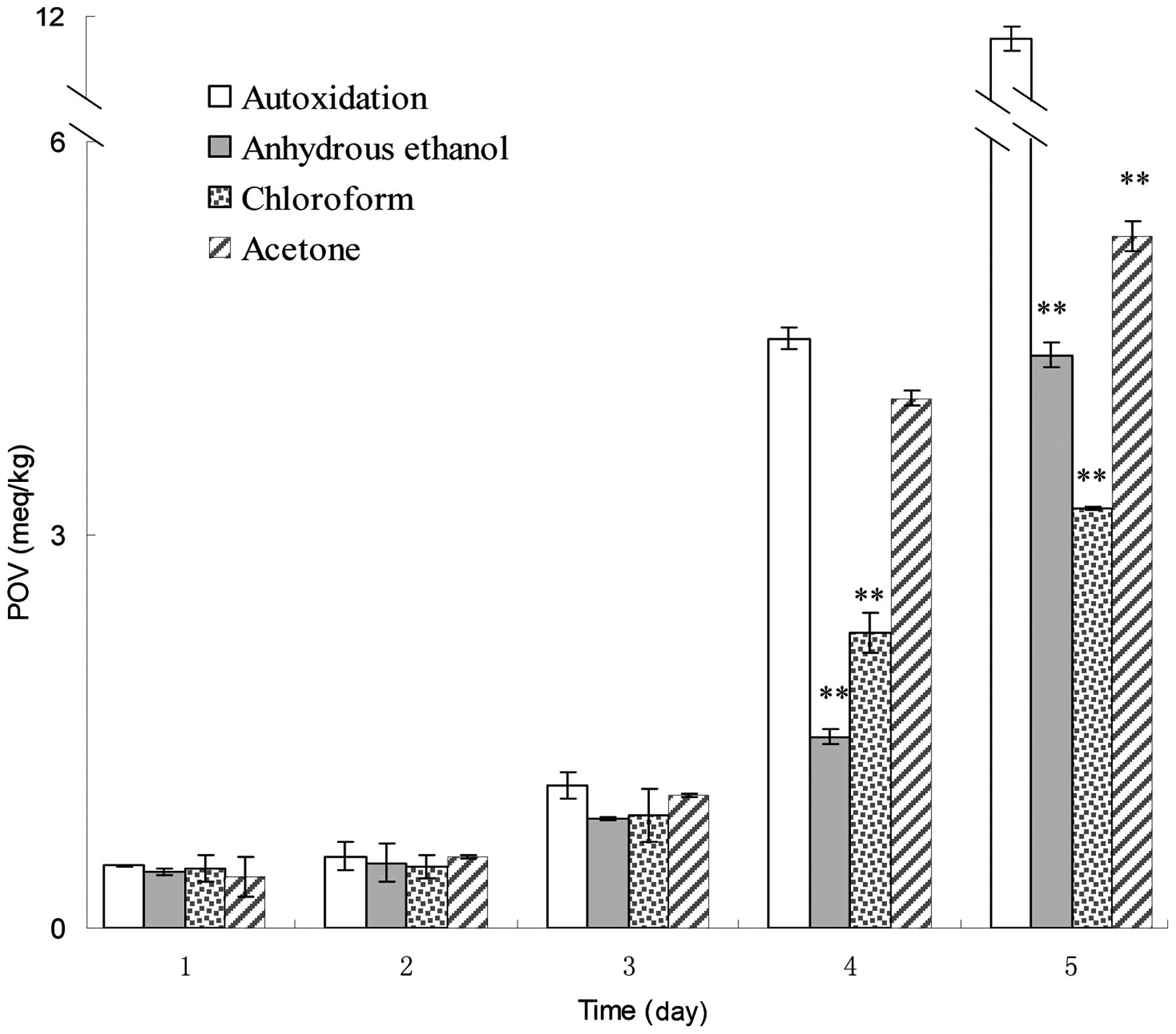
inhibiting other oxidation reactions (22). Amongst the eight extractions, the
anhydrous ethanol, chloroform and acetone extracts were capable of
inhibiting lipid peroxidation in the corn oil system, as their POVs
were lower than that of corn oil autoxidation (Fig. 4). The extractions did not
significantly reduce the peroxide formation at the early stages
(days 1–3) of the experiment. On the fourth day, the inhibition
rates of anhydrous ethanol, chloroform and acetone extracts were
67.5, 50 and 10.2%, respectively, as compared with the POV of corn
oil auto-oxidation. On the fifth day, anhydrous ethanol, chloroform
and acetone extracts caused 62, 71.2 and 54.1% reductions in the
formation of peroxides, respectively compared with oil
autooxidation. Following statistical analysis, the three
extractions on days 4 and 5 were observed to significantly inhibit
lipid peroxidation at a 0.01 significance level.
Discussion
H. erinaceus is a notable mushroom with
medicinal values; it contains various bioactive compounds with drug
efficacy. The nutrients in H. erinaceus include herinase
(23) and isohericenone (24). In the present study, eight reagents
with nonpolar, weak, middle and strong polarity were used to
extract the active compounds from H. erinaceus.
The compounds in H. erinaceus have their own
chemical structures and properties. They also have different
solubilities in different chemical solvents (10). Thus, each extraction inevitably was a
mixture of numerous constituents. Amongst the mixture, there were
antioxidants and synergists, which increase the antioxidant roles.
The extracts from H. erinaceus produced by eight reagents
all exhibited a reducing power and scavenging activity against DPPH
and superoxide anion free radicals. The antioxidant activities
varied with the extraction concentrations and the chemical reagents
that were used. The mixtures extracted by the solvents with similar
polarity presented different specific activities. For example, the
scavenging activities against DPPH and superoxide anion radical of
anhydrous ether extraction were low, although it had a middle-level
reducing power. The xylene extracts had a relatively high reducing
power and superoxide anion radical scavenging activity, however,
they did not effectively scavenge the DPPH radical. Similar results
were observed for other reagents with nonpolar, weak and middle
polarity. The results indicate that each extraction may have its
own specific function in different antioxidant assays, since the
extractions have complicated constituents that possess diverse
chemical characteristics. In the reducing power and free radical
scavenging assays, the activities of anhydrous ethanol and
distilled water extractions were among the highest. This may
indicate that polar ingredients contribute greatly to the
antioxidant activities. However, the water extraction did not
inhibit lipid peroxidation. It is possible that the efficient
compounds were not fully extracted at the low extraction
temperature that was used. Based on the results of the current
study, anhydrous ethanol appears to be the most efficient
extraction solvent that can be used to extract the effective
antioxidant compounds in the food and pharmaceutical
industries.
In the present study, four of the most frequently
used methods were performed to detect the antioxidant activity of
the extracts. Each method has a different mechanism, such as
termination of the free-radical mediated chain reaction, hydrogen
donation and elimination of peroxides (25). The results demonstrate that there is
no universal method to give an accurate and comprehensive picture
of an antioxidant profile (26).
Thus, multiple assays based on different antioxidant mechanisms are
crucial in order to provide a reliable approach of measuring the
antioxidant capacity of the extractions.
Under pathological conditions, free radicals and
lipid peroxides damage the proteins, membranes and nucleic acids
through numerous biological processes, thus giving rise to a
variety of diseases, such as gastrointestinal mucosal diseases
(27). Therefore, the demand for
discovering novel compounds with good antioxidant activity is
understandable (28). However,
nowadays many people are in the sub-health status, which is an
immediate stage between health and disease (29); and although the disease is in the
early stages in these patients, the oxidative pressure in the body
gradually increases and the antioxidant capacity gradually
decreases (30). There is growing
evidence that oxidative stress has a pivotal role in the
pathogenesis of numerous diseases (31). The risk of diseases, including type 2
diabetes, atherosclerosis and cancer, is increasing. Therefore, the
prevention of disease with potential natural, anti-oxidative
compounds would be of great significance to human health. In the
present study, extractions from H. erinaceus by different
solvents have been demonstrated to have dose-dependent antioxidant
activities. Since H. erinaceus is a source of natural
bioactive compounds, its antioxidant compounds may be prospective
protective agents that will help reduce the problem of suboptimal
health statuses in humans and even reduce oxidative
damage-associated diseases. The present study provides reference
data for the application of H. erinaceus in the
pharmaceutical industry and for disease prevention. Additionally,
the broad medicinal values of mushrooms such as H. erinaceus
have a promising future in alternative medicine. To understand the
mechanisms behind the properties of the active ingredients, further
study is required. Furthermore, a greater understanding of the
structures and functions of the components that are responsible for
the observed antioxidant activities is also required.
Acknowledgements
The present work was supported by the Foundation of
Anhui Educational Commission of China (grant no. KJ2013A075), the
Revitalization Plan of Anhui Province (grant no. gxyqZD2016222),
and the National Natural Science Foundation of China (grant no.
31250002).
References
|
1
|
Jiang SJ, Wang SH, Sun YJ and Zhang Q:
Medicinal properties of Hericium erinaceus and its potential
to formulate novel mushroom-based pharmaceuticals. Appl Microbiol
Biotechnol. 98:7661–7670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shimokawa H and Satoh K: Light and dark of
reactive oxygen species for vascular function: 2014 ASVB (Asian
Society of Vascular Biology). J Cardiovasc Pharmacol. 65:412–418.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holmes C: Review: Systemic inflammation
and Alzheimer's disease. Neuropathol Appl Neurobiol. 39:51–68.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang KA, Kim KC, Bae SC and Hyun JW:
Oxidative stress induces proliferation of colorectal cancer cells
by inhibiting RUNX3 and activating the Akt signaling pathway. Int J
Oncol. 43:1511–1516. 2013.PubMed/NCBI
|
|
5
|
Alzoghaibi MA: Concepts of oxidative
stress and antioxidant defense in Crohn's disease. World J
Gastroenterol. 19:6540–6547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdullah N, Ismail SM, Aminudin N, Shuib
AS and Lau BF: Evaluation of selected culinary-medicinal mushrooms
for antioxidant and ACE inhibitory activities. Evid Based
Complement Alternat Med. 2012:4642382012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fui HY, Shieh DE and Ho CT: Antioxidant
and free radical scavenging activities of edible mushrooms. Journal
of Food Lipids. 9:35–46. 2002. View Article : Google Scholar
|
|
8
|
Han ZH, Ye JM and Wang GF: Evaluation of
in vivo antioxidant activity of Hericium erinaceus
polysaccharides. Int J Biol Macromol. 52:66–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu H, Wu PR, Shen ZY and Chen XD: Chemical
analysis of Hericium erinaceum polysaccharides and effect of
the polysaccharides on derma antioxidant enzymes, MMP-1 and TIMP-1
activities. Int J Biol Macromol. 47:33–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan MA, Tania M, Liu R and Rahman MM:
Hericium erinaceus: An edible mushroom with medicinal
values. J Complement Integr Med. 10:253–258. 2013. View Article : Google Scholar
|
|
11
|
Snitsarev V, Young MN, Miller RM and
Rotella DP: The spectral properties of (−)-epigallocatechin
3-O-gallate (EGCG) fluorescence in different solvents: Dependence
on solvent polarity. PLoS One. 8:e798342013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang S, Ma Y and Yan D: Antioxidant and
antimicrobial properties of water soluble polysaccharide from
Arachis hypogaea seeds. J Food Sci Technol. 51:2839–2844.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Negro C, Tommasi L and Miceli A: Phenolic
compounds and antioxidant activity from red grape marc extracts.
Bioresour Technol. 87:41–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen H, Yan M, Zhu J and Xu X: Enhancement
of exo-polysaccharide production and antioxidant activity in
submerged cultures of Inonotus obliquus by lignocellulose
decomposition. J Ind Microbiol Biotechnol. 38:291–298. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi R, Zhang Q, Vriesekoop F, Yuan Q and
Liang H: Preparation of organogel with tea polyphenols complex for
enhancing the antioxidation properties of edible oil. J Agric Food
Chem. 62:8379–8384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vamanu E: In vitro antimicrobial and
antioxidant activities of ethanolic extract of lyophilized mycelium
of Pleurotus ostreatus PQMZ91109. Molecules. 17:3653–3671.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moein MR, Moein S and Ahmadizadeh S:
Radical scavenging and reducing power of Salvia mirzayanii
subfractions. Molecules. 13:2804–2813. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nahar L, Nasrin F, Zahan R, Haque A, Haque
E and Mosaddik A: Comparative study of antidiabetic activity of
Cajanus cajan and Tamarindus indica in
alloxan-induced diabetic mice with a reference to in vitro
antioxidant activity. Pharmacognosy Res. 6:180–187. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mensor LL, Menezes FS, Leităo GG, Reis AS,
dos Santos TC, Coube CS and Leitão SG: Screening of Brazilian plant
extracts for antioxidant activity by the use of DPPH free radical
method. Phytother Res. 15:127–130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rajendran P, Nandakumar N, Rengarajan T,
Palaniswami R, Gnanadhas EN, Lakshminarasaiah U, Gopas J and
Nishigaki I: Antioxidants and human diseases. Clin Chim Acta.
436:332–347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rudbäck J, Bergström MA, Börje A, Nilsson
U and Karlberg AT: α-Terpinene, an antioxidant in tea tree oil,
autoxidizes rapidly to skin allergens on air exposure. Chem Res
Toxicol. 25:713–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fritz KS and Petersen DR: Exploring the
biology of lipid peroxidation-derived protein carbonylation. Chem
Res Toxicol. 24:1411–1419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi BS, Sapkota K, Choi JH, Shin CH, Kim
S and Kim SJ: Herinase: A novel bi-functional fibrinolytic protease
from the monkey head mushroom, Hericium erinaceum. Appl
Biochem Biotechnol. 170:609–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim KH, Noh HJ, Choi SU and Lee KR:
Isohericenone, a new cytotoxic isoindolinone alkaloid from
Hericium erinaceum. J Antibiot (Tokyo). 65:575–577. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prior RL, Wu X and Schaich K: Standardized
methods for the determination of antioxidant capacity and phenolics
in foods and dietary supplements. J Agric Food Chem. 53:4290–4302.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moon JK and Shibamoto T: Antioxidant
assays for plant and food components. J Agric Food Chem.
57:1655–1666. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhattacharyya A, Chattopadhyay R, Mitra S
and Crowe SE: Oxidative stress: An essential factor in the
pathogenesis of gastrointestinal mucosal diseases. Physiol Rev.
94:329–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou D, Ruan J, Cai Y, Xiong Z, Fu W and
Wei A: Antioxidant and hepatoprotective activity of ethanol extract
of Arachniodes exilis (Hance) Ching. J Ethnopharmacol.
129:232–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bi J, Huang Y, Xiao Y, Cheng J, Li F, Wang
T, Chen J, Wu L, Liu Y, Luo R and Zhao X: Association of lifestyle
factors and suboptimal health status: A cross-sectional study of
Chinese students. BMJ Open. 4:e0051562014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tokarz P, Kaarniranta K and Blasiak J:
Role of antioxidant enzymes and small molecular weight antioxidants
in the pathogenesis of age-related macular degeneration (AMD).
Biogerontology. 14:461–482. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lim JL, Wilhelmus MM, de Vries HE,
Drukarch B, Hoozemans JJ and van Horssen J: Antioxidative defense
mechanisms controlled by NRF2: State-of-the-art and clinical
perspectives in neurodegenerative diseases. Arch Toxicol.
88:1773–1786. 2014. View Article : Google Scholar : PubMed/NCBI
|