Introduction
Cholangiocarcinoma, which is the second most common
hepatobiliary cancer, is difficult to diagnose in the early stages,
particularly in extrahepatic cholangiocarcinoma (1). The majority of patients with
cholangiocarcinoma present with jaundice and an advanced stage
tumor, resulting in low resectability and curability, and a poor
long-term survival rate (1,2). Gemcitabine has been increasingly used
to treat patients with cholangiocarcinoma (3,4), and a
chemotherapy regimen of gemcitabine is commonly used for inoperable
cholangiocarcinoma; however, the efficacy is not satisfactory
(5).
Gene therapy is a promising therapeutic strategy for
treatment of patients with cholangiocarcinoma (6,7), and
herpes simplex virus-thymidine kinase/ganciclovir (HSV-TK/GCV)
suicide gene therapy is considered one of the most promising
therapeutic strategies for the treatment of cholangiocarcinoma
(8,9). Expression of the HSV-TK gene induces
the production of thymidine kinase, which metabolizes GCV to GCV
monophosphate, and cellular kinases subsequently convert
monophosphorylated GCV into its diphosphate and triphosphate forms.
GCV triphosphate is incorporated into DNA during cell division,
resulting in single-strand DNA breaks and the inhibition of DNA
polymerase, which induces DNA chain termination (10). These effects induce apoptotic
mechanisms (11), thus producing an
antitumor effect. A previous study has suggested that gemcitabine
may improve the efficacy of HSV-TK/GCV gene therapies (12). Therefore, in the current study, the
efficacy of HSV-TK was evaluated alone, and in combination with
gemcitabine, in QBC929 cells (selected and derived from a patient
with cholangiocarcinoma) and a mouse model of cholangiocarcinoma.
The aim of the study was to determine whether HSV-TK/GCV plus
gemcitabine may be essential for anti-tumor growth, and may
therefore prove to be a novel therapeutic method to enhance the
efficacy of cholangicarcinoma chemotherapy.
Materials and methods
Cell culture and the efficiency of
gene transfer
QBC939 human cholangiocarcinoma cell line was
obtained from Suer Biological Inc. (Shanghai, China). Cells were
maintained in RPMI 1640 medium supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
100 U/ml penicillin and 100 µg/ml streptomycin (Shanghai Institute
Hui Biotechnology Co., Ltd., Shanghai, China) in a humidified
incubator (18 M; Sanyo Electric Co., Ltd., Osaka, Japan) containing
5% CO2 at 37°C.
A total of 5×104 cells/well were plated
in 24-well plates and incubated overnight. Cells were transfected
with lipofectamine 2000 (Sigma-Aldrich, St. Louis, MO, USA) and
HSV/luciferase/lentivirus supernatant (provided by Department of
Molecular Biology, Heilongjiang University of Chinese Medicine,
Harbin, China) at a dosage of 106 IU/cell in the
presence of 8 µg/ml polybrene. The viral supernatant was removed
from the wells after 6–8 h, and the cells were re-infected with
fresh supernatant containing polybrene. The following day, the
viral supernatant was removed and the appropriate complete growth
medium (Gibco; Thermo Fisher Scientific, Inc.) was added to the
cells prior to incubation at 37°C in an atmosphere containing 5%
CO2 for 72 h. Following incubation, the cells were
subcultured into 100-mm dishes and treated with 200 µg/ml G418
(Real-Times Biotechnology Co., Ltd., Beijing, China) for 2 weeks in
order to select for stable cell lines. Positive clones were
selected and expanded to establish the cell lines. Transfected
cells stably expressing HSV-TK, as verified by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR),
were named QBC939/HSV.
Cell proliferation assay
QBC939/HSV cells (5×103 cells/well) were cultured in
96-well plates. Media was changed on the second day following
plating and the cells were exposed to one of four following
treatment options: i) Gemcitabine (3 µg/ml; Eli Lilly & Co.,
Indianapolis, IN, USA) only for 24 h, ii) HSV-TK and GCV (50 µg/ml;
Wuhan Hiteck Biological Pharma Co., Ltd., Wuhan, China) only for 72
h, iii) HSV-T plus gemcitabine (3 µg/ml) for 24 h followed by media
removal and replacement with media containing 50 µgml GCV for 72 h,
and iv) phosphate-buffered saline (PBS) control group. GCV and
gemcitabine were dissolved in sterile distilled water and diluted
in culture medium immediately prior to use. Gemcitabine and GCV
dosages were selected according to previous studies (13,14).
Cell proliferation was evaluated by MTT assay
(Beijing CellChip Biotechnology Co., Ltd., Beijing, China).
Briefly, 1 mg/ml MTT was added to the wells and incubated in an
atmosphere containing 5% CO2 for 4 h. Absorbance was
measured at 490 nm using a microplate reader (FLx800; BioTek,
Winooski, VT, USA). Relative cell proliferation rates of the
various groups were evaluated using the equation
Atreated/Acontrol, where A is the absorbance.
All experiments were repeated six times for each cell group.
RT-qPCR
Total RNA was extracted from QBC939/HSV cells using
an AllPrep DNA/RNA Mini kit (Qiagen, Inc., Valencia, CA, USA). RNA
purification was performed using an RNeasy Kit (Qiagen, Inc.).
RT-qPCR was performed using an Mx3005P™ RT-qPCR system (Stratagene;
Agilent Technologies, Inc., Santa Clara, CA, USA). Reverse
transcription was performed at 42°C for 1 h. Super Taq Polymerase
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used in
the PCR reaction. RT-qPCR amplification mixtures (25 µl) contained
25 ng template cDNA, 2x SYBR Green I Master Mix buffer (12.5 µl;
Applied Biosystems; Thermo Fisher Scientific, Inc.) and 300 nM
forward and reverse primers. Reactions were run on an ABI PRISM
5700 Sequence Detector (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermal cycling conditions were as follows:
Activation for 10 min at 95°C, denaturation for 40 cycles at 95°C
for 15 sec, and annealing/extension at 60°C for 60 sec. The
following primers were used: HSV-TK, forward
5′-GGTGATGACCTCTGCCCAGAT-3′, and reverse
5′-TGTGAGGAGCCAGAACAGCAT-3′; and a human control GAPDH primer set
from the RT-qPCR kit. PCR cycling was performed as follows: Initial
denaturation at 50°C for 2 min followed by 35 cycles of 94°C for 30
sec, 54°C for 30 sec and 72°C for 30 sec, and final elongation at
72°C for 5 min. Each assay included (in duplicate): A standard
curve of four serial dilution points of cDNA (ranging, 50 ng-50
pg), a no-template control, a no RT control and 25 ng of each test
cDNA. PCR products were analyzed using 3% agarose gel
electrophoresis stained with ethidium bromide using Applied
Biosystems Sequence Detection Software version 1.3 (Applied
Biosystems; Thermo Fisher Scientific, Inc.).
Animal model and therapy
The animal protocol of the present study was
approved by the Institutional Animal Care and Use Committee. At the
end of the experiments, mice were sacrificed by asphyxiation with
CO2 (99.9%). Cholangiocarcinoma tumor xenografts were
established in 24 mice (age, 4–6 weeks) by subcutaneously
inoculating the left back of each mouse with QBC939 cells (5×106
cells/well). Once the tumor size was 8–10 mm in diameter, the mice
were randomly divided into four groups: i) Gemcitabine group,
intratumoral injection of 100 µl phosphate-buffered saline (PBS),
followed by intraperitoneal injection of 50 mg/kg gemcitabine; ii)
gene, intratumoral injection of HSV-TK gene (108) in 100 µl PBS,
followed by intraperitoneal injection of 50 mg/kg GCV; iii)
gemcitabine + gene, intratumoral injection of HSV-TK gene (108) in
100 µl PBS, followed by intraperitoneal injection of 50 mg/kg GCV
and 50 mg/kg gemcitabine; and iv) control, intratumoral injection
of 100 µl PBS, followed by intraperitoneal injection of PBS.
Gemcitabine was injected once, and GCV was injected for 14 days.
Gemcitabine and GCV dosages were selected according previous
studies (15,16). The general conditions of the mice
were monitored daily.
Imaging studies
Mice were anesthetized with 5% isoflurane gas
(Shandong Keyuan Pharmaceutical Co., Jinan, China) and maintained
with 2% isoflurane in a supine position in order to perform optical
and ultrasound (US) imaging. Images were captured at day 1 prior to
treatment and at days 4, 7, 10 and 14 following treatment.
Optical imaging was performed using an Fx Pro
molecular imaging system (Bruker Bio Spin Corporation, Billerica,
MA, USA). Intraperitoneal injection of 300 mg/kg d-luciferin
(Shanghai Yeasen Biotechnology Co., Ltd., Shanghai, China) was
administered and images were captured at 20 min (binning, 4×4).
Exposure time was adjusted for each image in order to ensure that
the acquired images were presented in the same scale. X-ray was
exposed for 2 min. The region of interest (ROI) was drawn using the
imaging software (Fx Pro; Bruker Bio Spin Corporation), and the
photon flux (photon/s) was subsequently measured. An untreated site
on each image was used to normalize the signals against background
noise.
Tumor sizes were measured using a Mindray DC-T6 US
machine (Mindary Medical International, Ltd., Beijing China). The
tumor mass was coated in warmed (37°C) Aquasonic ultrasound gel
(Parker Laboratories, Inc., Fairfield, NJ, USA) and centered on the
imaging plane. The two longest perpendicular axes were positioned
at the X and Y planes, and the depth axes, defined as the Z of each
tumor mass, were measured. The volume of each tumor mass was then
calculated according to following equation: Volume = X × Y × Z ×
π/6.
Histological conformation
Tumor masses of the mice were harvested at day 14
following treatment. Tumor tissues were embedded in optical cutting
temperature compound (Sakura Finetek USA, Inc., Torrance, CA, USA),
frozen in liquid nitrogen and maintained at −80°C. Tumor tissues
were then cryosectioned into 10-µm sections for apoptosis, and
hematoxylin and eosin (Boster Biotechnology Co., Ltd., Wuhan,
China) staining. Level of apoptosis was determined via a terminal
deoxynucleotidyl transferase dUTP nick end-labeling assay using a
TACS XL Blue Label kit (Trevigen, Gaithersburg, MD, USA) according
to the manufacturer's protocol. For each slide, images from random
six fields were captured using an Olympus digital camera (IX53;
Olympus Corporation, Tokyo, Japan). Apoptosis results were analyzed
using the apoptotic index, which was defined as the number of
apoptotic cells/total number of cells in each field.
Statistical analysis
SPSS version 19.0 (IMB SPSS, Amronk, NY, USA) was
used to perform all data analysis. Data were presented as the mean
± standard deviation. A non-parametric Mann-Whitney U test was used
to compare the relative proliferation rates between the different
in vitro cell groups and the relative signal intensities
(RSI) of fluorescence between the different in vivo mice
groups. RSI values were normalized using the following equation:
RSI = SI Dn/SI D0, where SI is the signal intensity, Dn represents
the days after treatment and D0 is the day prior to treatment.
Between-in vivo group differences in the tumor volumes were
analyzed using Student's t-test. Р≤0.05 was considered to indicate
a statistically significant difference.
Results
Administration of gemcitabine + HSV-TK
significantly decreases cell proliferation in vitro
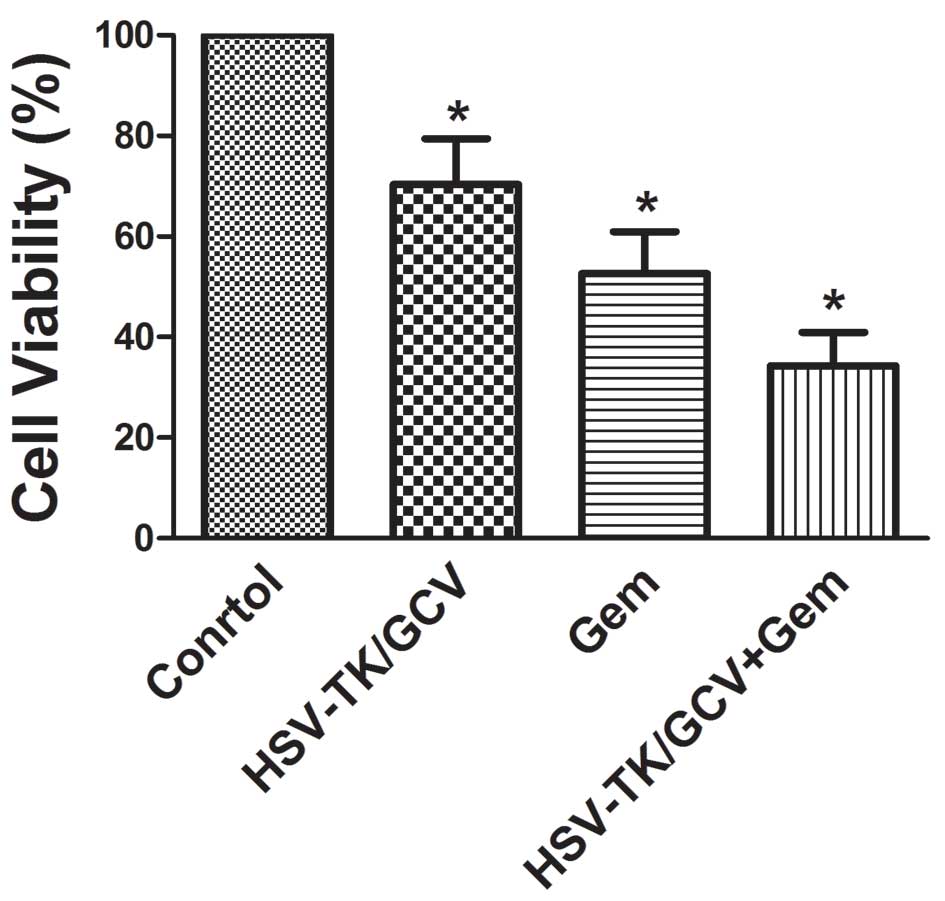
In vitro, the relative viability of cells in
the gene treatment, gemcitabine and gemcitabine + gene groups
(70.37±9.07, 52.64±8.28 and 34.21±6.63%, respectively were
significantly decreased, as compared with the control group
(Р<0.05; Fig. 1). Furthermore,
cell viability was lowest in the combined treatment group and was
significantly reduced, as compared with the gene treatment and
gemcitabine monotherapy groups (Р<0.05). Relative cell viability
values were significantly lower in the cell group receiving
gemcitabine, as compared with the HSV-TK + GCV group (52.64±8.28
vs. 70.37±9.07%; Р<0.05). HSV-TK mRNA expression levels in the
transduced QBC939 cells were assessed using RT-qPCR and agarose gel
electrophoresis was used to detect the HSV-TK mRNA 237-bp
product.
Administration of gemcitabine + HSV-TK
significantly decreases tumor signals in a mouse model of
subcutaneous cholangiocarcinoma
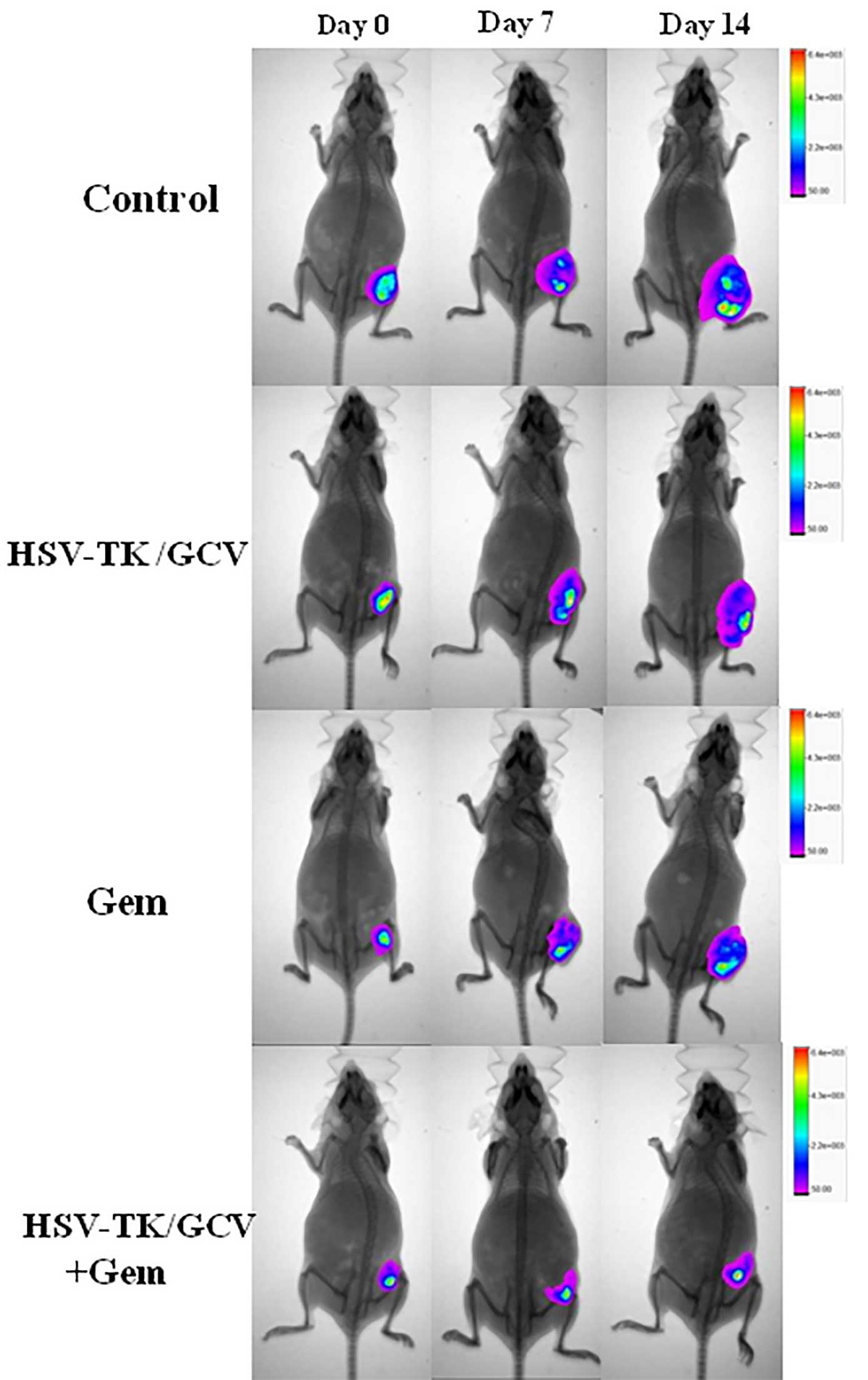
All mice survived the in vivo experiments
performed in the present study. Fig.
2 shows the optical images captured of the various groups.
Follow-up optical imaging on day 14 after treatment demonstrated
significantly decreased optical signals in the gemcitabine + gene
group, as compared with the other three groups (1.68±0.74 vs.
2.27±0.58, 2.87±0.82 and 3.79±0.72, respectively; Р<0.05).
Gemcitabine + HSV-TK combination
therapy significantly reduces tumor volume in a mouse model of
subcutaneous cholangiocarcinoma
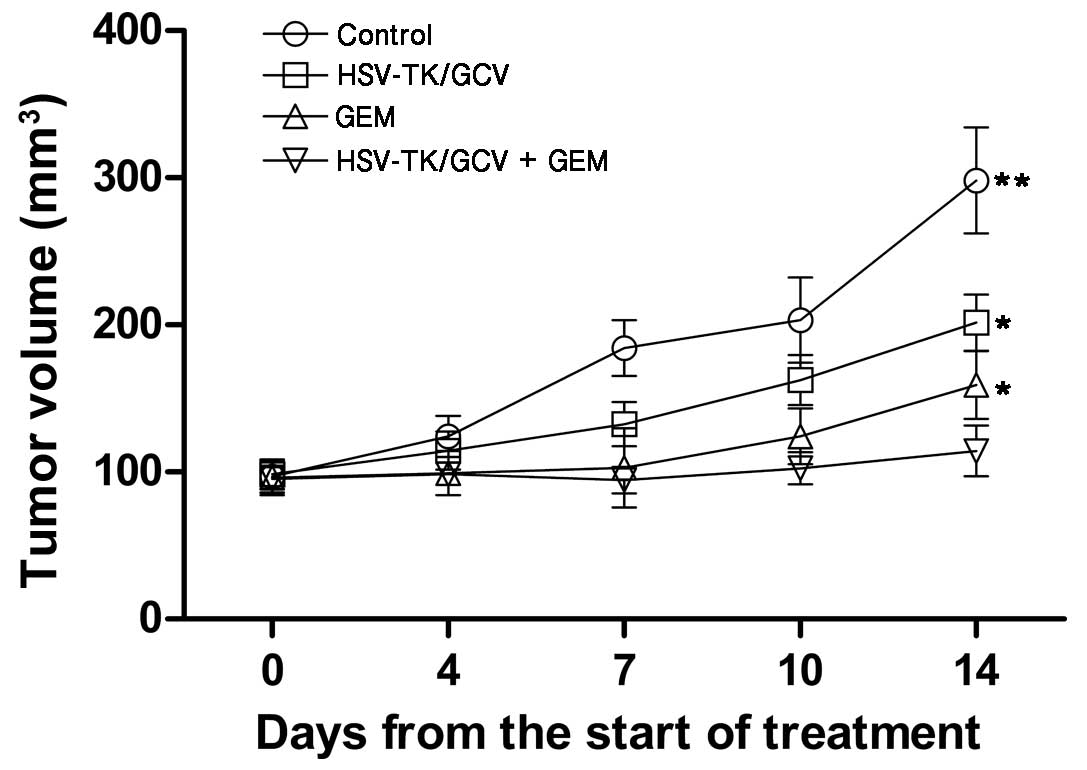
The mean volumes of the tumors prior to treatment in
the control, gemcitabine-only, HSV-TK + GCV and combination therapy
groups were 96.71±11.12, 87.68±12.27, 98.39±10.20 and 95.32±9.81
mm3, respectively. Although gene or gemcitabine monotherapy
significantly inhibited tumor growth (1.68±0.74 and 2.27±0.58,
respectively; P<0.05), combination therapy induced greater
inhibition of tumor development and the most significant delay in
tumor growth (2.87±0.82; P<0.05), as determined by tumor volume
on days 7, 10 and 14 following initial treatment (Fig. 3). Tumor volume on day 14 following
treatment was significantly reduced in the gemcitabine + HSV-TK
group, as compared with the gemcitabine monotherapy group
(114.32±17.17 vs. 159±23.74; Р<0.05), gene only (114.32±17.17
vs. 201.63±19.26; Р<0.05) and the control group (114.32±17.17
vs. 298.23±46.35; P<0.01) (Figs.
3 and 4). Furthermore, the
apoptosis index was significantly increased in the gemcitabine +
HSV-TK group, as compared with the gemcitabine (41±8 vs. 24±6%;
Р<0.05), HSV-TK + GCV (41±8 vs. 16±5%; Р<0.05) and control
(41±8 vs. 4±1%; Р<0.05) groups. These results indicated the
enhanced cell killing effects of gemcitabine + HSV-TK combination
therapy.
Discussion
Cholangiocarcinoma is one of the most difficult
malignancies to treat and mortality rates remain very high. The
most effective treatment for cholangiocarcinoma is curative
surgical resection of the primary tumor; however, this procedure is
complex and is dependent on the site and extent of the tumor.
Furthermore, once diagnosed, the majority of patients are already
at the late stages of disease and are no longer candidates for
surgery (2). Therefore, the clinical
management of cholangiocarcinoma remains a major concern (17).
Gemcitabine is the only chemotherapeutic agent that
has been demonstrated to have a significant impact on either
survival or disease-related symptoms in patients with pancreatic
carcinoma (18). Biliary tract
cancers are considered similar to pancreatic cancer in
aggressiveness and sensitivity to chemotherapy (19). Due to the lack of effective treatment
options for cholangiocarcinoma, gemcitabine-based chemotherapy for
advanced cholangiocarcinoma has been widely used in the past decade
and is accepted as the standard chemotherapeutic agent for the
treatment of cholangiocarcinoma (4,20).
Gemcitabine, either alone or in combination with other therapeutic
agents, including fluoropyrimidines or cisplatin, has been
demonstrated to have positive activity and response in treating
advanced cholangiocarcinoma. Response rates of patients
administered single-agent gemcitabine have varied between 0–30%,
with median overall survival times ranging between 5–14 months
(17). However, the outcome of
gemcitabine-based chemotherapy remains poor due to the high
resistance of cancer cells to gemcitabine; therefore, novel
therapeutic approaches are necessary (5).
Tang et al (21) established a rat model of bladder
tumors. The anaerobic Bifidobacterium infantis-mediated
HSV-TK was injected into tumor-bearing rats via the tail vein,
followed by intraperitoneal injection of GCV. The results
demonstrated that bladder tumor burdens were significantly lower in
the rats treated with HSV-TK + GCV compared with the control group
(P<0.05). While various degrees of apoptosis of the tumor cells
were detected in all groups using an in situ TUNEL assay,
apoptosis was mostly notable in the Bifidobacterium
infantis-mediated HSV-TK + GCV treatment group. The results
demonstrated that HSV-TK + GCV suicide gene therapy system can
effectively inhibit rat bladder tumor growth. In the present study,
HSV-TK gene therapy was administered in combination with
gemcitabine to human cholangiocarcinoma QBC939 cell line. As
compared with the other groups, the in vitro gemcitabine +
HSV-TK group exhibited significantly decreased viability. This
suggested that combination therapy may have a more potent
anti-tumor effect. In order to study the effects of combined HSV-TK
gene therapy and gemcitabine in vivo, we developed a mouse
model of subcutaneous cholangiocarcinoma was established using
tumor xenografts, which confirmed that the combination of HSV-TK
and gemcitabine was superior to either HSV-TK or gemcitabine
monotherapy. Although gemcitabine immunotherapy temporarily
suppressed tumor growth, the tumors relapsed after 7 days.
Similarly, HSV-TK monotherapy did not completely suppress tumor
growth, demonstrating that HSV-TK/GCV has limited antitumor
activity in vivo as a monotherapy. However, the combination
of HSV-TK and gemcitabine significantly suppressed the tumors,
which confirmed that HSV-TK increases the chemosensitization of the
tumors.
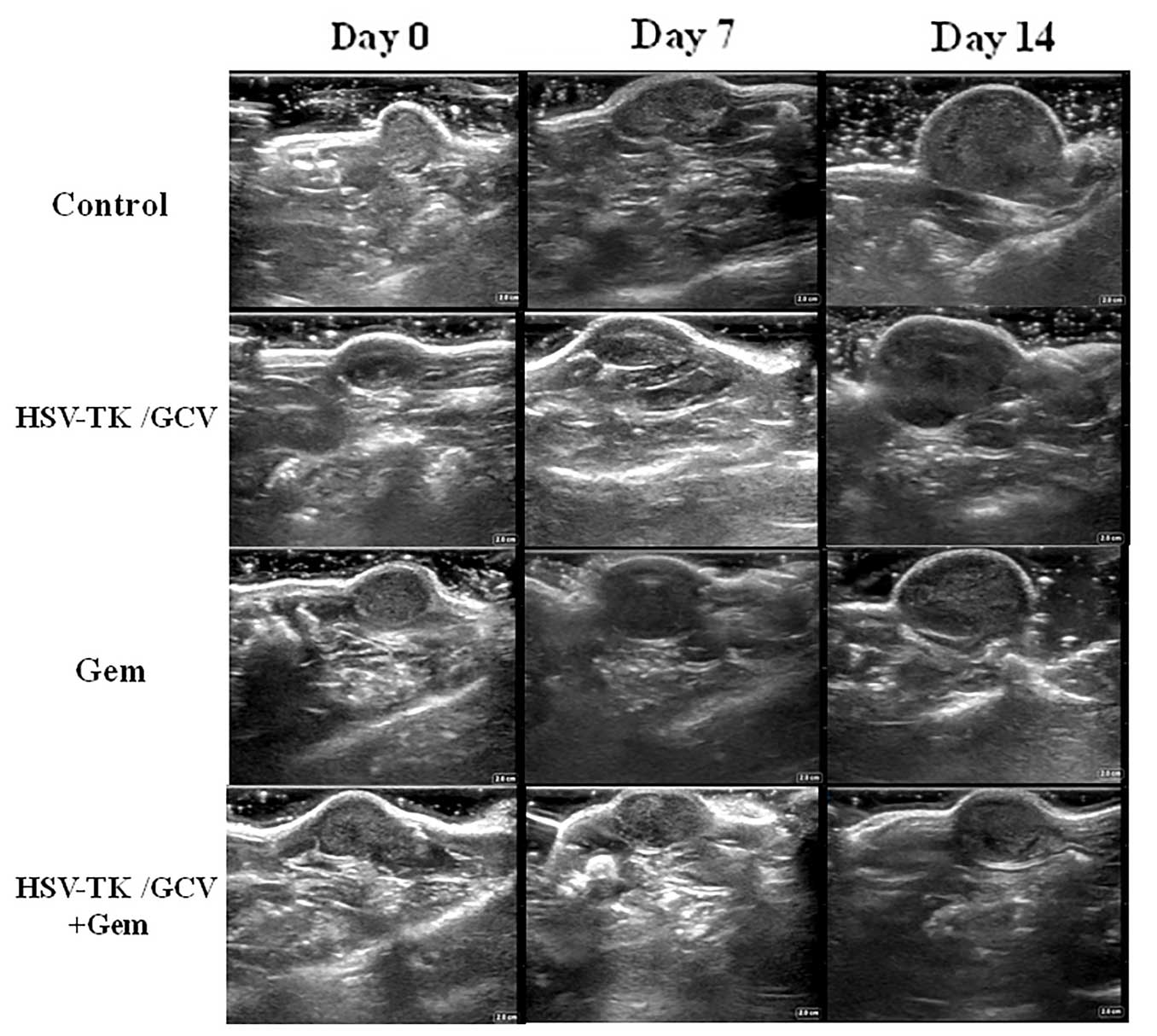
Optical imaging was used in the present study to
assess tumor growth in individual mice, due to its noninvasive,
objective and quantitative features (22). In the present study, QBC939 cells
expressing HSV-TK/luciferase were subcutaneously implanted into
mice. When injected with luciferin, the tumors emit a visual light
signal that can be monitored using a sensitive optical imaging
system. As the photon flux emitted from the tumor is proportional
to the number of light-emitting cells, this technique can be used
to monitor tumor growth and the effect of therapy. In the present
study, a decreased photon flux was detected in the murine group
treated with HSV-TK + gemcitabine. At days 7 and 14 post-treatment,
the mean photon flux value of the murine group administered HSV-TK
+ gemcitabine significantly decreased, as compared with the other
groups. Photon flux values emitted from tumors in the gemcitabine
monotherapy groups were decreased, as compared with the HSV-TK-only
group. According to our experience, the tumor growth may be
monitored using optical imaging for several days before the tumor
size becomes palpable or is measurable by US. Furthermore, optical
imaging is significantly more sensitive than US in detecting small
metastases due to the high signal-to-noise ratio. In the present
study, optical imaging successfully detected metastasis in a mouse
that US was unable to detect. However, major limitations of optical
imaging include its low penetration depth and its inaccuracy at
detecting cystic tumors (23).
Optical imaging offers high sensitivity for superficially localized
lesions, whereas US detects the accurate size of the tumor
(24). Therefore, US and optical
imaging techniques provided complementary information in the
present study.
In conclusion, the results of the present study
suggested that gemcitabine is capable of enhancing the therapeutic
effects of HSV-TK/GCV in the treatment of cholangiocarcinoma, which
can be efficiently monitored by optical imaging and US in
vivo. The present study may a novel therapeutic strategy for
the management and treatment of cholangiocarcinoma using
gemcitabine and HSV-TK/GCV combination therapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81373714).
Glossary
Abbreviations
Abbreviations:
|
HSV-TK
|
herpes simplex virus-thymidine
kinase
|
|
GCV
|
ganciclovir
|
|
PBS
|
phosphate-buffered saline
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
ROI
|
region of interest
|
|
RSI
|
relative signal intensities
|
References
|
1.
|
Nakeeb A, Pitt HA, Sohn TA, Coleman J,
Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ and Cameron
JL: Cholangiocarcinoma. A spectrum of intrahepatic, perihilar and
distal tumors. Ann Surg. 224:463–473; discussion 473–465. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Seyama Y and Makuuchi M: Current surgical
treatment for bile duct cancer. World J Gastroenterol.
13:1505–1515. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Woo SM, Lee WJ, Kim JH, Kim DH, Han SS,
Park SJ, Kim TH, Lee JH, Koh YH and Hong EK: Gemcitabine plus
cisplatin versus capecitabine plus cisplatin as first-line
chemotherapy for advanced biliary tract cancer: A retrospective
cohort study. Chemotherapy. 59:232–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kim MJ, Oh DY, Lee SH, Kim DW, Im SA, Kim
TY, Heo DS and Bang YJ: Gemcitabine-based versus
fluoropyrimidine-based chemotherapy with or without platinum in
unresectable biliary tract cancer: A retrospective study. BMC
Cancer. 8:3742008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Nagi P, Vickers SM, Davydova J, Adachi Y,
Takayama K, Barker S, Krasnykh V, Curiel DT and Yamamoto M:
Development of a therapeutic adenoviral vector for
cholangiocarcinoma combining tumor-restricted gene expression and
infectivity enhancement. J Gastrointest Surg. 7:364–371. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Andersen JB and Thorgeirsson SS: A
perspective on molecular therapy in cholangiocarcinoma: Present
status and future directions. Hepat Oncol. 1:143–157. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Pan JG, Luo RQ, Zhou X, Han RF and Zeng
GW: Suppression of bladder cancer growth by adeno-associated virus
vector-mediated combination of HSV-TK and endostatin in vitro. Clin
Lab. 59:1077–1089. 2013.PubMed/NCBI
|
|
9.
|
Jarnagin WR, Zager JS, Hezel M, Stanziale
SF, Adusumilli PS, Gonen M, Ebright MI, Culliford A, Gusani NJ and
Fong Y: Treatment of cholangiocarcinoma with oncolytic herpes
simplex virus combined with external beam radiation therapy. Cancer
Gene Ther. 13:326–334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Qu L, Wang Y, Gong L, Zhu J, Gong R and Si
J: Suicide gene therapy for hepatocellular carcinoma cells by
survivin promoter-driven expression of the herpes simplex virus
thymidine kinase gene. Oncol Rep. 29:1435–1440. 2013.PubMed/NCBI
|
|
11.
|
Duarte S, Carle G, Faneca H, de Lima MC
and Pierrefite-Carle V: Suicide gene therapy in cancer: Where do we
stand now? Cancer Lett. 324:160–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Boucher PD and Shewach DS: In vitro and in
vivo enhancement of ganciclovir-mediated bystander cytotoxicity
with gemcitabine. Mol Ther. 12:1064–1071. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wang J, Yang W, Huang Q and Zhai R:
Radiosensitization of gemicitabine in human cholangiocarcinoma cell
line. Zhonghua Fangshe Zhongliu Xue Za Zhi. 17:123–125. 2008.(In
Chinese).
|
|
14.
|
Chen L, Guo G, Liu T, Guo L and Zhu R:
Radiochemotherapy of hepatocarcinoma via lentivirus-mediated
transfer of human sodium iodide symporter gene and herpes simplex
virus thymidine kinase gene. Nucl Med Biol. 38:757–763. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Wang J, Yang W, Huang Q, Qian X and Zhai
R: Radioenhancement on cholangiocarcinoma by gemcitabine in vivo.
Jieru Fang She Xue Za Zhi. 10:685–687. 2007.(In Chinese).
|
|
16.
|
Shao D, Li J, Xiao X, Zhang M, Pan Y, Li
S, Wang Z, Zhang X, Zheng H, Zhang X and Chen L: Real-time
visualizing and tracing of HSV-TK/GCV suicide gene therapy by
near-infrared fluorescent quantum dots. ACS Appl Mater Interfaces.
6:11082–11090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Skipworth JR, Keane MG and Pereira SP:
Update on the management of cholangiocarcinoma. Dig Dis.
32:570–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Valle JW, Furuse J, Jitlal M, Beare S,
Mizuno N, Wasan H, Bridgewater J and Okusaka T: Cisplatin and
gemcitabine for advanced biliary tract cancer: A meta-analysis of
two randomised trials. Ann Oncol. 25:391–398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Weiss GA, Rossi MR, Khushalani NI, Lo K,
Gibbs JF, Bharthuar A, Cowell JK and Iyer R: Evaluation of
phosphatidylinositol-3-kinase catalytic subunit (PIK3CA) and
epidermal growth factor receptor (EGFR) gene mutations in
pancreaticobiliary adenocarcinoma. J Gastrointest Oncol. 4:20–29.
2013.PubMed/NCBI
|
|
20.
|
Malka D, Cervera P, Foulon S, Trarbach T,
de la Fouchardière C, Boucher E, Fartoux L, Faivre S, Blanc JF,
Viret F, et al: Gemcitabine and oxaliplatin with or without
cetuximab in advanced biliary-tract cancer (BINGO): A randomised,
open-label, non-comparative phase 2 trial. Lancet Oncol.
15:819–828. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tang W, He Y, Zhou S, Ma Y and Liu G: A
novel Bifidobacterium infantis-mediated TK/GCV suicide gene therapy
system exhibits antitumor activity in a rat model of bladder
cancer. J Exp Clin Cancer Res. 28:1552009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Choy G, Choyke P and Libutti SK: Current
advances in molecular imaging: Noninvasive in vivo bioluminescent
and fluorescent optical imaging in cancer research. Mol Imaging.
2:303–312. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Keereweer S, Van Driel PB, Snoeks TJ,
Kerrebijn JD, de Baatenburg Jong RJ, Vahrmeijer AL, Sterenborg HJ
and Löwik CW: Optical image-guided cancer surgery: Challenges and
limitations. Clin Cancer Res. 19:3745–3754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Keereweer S, Hutteman M, Kerrebijn JD, van
de Velde CJ, Vahrmeijer AL and Löwik CW: Translational optical
imaging in diagnosis and treatment of cancer. Curr Pharm
Biotechnol. 3:498–503. 2012. View Article : Google Scholar
|