Introduction
Despite significant improvements in the diagnosis
and treatment of heart failure (HF), the mortality and morbidity
rates for patients with HF remain high (1–3). For
patients with hypertension, in particular elderly patients, the
long-term overload of the heart caused by increased afterload, as
well as reduced preservation of cardiac function, increases their
susceptibility to cardiac insufficiency, which manifests clinically
as acute decompensated HF (ADHF) (4). The hemodynamic characteristics of
patients with ADHF have been shown to be different from those of
patients with chronic HF (CHF) (5).
In contrast to the reduced left ventricular systolic function
observed in patients with CHF, patients with ADHF typically have
elevated filling pressures, high systemic vascular resistance and
hypertension, which may lead to pump failure and further reduction
of the perfusion of blood to vital organs, resulting in vascular
failure (6,7). Therefore, vasodilators may be
beneficial in these conditions due to their pharmacological actions
of reducing cardiac afterload, as well as improving peripheral
perfusion (8,9).
Vasodilators have been considered to be an effective
class of drugs in patients with hypertension and ADHF (10–12). The
most commonly used vasodilator in patients with ADHF is
nitroglycerin (11–13). Although nitroglycerin has been
reported to be effective and applicable in the majority of cases,
there have been reports regarding its unfavorable effect on the
heart rate (HR) (13,14). Furthermore, no evident benefits of
nitroglycerin on the cardiac systolic and diastolic function have
been reported (13). Therefore,
there is an urgent requirement for the development of novel
pharmacological agents for the treatment of hypertension and ADHF.
Urapidil, which is a vasodilator that exerts peripheral
α-adrenergic receptor and central serotonin receptor 1A (5-HT1A)
antagonizing effects, has been proposed to be potentially effective
for patients with hypertension and ADHF (15,16).
However, its effect on cardiac function, particularly in elderly
patients with ADHF, has yet to be determined. Therefore, the aim of
the current randomized controlled trial (RCT) was to compare the
therapeutic effects of intravenous urapidil and nitroglycerin in
elderly patients with hypertension and ADHF.
Patients and methods
Study design
The present study was designed as a multicenter
clinical trial and was performed in 10 research centers across
mainland China: Department of Cardiology and Emergency, Xuanwu
Hospital of Capital Medical University (Beijing, China); Department
of Geriatric Cardiology, Beijing Anzhen Hospital Affiliated to
Capital Medical University (Beijing, China); Department of Internal
Medicine, Beijing Tongren Hospital Affiliated to Capital Medical
University (Beijing, China); Department of Cardiology, The First
Affiliated Hospital of Chongqing Medical University (Chongqing,
China); Department of Cardiology, Sir Run Run Shaw Hospital in
Zhejiang Province (Hangzhou, China); Department of Cardiology, The
Tenth People's Hospital Affiliated to Shanghai Tongji University
(Shanghai, China); Department of Cardiology, Qilu Hospital of
Shandong University (Jinan, China); Department of Cardiology, The
First Hospital of Jilin University (Changchun, China); Department
of Cardiology, The First Affiliated Hospital of Harbin Medical
University (Harbin, China); and Department of Cardiology, Guangdong
Provincial People's Hospital (Guangzhou, China). This open-label
RCT aimed to evaluate the efficacy and safety of intravenous
urapidil, as compared with that of the conventional vasodilator
nitroglycerin, for the treatment of elderly hypertensive patients
who also suffered from ADHF. Patients were enrolled in the present
study between August 1st, 2011 and November 1st, 2013. The ethics
committees of all the included research centers approved the
protocols of the study prior to its initiation. All patients
provided written informed consent prior to their enrollment. The
protocol of the clinical trial was registered in the Chinese
Clinical Trial Registry (no. ChiCTR-TRC-11001781) before the
enrollment of the first patients. A subgroup study of present
study, which included only AHF patients with DM, has been published
previously (17).
Inclusion and exclusion criteria of
the patients
The inclusion criteria for the present study were as
follows: i) Age, >65 years; ii) a previous diagnosis of
hypertension, defined as a systolic blood pressure (SBP) of >140
mmHg and/or a diastolic blood pressure (DBP) of >90 mmHg, or
regular use of antihypertensives (18); iii) a diagnosis of systolic ADHF; and
iv) the patient had been scheduled for inpatient treatment at one
of the included medical centers. The diagnosis of systolic ADHF was
based on the decompensated clinical manifestations of the patients
[New York Heart Association (NYHA) classification of II–IV
(19)] and a reduced left
ventricular ejection fraction (LVEF) of <50%, as assessed by
transthoracic echocardiography (2).
Patients were excluded from the present study if
they presented any of the following: i) Complications including
cardiogenic shock, an SBP of ≤100 mmHg, cerebral ischemia or severe
stenosis of the carotid arteries, or other clinical disorders that
were contraindications for intravenous administration of
vasodilators; ii) confirmed acute coronary syndrome; iii)
comorbidities of severe structural heart diseases, including severe
valvular stenosis, hypertrophic obstructive cardiomyopathy,
restrictive cardiomyopathy or pericarditis; iv) a severe condition
of other systems or organs, including severe chronic asthmatic
bronchial and pulmonary diseases, severe liver dysfunction
(>3-fold maximum normal levels of alanine aminotransferase and
aspartate aminotransferase) or kidney insufficiency (>2-fold
maximum normal level of creatinine); v) a known or suspected
allergy to any of the tested medications and their ingredients; vi)
unsuitable for participation as determined by researchers (for
example, due to the presence of cancer cachexia or severe mental
illness); vii) use of any of the tested medications within 60 days
prior to enrollment; and viii) participation in another clinical
research program.
Randomization, treatment assignment
and medications
During the study process, all patients continued to
receive conventional antihypertensives (such as calcium-channel
blockers, angiotensin converting enzyme inhibitors, angiotensin
receptor blockers and diuretics) and other cardiovascular
medications (including digoxin, amiodarone and statins). However,
β-blockers were not prescribed during the study period, since they
may increase the risk of a deterioration of ADHF (19). The included patients were randomly
assigned to either an urapidil-based treatment group or a
nitroglycerin-based control group, according to a random number
generated by a computer. The assignment of the patients was open to
both the investigators and the participants.
For patients in the urapidil group, urapidil
hydrochloride (100 mg diluted in 50 ml normal saline; Ebrantil;
Altana AG, Konstanz, Germany) was continuously administered
intravenously as required and the dosages of the urapidil were
adjusted according to the blood pressure of the patients.
Typically, the starting dose of urapidil was set at a small dose in
accordance with the clinical situation and then increased to the
target dose (50–100 µg/min) within 6 h, with a maximum dose of 400
µg/min. Intravenous urapidil was discontinued if the SBP was <90
mmHg. Furthermore, if the symptoms of ADHF were not improved during
the initial 48 h of administration, the intravenous urapidil was
discontinued and the subsequent treatment was based on the
judgement of the investigators. Similarly, for patients assigned to
the nitroglycerin group, intravenous nitroglycerin (10 mg diluted
in 50 ml 5% glucose solution; Beijing Yimin Pharmaceutical Co.,
Ltd., Beijing, China) was continuously administered as required.
The starting dose of nitroglycerin was set at a small dose in
accordance with the clinical situation and then increased to the
target dose (5–10 µg/min) within 6 h, with a maximum dose of 40
µg/min. The protocols for dosage adjustment and the criteria for
medication discontinuation were the same as those for the
intravenous urapidil group. Only patients who received the tested
medications for >24 h were included in subsequent analyses.
Study protocols
Each participant underwent repeated evaluations of
hemodynamic parameters, including HR, SBP and DBP, at hospital
admission (day 0) and at 1, 2, 3 and 7 days after intravenous
vasodilator administration was initiated, as previously described
(17). In addition, serum levels of
N-terminal B type natriuretic peptide (NT-proBNP) were evaluated at
the same time points. The left ventricular function was evaluated
by means of transthoracic echocardiography, while the parameters of
LVEF and left ventricular end-diastolic volume (LVEDV) were also
measured at hospital admission and at 2 and 7 days after
intravenous vasodilator administration. Furthermore, the serum
levels of indices that reflect the metabolism of glucose and
lipids, including fasting plasma glucose (FPG), glycosylated
hemoglobin (GHB), postprandial 2 h plasma glucose (P2hPG),
triglycerin (TG), total cholesterol (TC), low-density lipoprotein
cholesterol (LDL-C) and high-density lipoprotein cholesterol
(HDL-C), were evaluated at hospital admission and on days 2 and 7
after intravenous vasodilator administration. Measurements of the
above parameters were recorded as previously described (17). No other intravenous vasodilators were
used during the study period.
Echocardiological evaluation of left
ventricular function
Global left ventricular function was assessed by
transthoracic echocardiography (Philips IE33 Ultrasound system;
Philips Healthcare, Amsterdam, The Netherlands). The apical 2- and
4-chamber views were selected for the measurement of LVEF, which
was used as an index of global left ventricular function. To
calculate the LVEF, the end-diastolic and end-systolic frames were
selected, the end-diastolic and end-systolic endocardial borders
were manually traced and the biplane LVEF was calculated. In
addition, the LVEDV was measured for each patient, as previously
described (17).
Follow-up and clinical outcomes
The patients were followed up for 6 months after
discharge from the hospital. Clinical outcomes, including
HF-associated rehospitalization, cardiovascular mortality,
non-fatal myocardial infarction (MI) and malignant arrhythmia
(including ventricular tachycardia and ventricular fibrillation)
were evaluated for all patients.
Statistical analysis
Continuous data are presented as the mean ± standard
deviation and the categorical data are presented as the number and
percentage. Each data set was assessed for normality. Differences
in the continuous and categorical data between the two groups were
analyzed using the Student's t-test or the χ2 test.
Differences in the data from multiple time points between the two
groups were analyzed using the repeated measures analysis of
variance (ANOVA) and independent samples t-test. Statistical
analyses were performed using SPSS version 16.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics of the
included patients
A total of 120 elderly patients, including 70 males
and 50 females, with hypertension and confirmed ADHF were included
in the present study during the enrollment period from the 10
medical centers. The ages of the included patients ranged between
65 and 84 years. A total of 62 patients were randomly assigned to
the nitroglycerin group, whereas the remaining 58 patients were
assigned to the urapidil group. The baseline characteristics of the
included patients are presented in Table
I. There were no significant differences in the demographic
factors, including age, gender, duration of hypertension, clinical
manifestations of ADHF (as evaluated based on the LVEF and
distribution of NYHA classification), comorbidities of coronary
heart disease (CHD), diabetes mellitus and atrial fibrillation, as
well as the use of cardiovascular medications, such as
antihypertensives, digoxin, amiodarone and statins, between the two
groups (all P>0.05). All patients received predetermined
protocols involving intravenous vasodilators and no adverse events
were observed. The mean treatment duration for nitroglycerin was 87
h and the mean dosage was 88.7 mg per patient. In comparison, the
treatment duration for urapidil was 90 h and mean dosage was 405.2
mg per patient.
 | Table I.Baseline characteristics of elderly
hypertensive patients in both groups. |
Table I.
Baseline characteristics of elderly
hypertensive patients in both groups.
| Characteristic | Nitroglycerin group
(n=62) | Urapidil group
(n=58) |
|---|
| Males | 36 (58.1) | 34 (58.6) |
| Age (years) | 78.1±10.2 | 78.0±10.0 |
| Duration of
hypertension (years) | 17.1±10.6 | 18.8±7.4 |
| HGB (g/l) | 109.0±5.2 | 107.2±4.6 |
| eGFR (ml/min) | 47.2±4.8 | 45.0±6.2 |
| LVEF (%) | 39.7±5.2 | 41.9±4.2 |
| NYHA
classification |
|
|
| Class
II | 34 (54.8) | 32 (55.2) |
| Class
III | 18 (29.0) | 16 (27.6) |
| Class
IV | 10 (16.2) | 10 (17.2) |
| Comorbidities |
|
|
|
CHD | 18 (29.0) | 17 (29.3) |
| DM | 27 (43.5) | 26 (44.8) |
| AF | 10 (16.1) | 11 (18.9) |
| Number of
antihypertensives used |
|
|
| 1 | 15 (24.2) | 17 (29.3) |
| 2 | 25 (40.3) | 23 (39.7) |
| 3 | 22 (35.5) | 18 (31.0) |
| Concurrent CV
medications |
|
|
|
CCBs | 48 (77.4) | 46 (79.3) |
|
ACEIs/ARBs | 37 (59.7) | 39 (67.2) |
|
β-blockers | 18 (29.0) | 16 (27.8) |
|
Diuretics | 39 (62.9) | 37 (63.8) |
|
Digoxin | 17 (27.4) | 14 (24.1) |
|
Amiodarone | 12 (19.4) | 13 (22.4) |
|
Statins | 45 (72.5) | 43 (74.1) |
Effect of urapidil versus
nitroglycerin on SBP, DBP and HR
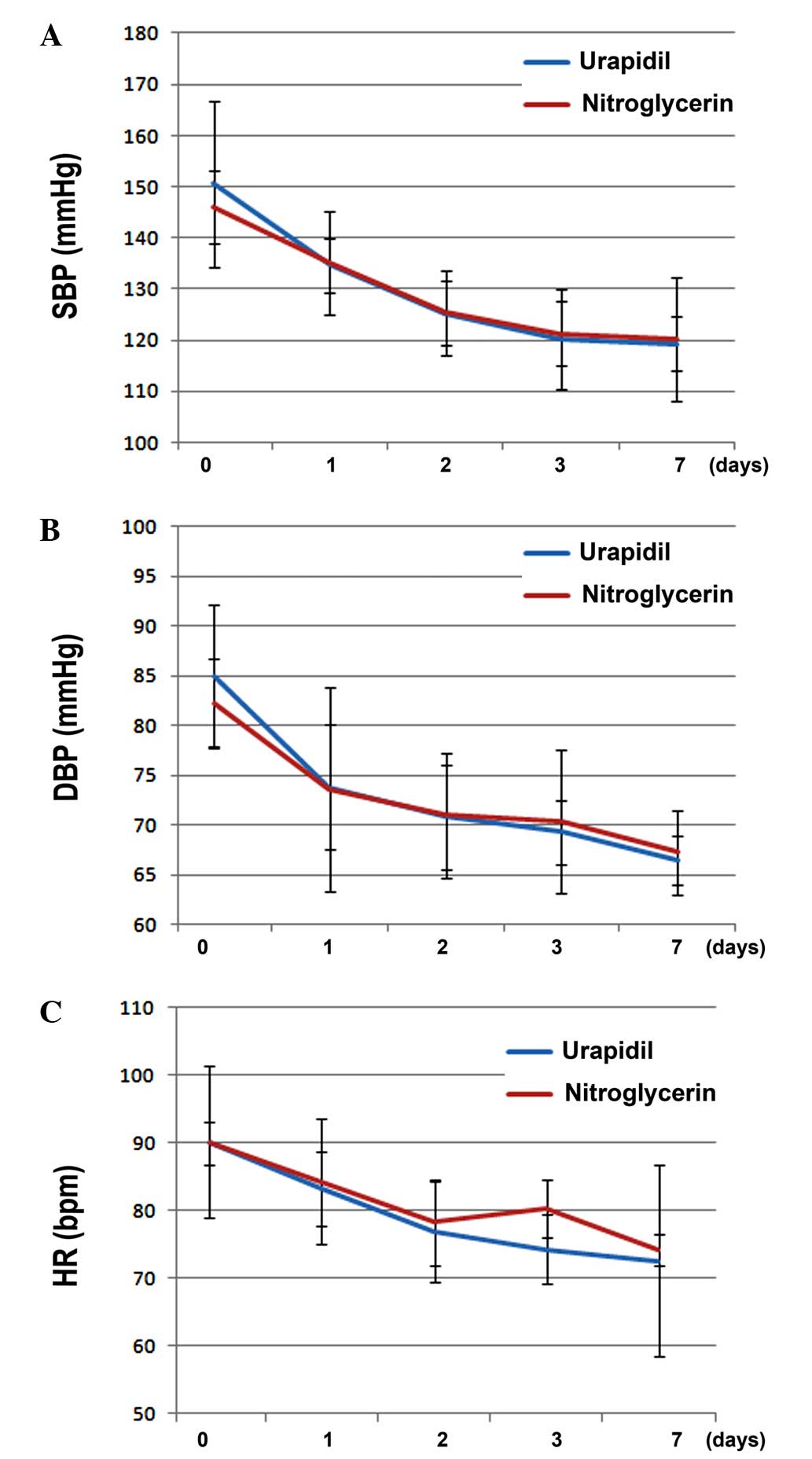
A multivariate data analysis of the intragroup
elements, using the repeated measures ANOVA, demonstrated that
there were significant differences in the SBP (F=27.42), DBP
(F=29.32) and HR (F=31.24) among the various time points
(P<0.05) in the two groups. This suggested that the hemodynamic
parameters of SBP, DBP and HR were significantly lowered within 7
days after intravenous administration of vasodilators, as compared
with the values at admission (Fig.
1A–C). There was no association between time and group, which
suggested that the time trends for the two medications were
identical in their effects on the hemodynamic parameters of SBP,
DBP and HR (P>0.05). Furthermore, intergroup ANOVA demonstrated
that there were no significant differences regarding the effects of
urapidil and nitroglycerin on the SBP, DBP and HR (P>0.05), thus
suggesting that intravenous urapidil and nitroglycerin exerted
similar blood pressure-lowering and HR-regulating effects within 7
days of treatment in elderly patients with hypertension and ADHF
(Fig. 1A–C).
Effect of urapidil versus
nitroglycerin on serum NT-proBNP levels
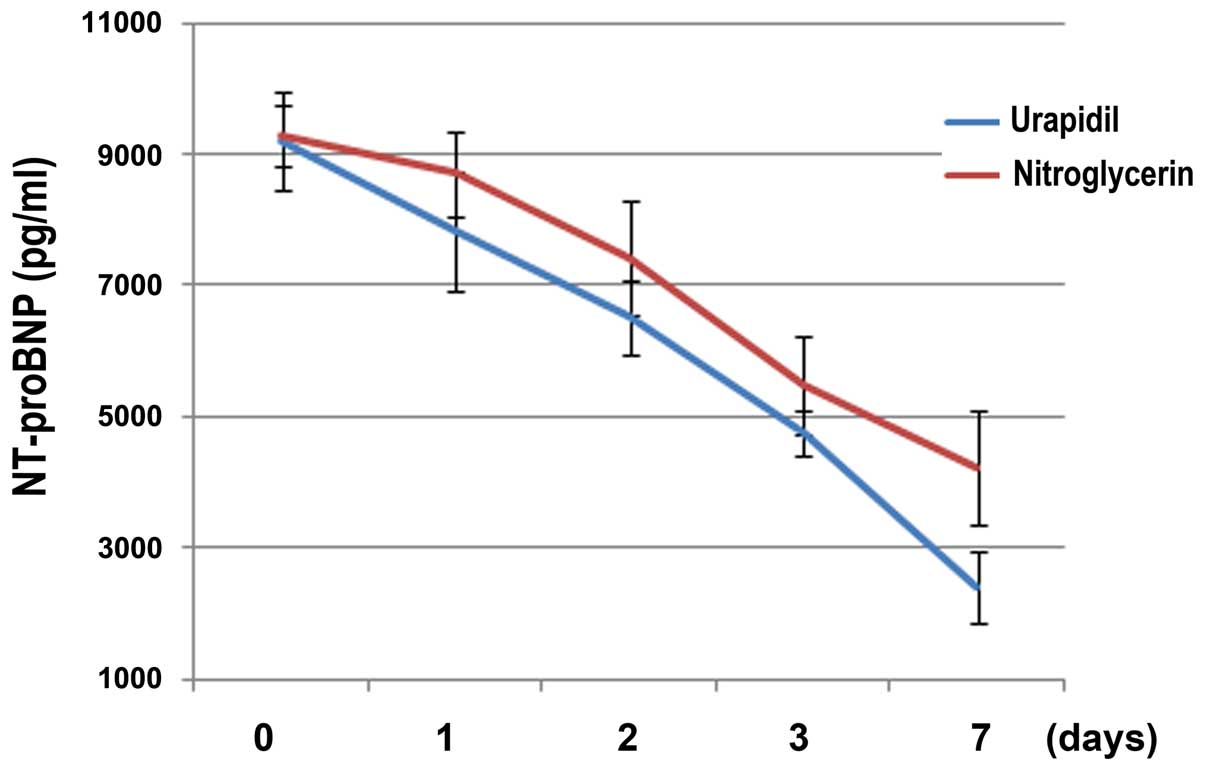
A multivariate analysis of intragroup elements,
using the repeated measures ANOVA, demonstrated that the serum
level of NT-proBNP (F=59.14) was significantly reduced after 7 days
of treatment with intravenous vasodilators in the two groups, as
compared with the level at admission (P<0.05; Fig. 2). However, there was no significant
association between time and group, which indicated that the time
trends for the two medications were identical in their effects on
the serum levels of NT-proBNP (P>0.05). Intergroup ANOVA
demonstrated that urapidil (F=57.24) was able to reduce the serum
levels of NT-proBNP to a greater extent when compared with
nitroglycerin (P<0.05), thus suggesting that intravenous
urapidil may be more effective in improving cardiac function in
elderly patients with hypertension and ADHF (Fig. 2).
Effect of urapidil versus
nitroglycerin on LVEF and LVEDV
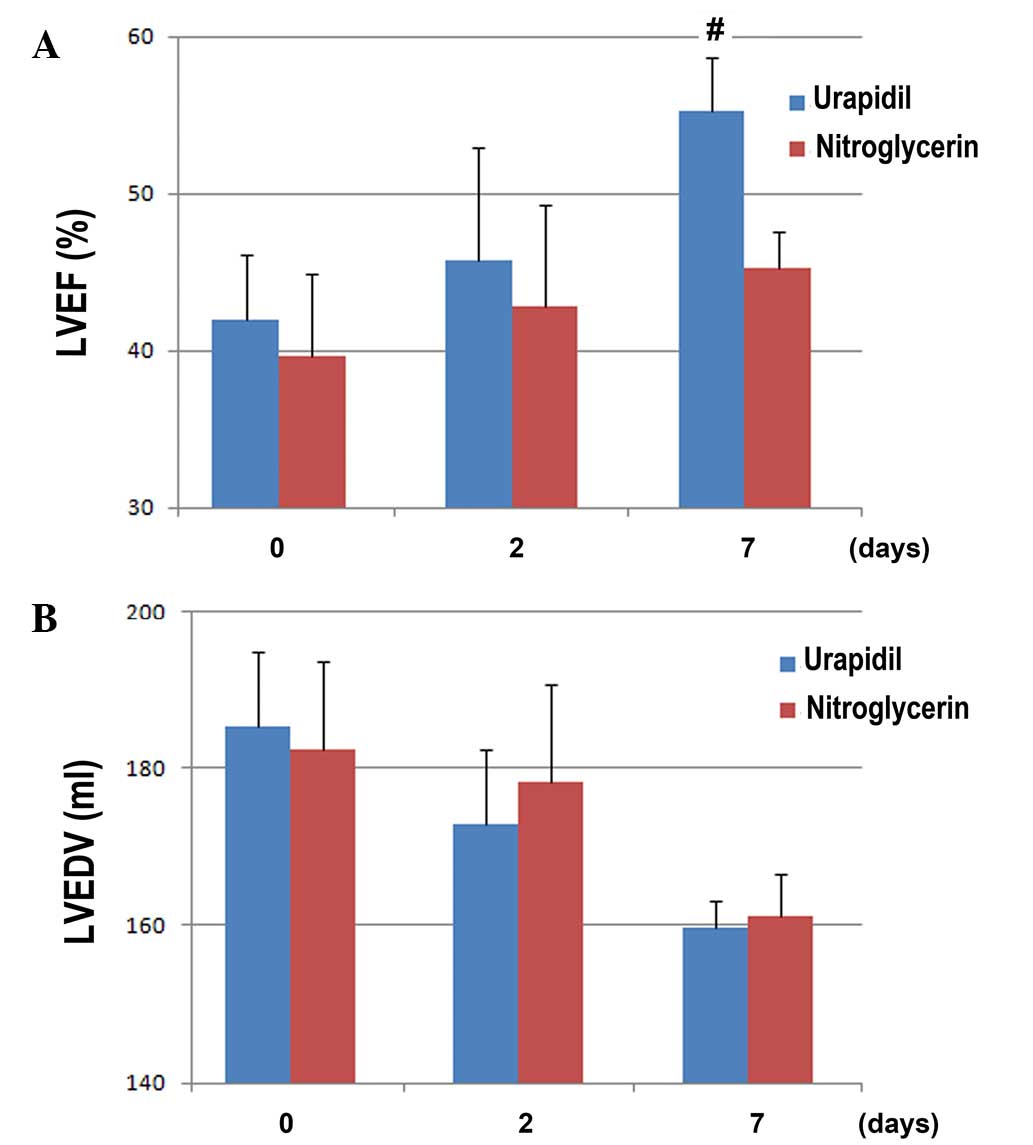
The effect of urapidil, as compared with
nitroglycerin, on the index of left ventricular function, LVEF, are
presented in Fig. 3A. Although no
significant differences were observed in the LVEF between the two
groups at admission and 2 days after intravenous vasodilator
treatment, the LVEF for patients allocated to the urapidil group
was significantly improved at 7 days after treatment, as compared
with the nitroglycerin group (55.3±3.4 vs. 45.2±2.4%, respectively;
t=−3.104; P<0.05; Fig. 3A). These
results indicate that urapidil exerts a more beneficial effect on
left ventricular function, as compared with nitroglycerin.
Conversely, no significant differences were detected in the LVEDV
between the two groups at admission or on days 2 and 7 after
treatment with intravenous vasodilators (all P>0.05; Fig. 3B).
Effect of urapidil on the serum
indices of glucose and lipid metabolism
The independent samples t-test was used to compare
the differences in glucose metabolism indices, including FPG, P2hPG
and GHB, in the patients with hypertension and ADHF at admission
and on days 2 and 7 after intravenous vasodilator treatment. As
compared with the nitroglycerin group, patients in the urapidil
group showed no significant differences in any of the glycemic
indices on days 2 and 7 after treatment (all P>0.05; Table II); however, a greater reducing
trend in the urapidil group, as compared with the nitroglycerin
group, could be predicted from the changes in the glycemic data.
Similarly, the serum levels of lipids, including TG, TC, LDL-C and
HDL-C, were not significantly different between the two groups at
admission and on days 2 and 7 after treatment (all P>0.05;
Table II).
 | Table II.Effects of urapidil on indices of
glucose and lipid metabolism, compared with the effect of
nitroglycerin. |
Table II.
Effects of urapidil on indices of
glucose and lipid metabolism, compared with the effect of
nitroglycerin.
|
| Nitroglycerin group
(n=62) | Urapidil group
(n=58) |
|---|
|
|
|
|
|---|
| Index | Baseline | 2 days | 7 days | Baseline | 2 days | 7 days |
|---|
| FPG (mmol/l) |
7.86±4.68 |
7.28±2.34 |
6.99±2.34 |
7.99±1.24 |
7.55±2.12 |
6.12±2.56 |
| GHB (mmol/l) |
7.14±2.09 |
7.05±3.34 |
6.93±1.47 |
7.16±1.24 |
6.74±3.52 |
6.53±1.24 |
| P2hPG (mmol/l) |
11.29±5.44 |
10.61±5.62 |
10.04±1.25 |
11.52±4.08 |
10.66±1.52 |
9.25±3.51 |
| TG (mmol/l) |
1.38±1.30 |
1.43±0.27 |
1.40±0.68 |
1.44±0.26 |
1.38±0.76 |
1.37±0.58 |
| TC (mmol/l) |
3.93±1.04 |
3.88±0.91 |
4.06±1.01 |
4.11±1.12 |
4.07±1.36 |
4.04±1.31 |
| LDL-C (mmol/l) |
2.37±0.34 |
2.32±0.75 |
2.29±0.81 |
2.91±1.16 |
2.78±1.26 |
2.78±1.23 |
| HDL-C (mmol/l) |
1.13±0.23 |
1.12±0.34 |
1.16±0.21 |
1.13±0.46 |
1.23±0.24 |
1.26±0.36 |
Follow-up data within 6 months
following discharge
The follow-up data for patients in the urapidil and
nitroglycerin groups showed no significant differences regarding
the incidences of rehospitalization for HF,
cardiovascular-associated mortality, non-fatal myocardial
infarction or malignant arrhythmia (Table III).
 | Table III.Incidence of adverse cardiovascular
events during the 6-month follow-up. |
Table III.
Incidence of adverse cardiovascular
events during the 6-month follow-up.
| Index | Nitroglycerin group
(n=62) | Urapidil group
(n=58) |
|---|
| Rehospitalization
for heart failure | 12 (19.4) | 7
(12.1) |
|
Cardiovascular-related death | 2 (3.2) | 1 (1.7) |
| Non-fatal
myocardial infarction | 4 (6.5) | 1 (1.7) |
| Malignant
arrhythmia | 3 (4.8) | 2 (3.4) |
Discussion
The present multicenter, controlled clinical trial
compared the therapeutic effects of intravenous administration of
urapidil and nitroglycerin in elderly patients with ADHF and
hypertension. The results indicated that intravenous urapidil
exerted similar blood pressure-lowering effects as nitroglycerin in
patients with ADHF. Furthermore, the two treatments were not
associated with an evident increase in HR and there were no reports
of acute adverse events. Notably, short-term administration of
urapidil was associated with a greater improvement in cardiac
systolic function compared with nitroglycerin, as demonstrated by
the greater extent by which urapidil increased the LVEF and reduced
the serum level of NT-proBNP level on day 7 of treatment in elderly
patients with ADHF. However, the follow-up data suggested that the
clinical outcomes of the patients in the two groups were not
significantly different. These results suggested that, as compared
with intravenous nitroglycerin, intravenous urapidil may be more
effective for improving cardiac systolic function in elderly
patients with hypertension and ADHF. Further large-scale RCTs are
required in order to determine whether the acute beneficial effects
of urapidil on cardiac function lead to improved clinical outcomes
for elderly patients with ADHF and hypertension in the
long-term.
Previous epidemiological studies and registry trials
reported that ADHF, which is characterized by acute insufficiency
of cardiac systolic function and failure of peripheral perfusion of
organs, has emerged as one of the most important causes of
mortality and morbidity in the elderly population (2,3,19). In China, the outcome of acute HF is
typically poor, with a hospital mortality rate of 3%, a 6-month
readmission rate of ≤50% and a 5-year mortality rate as high as 60%
(2). Therefore, there is an urgent
requirement for the development of novel treatment strategies for
patients with ADHF. The majority of ADHF cases are complicated by
elevated blood pressure, which may cause overloading of the heart
and increased peripheral vascular resistance, thereby exacerbating
the hemodynamic conditions (6,20).
Vasodilators have been recommended as the first-line medication for
patients with ADHF, which may be due to their ability to stabilize
the hemodynamic status (4,9). The present study demonstrated that
intravenous administration of urapidil and nitroglycerin conferred
similar blood pressure-lowering effects in elderly patients with
ADHF and hypertension, without having adverse effects on HR. These
results are consistent with those of previous studies, which
similarly reported the beneficial effects of urapidil and
nitroglycerin on the hemodynamic status in patients with ADHF
(16,21,22). In
a previous study investigating patients with CHF, 25 mg intravenous
urapidil administered twice within 15 min was associated with a
significant decrease of 16% in the SBP, 13% in the mean arterial
pressure, 38% in the left ventricular end-diastolic pressure, 31%
in the mean pulmonary artery pressure and 40% in the wedge pressure
(16). The mechanisms underlying the
beneficial effects of urapidil on hemodynamic conditions have been
considered based on its elucidated pharmacological characteristics,
including antagonizing effects on the peripheral α-adrenergic
receptor and 5-HT1A, which enable it to be effective in the
regulation of heart overloading (15).
As well as stabilizing the hemodynamic status of
patients with ADHF and hypertension, another important therapeutic
strategy for these patients is to improve the cardiac systolic
function (23,24). An early animal study (25) and observational studies (16,22,26,27)
reported potential beneficial effects of urapidil on cardiac
output; however, its effect on the left ventricular systolic
function in patients with ADHF has not been systematically
evaluated in controlled clinical trials. To the best of our
knowledge, the present study was the first to evaluate the effects
of acute administration of urapidil on cardiac function, as
compared with nitroglycerin administration, in ADHF patients in a
randomized clinical trial. The results demonstrated that, on day 7
after treatment, patients who received intravenous urapidil
exhibited a greater improvement in left systolic function, as
compared with those who received nitroglycerin, which was shown by
the greater improvement of the LVEF and a greater reduction in the
serum level of NT-proBNP. LVEF and serum NT-proBNP have been
confirmed to be reflective of cardiac systolic function in patients
with ADHF (28,29). Furthermore, these indices were shown
to have a prognostic value in these patients (30). The results of the present study were
consistent with those of a previous study that showed that
long-term urapidil administration was able to improve the LVEF in
the 3 months following coronary revascularization in patients with
CHD who had undergone coronary stenting (31). The exact mechanisms underlying the
beneficial effects of urapidil on cardiac function are currently
unknown. However, a previous study in patients with acute coronary
syndrome suggested that urapidil treatment may improve the left
ventricular function by increasing the coronary flow and myocardial
perfusion, thereby improving the energy metabolism of the
myocardium (32).
The present study observed that there were no
significant differences in the effects of urapidil and
nitroglycerin on the indices of glucose and lipid metabolism on day
7 after treatment between the two groups. Previous studies have
suggested potential benefits of urapidil against insulin resistance
in patients with hypertension (33);
however, its metabolic benefits in patients with ADHF need to be
observed in further studies. Furthermore, the long-term influence
of intravenous urapidil on clinical outcomes requires clarification
in large-scale RCTs in the future.
However, the current study had several limitations
that must be considered when interpreting the results. Firstly, the
study was designed as an open-label trial, which may lead to bias
and the results of the study may, therefore, have been affected by
subjective factors introduced by the patients and researchers.
Furthermore, the study was designed as a pilot study and only a
limited number of patients was included. Therefore, the possibility
of the lack of statistical power in the present study can not be
excluded and certain negative results should be interpreted with
caution. Finally, differences in the effects of urapidil on
patients with ischemia and without ischemia were not evaluate due
to the limited number of patients included. Therefore, further
high-quality, large-scale RCTs are required.
In conclusion, the present study demonstrated that
intravenous urapidil was as effective as nitroglycerin in lowering
blood pressure and was more effective in improving cardiac systolic
function in elderly patients with hypertension and ADHF, suggesting
that urapidil may be more a more effective therapeutic strategy
than nitroglycerin in hypertensive patients with ADHF. Further
large-scale RCTs are required to confirm the results and to observe
the long-term effects of urapidil on clinical outcomes in these
patients.
Acknowledgements
The present study was supported by a grant from the
Capital Medical Development Foundation (863 project no.
2012BAI37B03).
References
|
1.
|
Jiang H and Ge J: Epidemiology and
clinical management of cardiomyopathies and heart failure in China.
Heart. 95:1727–1731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Chinese Society of Cardiology of Chinese
Medical Association; Editorial Board of Chinese Journal of
Cardiology: Chinese guidelines for the diagnosis and treatment of
heart failure 2014. Zhonghua Xin Xue Guan Bing Za Zhi. 42:98–122.
2014.(In Chinese). PubMed/NCBI
|
|
3.
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ,
Howard VJ, et al: American Heart Association Statistics Committee
and Stroke Statistics Subcommittee: Heart disease and stroke
statistics-2015 update: A report from the American Heart
Association. Circulation. 131:e29–322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Cicci JD, Reed BN, McNeely EB, Oni-Orisan
A, Patterson JH and Rodgers JE: Acute decompensated heart failure:
Evolving literature and implications for future practice.
Pharmacotherapy. 34:373–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Felker GM, Adams KF Jr, Konstam MA,
O'Connor CM and Gheorghiade M: The problem of decompensated heart
failure: Nomenclature, classification and risk stratification. Am
Heart J. 145(Suppl 2): S18–S25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Adams KF Jr, Fonarow GC, Emerman CL,
LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M and
Horton DP: ADHERE Scientific Advisory Committee and Investigators:
Characteristics and outcomes of patients hospitalized for heart
failure in the United States: rationale, design and preliminary
observations from the first 100,000 cases in the Acute
Decompensated Heart Failure National Registry (ADHERE). Am Heart J.
149:209–216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Zannad F, Mebazaa A, Juillière Y,
Cohen-Solal A, Guize L, Alla F, Rougé P, Blin P, Barlet MH,
Paolozzi L, et al: Clinical profile, contemporary management and
one-year mortality in patients with severe acute heart failure
syndromes: The EFICA study. Eur J Heart Fail. 8:697–705. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Piper S and McDonagh T: The role of
intravenous vasodilators in acute heart failure management. Eur J
Heart Fail. 16:827–834. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Levy PD, Laribi S and Mebazaa A:
Vasodilators in acute heart failure: Review of the latest studies.
Curr Emerg Hosp Med Rep. 2:126–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Elkayam U, Janmohamed M, Habib M and
Hatamizadeh P: Vasodilators in the management of acute heart
failure. Crit Care Med. 36(Suppl 1): S95–S105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hollenberg SM: Vasodilators in acute heart
failure. Heart Fail Rev. 12:143–147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Metra M, Teerlink JR, Voors AA, Felker GM,
Milo-Cotter O, Weatherley B, Dittrich H and Cotter G: Vasodilators
in the treatment of acute heart failure: What we know, what we
don't. Heart Fail Rev. 14:299–307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Elkayam U, Bitar F, Akhter MW, Khan S,
Patrus S and Derakhshani M: Intravenous nitroglycerin in the
treatment of decompensated heart failure: Potential benefits and
limitations. J Cardiovasc Pharmacol Ther. 9:227–241. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
den Uil CA and Brugts JJ: Impact of
intravenous nitroglycerin in the management of acute decompensated
heart failure. Curr Heart Fail Rep. 12:87–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Buch J: Urapidil, a dual-acting
antihypertensive agent: Current usage considerations. Adv Ther.
27:426–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Tebbe U, Wurst W and Neuhaus KL: Acute
haemodynamic effects of urapidil in patients with chronic left
ventricular failure. Eur J Clin Pharmacol. 35:305–308. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yang W, Zhou YJ, Fu Y, Qin J, Qin S, Chen
XM, Guo JC and Hua Q: A multicenter, randomized, trial comparing
urapidil and nitroglycerin in multifactor heart failure in the
elderly. Am J Med Sci. 350:109–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Liu LS: Writing Group of 2010 Chinese
Guidelines for the Management of Hypertension: 2010 Chinese
guidelines for the management of hypertension. Zhonghua Xin Xue
Guan Bing Za Zhi. 39:579–615. 2011.(In Chinese). PubMed/NCBI
|
|
19.
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL
and Johnson MR: 2013 ACCF/AHA guideline for the management of heart
failure: A report of the American College of Cardiology
Foundation/American Heart Association Task Force on practice
guidelines. J Am Coll Cardiol. 62:e147–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Cherney D and Straus S: Management of
patients with hypertensive urgencies and emergencies: a systematic
review of the literature. J Gen Intern Med. 17:937–945. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zink M, Gombotz H, Wasler A, Grasser B,
Rehak P and Metzler H: Urapidil reduces elevated pulmonary vascular
resistance in patients before heart transplantation. J Heart Lung
Transplant. 21:347–353. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Drobinski G, Montalescot G, Fossier JM and
Grosgogeat Y: Effects of urapidil by intravenous injection on
pulmonary circulation and cardiac function in left ventricular
failure. Ann Cardiol Angeiol (Paris). 42:167–172. 1993.PubMed/NCBI
|
|
23.
|
Carlson MD and Eckman PM: Review of
vasodilators in acute decompensated heart failure: The old and the
new. J Card Fail. 19:478–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Fermann GJ and Collins SP: Initial
management of patients with acute heart failure. Heart Fail Clin.
9:291–301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Carlyle PF and Cohn JN: Systemic and
regional hemodynamic effects of alpha-adrenoceptor blockade in
chronic left ventricular dysfunction in the conscious dog. Am Heart
J. 120:619–624. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wang RY, Chow JS, Chan KH, Pan HY and Wong
RP: Acute haemodynamic and myocardial metabolic effects of
intravenous urapidil in severe heart failure. Eur Heart J.
5:745–751. 1984.PubMed/NCBI
|
|
27.
|
M'Buyamba-Kabangu JR, Bielen E, Staessen
J, Fagard R, Lijnen P, Van Hoof R and Amery A: Hemodynamic effects
of urapidil in men. Presse Med. 19:1407–1411. 1990.PubMed/NCBI
|
|
28.
|
Toma M, Ezekowitz JA, Bakal JA, O'Connor
CM, Hernandez AF, Sardar MR, Zolty R, Massie BM, Swedberg K and
Armstrong PW: The relationship between left ventricular ejection
fraction and mortality in patients with acute heart failure:
Insights from the ASCEND-HF Trial. Eur J Heart Fail. 16:334–341.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Bhardwaj A and Januzzi JL Jr: Natriuretic
peptide-guided management of acutely destabilized heart failure:
Rationale and treatment algorithm. Crit Pathw Cardiol. 8:146–150.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Parissis JT, Nikolaou M, Mebazaa A,
Ikonomidis I, Delgado J, Vilas-Boas F, Paraskevaidis I, McLean A,
Kremastinos D and Follath F: Acute pulmonary oedema: Clinical
characteristics, prognostic factors and in-hospital management. Eur
J Heart Fail. 12:1193–1202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kozàkovà M, Marco J, Heusch G, Bernies M,
Bossi IM, Palombo C, Anguissola GB, Donatelli F, Laurent JP and
Gregorini L: The alpha1-adrenergic blocker urapidil improves
contractile function in patients 3 months after coronary stenting:
A randomized, double-blinded study. Am Heart J. 147:E62004.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Yao DK, Jia SQ, Wang L, Li HW, Zhang YC,
Wang YL and Wang LX: Therapeutic effect of urapidil on myocardial
perfusion in patients with ST-elevation acute coronary syndrome.
Eur J Intern Med. 20:152–157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Oren S, Turkot S, Paran E, Flandra O,
Slezak L and Hof B: Efficacy and tolerability of slow release
urapidil (ebrantil) in hypertensive patients with non-insulin
dependent diabetes mellitus (NIDDM). J Hum Hypertens. 10:123–127.
1996.PubMed/NCBI
|