Introduction
Post-transplant lymphoproliferative disorder (PTLD)
is a well-recognized and potentially life-threatening morbidity
that can occur following transplantation of allogeneic
hematopoietic stem cells and solid organs, including the heart
(1–3). It is estimated to occur in 1–6.3%
cardiac recipients, and manifests as a spectrum of
lymphoproliferative disorders, from benign lymphoid hyperplasia to
invasive lymphoma (1–3). An Epstein-Barr virus (EBV) infection
resulting from post-transplantation use of immunosuppressive agents
is understood to drive the neoplastic pathogenesis of B cells in
PTLD (2). Treatment for PTLD
primarily includes the reduction or cessation of immunosuppressive
therapy, anti-EBV therapy, anti-neoplastic therapy, such as
chemoradiation therapy and surgery, and immunotherapy (4).
Two subtypes of PTLD, early- and late-onset PTLD,
occur within the first year post-transplantation and afterwards,
respectively, and exhibit distinct pathological profiles and
prognoses (5,6). It has been reported that EBV-positive
patients are more frequently afflicted with early-onset PTLD than
their EBV-negative counterparts (5,6).
Conversely, late-onset PTLD occurs more frequently in older
patients who are typically EBV-negative, following a longer latency
period; these patients generally have a poor treatment response and
unfavorable prognosis, as late-onset PTLD frequently acts like
aggressive lymphoma (7).
The present study reports a case of an EBV-negative,
middle-aged Han Chinese man who developed late-onset PTLD 12 years
after heart transplantation. Previous treatment with cyclosporine
and prednisone was replaced by tacrolimus and mycophenolate
mofetil. Rituximab (a monoclonal CD20 antibody) and a modified
mini-CHOP (R-mini-CHOP) chemotherapy regimen was well tolerated and
resulted in a 5-year clinical remission, free of allograft
rejection.
Case report
A 54-year-old Han Chinese patient received
orthotopic heart transplantation due to a diagnosis of end-stage
dilated cardiomyopathy at the Zhejiang Provincial People's Hospital
(Hangzhou, China) in June 1997. The patient underwent an uneventful
perioperative course and remained on immunosuppressive therapy with
cyclosporine and prednisone. The patient exhibited a good general
condition free of allograft rejection, infection, or other
clinically significant transplant-associated complications.
However, at a follow-up visit in April 2009, the patient complained
of multiple, bilateral, painless, cervical masses, in the absence
of other discomforts such as fever, toothache, cough or dyspnea.
These masses were 1–3 cm in size and less mobile on palpation, and
the local skin showed no redness or rash. Histologic examination
with hematoxylin (Regal Biotechnology Co., Ltd., Bengbu, China) and
eosin (Shanghai Xinsheng Chemical Technology Co., Ltd., Shanghai,
China) was performed on a paraffin-embedded lymph node biopsy
specimen (4 µm; Leica RM2135 microtome; Leica Microsystems GmbH,
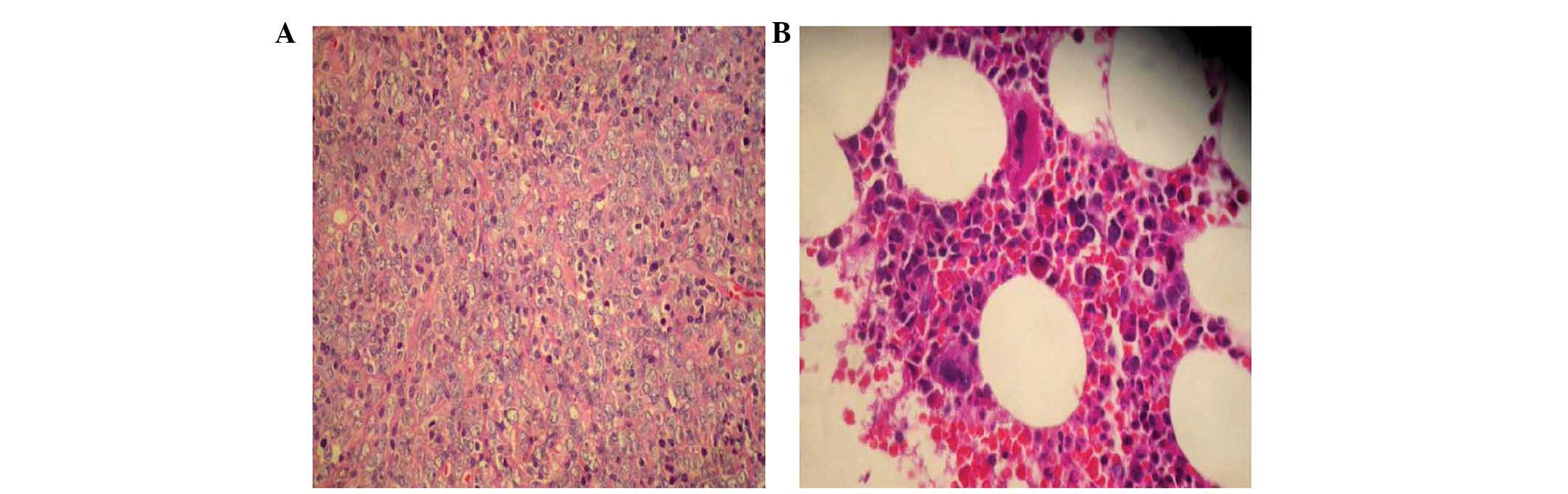
Wetzlar, Germany), and showed non-Hodgkin lymphoma-diffuse large
B-cell lymphoma (DLBCL; Fig. 1A)
under the microscope (BX5; Olympus Corporation, Tokyo, Japan).
Immunohistochemistry was performed on lymph node paraffin-embedded
tissue (4 µm) by blocking with hydrogen peroxide, followed by
incubation with primary antibodies against CD20 (1:150; rabbit
monoclonal/polyclonal; cat. no. EP7), CD10 (1:50; rabbit
monoclonal; cat. no. SP67), CD15 (1:200; mouse monoclonal; cat. no.
MMA+BY87), CD3 (1:100; rabbit monoclonal; cat. no. EP41), CD30
(1:150; rabbit monoclonal; cat. no. EP154), CD38 (1:150; mouse
monoclonal; cat. no. SPC32), CD79a (1:150; rabbit monoclonal; cat.
no. EP82), CD45RO (1:150; mouse monoclonal; cat. no. UCHL1), PAX-5
(1:50; rabbit monoclonal; cat. no. EP156), BCL-6 (1:100; mouse
monoclonal; cat. no. LN22), MUM-1 (1:200; rabbit monoclonal; cat.
no. EP190), ALK (1:150; mouse monoclonal; cat. no. OT1/A4), TDT
(1:150; mouse monoclonal; cat. no. SEN28), Ki67 (1:100; rabbit
monoclonal; cat. no. EP5), kappa (1:150; mouse monoclonal; cat. no.
CH15), lambda (1:150; mouse monoclonal; cat. no. SHL53) and EMA
(1:150; mouse monoclonal; cat. no. GP1.4) for 15 h at 4°C (all
purchased from OriGene Technologies, Inc. (Rockville, MD, USA). The
results identified strong immuno-positivity for CD20 and CD79a
(Table I) using ABC series
(avidin-biotin complex; Vector Laboratories, Ltd., Peterborough,
UK) and a microscope (BX5; Olympus Corporation). Bone marrow
examination showed mild marrow hyperplasia (Fig. 1B), and chest/abdominal computed
tomography (GE HiSpeec TC/i; The General Electric Company,
Coventry, UK) detected no distant metastasis. Thus, a diagnosis of
monomorphic PTLD-DLBCL was established based on the previous
history of heart transplantation and medication with
immunosuppressive agents, in accordance with the World Health
Organization classification of PTLD (8). The present study was approved by the
Ethics Committee of Zhejiang Provincial People's Hospital
 | Table I.Immunohistochemistry results of a
lymph node biopsy specimen. |
Table I.
Immunohistochemistry results of a
lymph node biopsy specimen.
|
Immunohistochemical | Positive rate |
|---|
| CD20 | ++ |
| TdT | − |
| MUM-1 | + |
| EBER | − |
| CD15 | − |
| CD30 | − |
| CD79a | ++ |
| CD10 | − |
| EMA | − |
| CD38 | ± |
| Ki67 | +/60% |
| UCHL1 | ± |
| Pax-5 | + |
| ALK | − |
| CD3 | ± |
| BCL-6 | ± |
| kappa | + |
| lambda | − |
Routine hematologic (Sysmex 2100; Sysmex Corporation
Co., Ltd., Kobe, Japan), clinical biochemistry (AU5800; Beckman
Coulter, Brea, CA, USA), serologic (Abbott i2000; Beckman Coulter),
and virologic (EBV and cytomegalovirus) tests (Euroimmun UK, Ltd.,
London, UK) showed no clinically significant abnormality. The
immunosuppressive regimen of the patient was changed to
dose-reduced tacrolimus (2 mg daily) and mycophenolate mofetil (1.5
g daily) prior to the initiation of chemotherapy. A rituximab
(Roche Diagnostics, Basel, Switzerland) and R-mini-CHOP
chemotherapy regimen for non-Hodgkin lymphoma was administered as
follows: 375 mg/m2 rituximab, 400 mg/m2
cyclophosphamide (Jiangsu Hengrui Medicine Co., Ltd., Shanghai,
China), 30 mg/m2 pirarubicin (Shenzhen Wanle
Pharmaceutical Co., Ltd., Shenzen, China), and 1 mg vincristine
(Hangzhou Minsheng Pharmaceutical Group Co., Ltd., Hangzhou, China)
on day 1 and 40 mg/m2 prednisone (Shanghai Xinyi
Pharmaceutical Co., Ltd., Shanghai, China) on days 1–5. The
induction therapy was given every 4 weeks for a total of 6 cycles.
Eight-cycle 375-mg/m2 rituximab infusion was
subsequently administered as maintenance therapy. The R-mini-CHOP
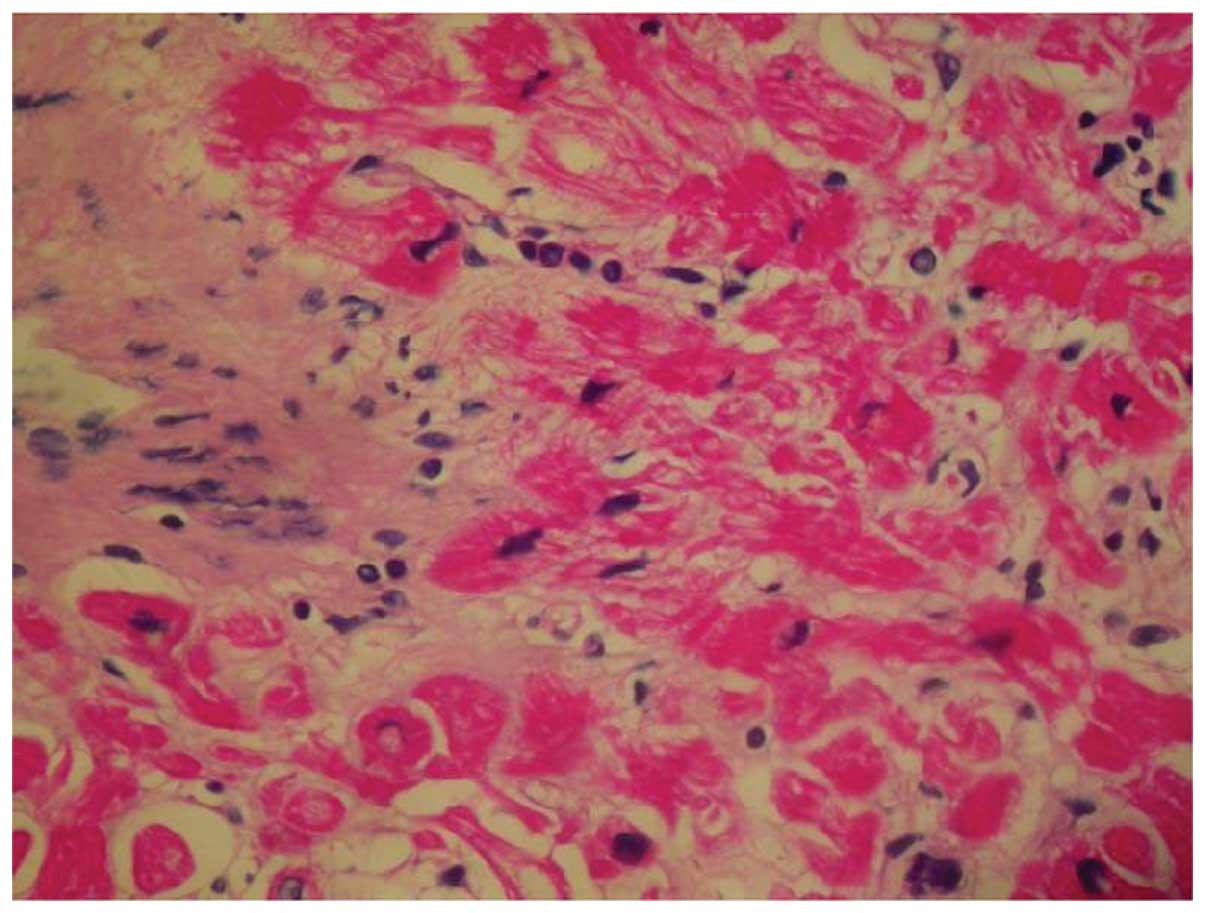
therapy was well tolerated, and myocardial biopsy confirmed a grade
0–1 allograft rejection at 2.5 years after the previous rituximab
maintenance therapy (Fig. 2). The
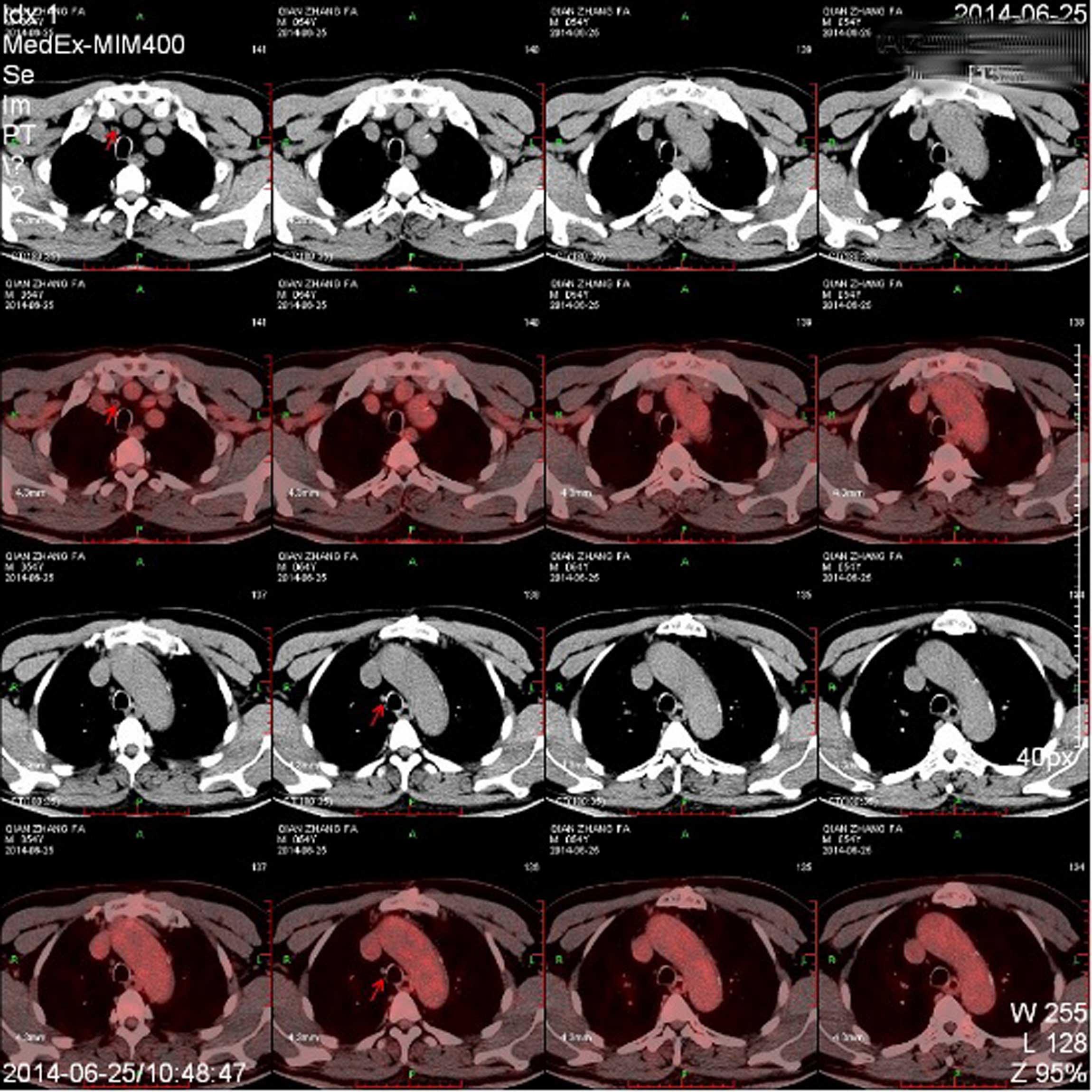
patient remained in clinical remission, as shown by follow-up
positron emission tomography (Biograoh MCT; Siemens AG, Munich,
Germany) and computed tomography 1 and 5 years after R-mini-CHOP
therapy (Fig. 3).
Discussion
The first heart transplantation was performed in
1967 (9), and PTLD was first
reported in a kidney recipient in 1979 (10). PTLD is the most common malignancy
second to skin/lip cancer in patients who have undergone solid
organ transplantation, with an estimated incidence rate of
159/100,000 persons per year (11).
Skin and splenic lymph nodes (56%) are most frequently involved in
PTLD at the time of presentation (8). In addition, non-renal transplant
recipients, in particular heart and lung recipients, have a higher
risk of developing PTLD than renal transplant recipients (12). To the best of our knowledge, the
present study reports the first case of late-onset PTLD following
heart transplantation in a Chinese patient, although ~200 cases of
PTLD have been reported among heart recipients in the English
literature.
PTLD is the overproliferation of lymphoid cells,
with disease severity ranging from benign polyclonal histology to
malignant lymphoma (13). The most
common pathologies of PTLD include DLBCL and immunoblastic lymphoma
(14), whereas T-cell lymphomas,
Hodgkin disease and plasma cell neoplasms are less frequently
encountered (15).
Post-transplantation, patients are at the highest risk of
developing early-onset PTLD, which occurs within the first
postoperative year (3,16,17). The
early-onset subtype more frequently affects pediatric and younger
adult patients as polymorphic lymphoid proliferation with a
possible causative association with EBV infection (3), whereas the late-onset subtype, which
manifests as monomorphic lymphomas of B-cells, or less often,
T-cells, primarily occurs in older patients without a definitive
association with EBV infection (18,19).
Late-onset PTLD patients (21%) are more likely to be negative on
the Epstein-Barr encoding region (EBER) in situ
hybridization, compared with early-onset patients (10%) (12).
As the EBV-associated B-cell tumor is understood to
result from impaired immunity following immunosuppressive therapy,
the reduction or cessation of immunosuppressive therapy is
essential for the treatment of PTLD and may lead to spontaneous
remission in early cases; however, reduced immunosuppression may
elicit allograft rejection (4). Au
et al (20) reported a case
of brain PTLD (EBER+, CD20+, DLBCL) in a Chinese patient following
heart transplantation, and the patient achieved complete remission
following a reduction of immunosuppression. However, the patient
succumbed to allograft rejection after 5 months. Intensive
chemotherapy is typically required if reduced immunosuppression
fails to control early-onset disease, and is the initial therapy
for the majority of cases of late-onset PTLD (2).
An antracyclin-based multi-drug regimen, such as the
CHOP regimen with granulocyte-colony stimulating factor (21), is used in the majority of
transplantation centers. An underlying adverse effect of cytotoxic
chemotherapy is enhanced hematotoxicity in patients with PTLD, as
previous long-term immunosuppression may result in kidney and
marrow toxicities (22). These
patients also have a higher susceptibility to infectious
complications and sepsis, due to the underlying impairment of
immunity (22). A low-dose CHOP,
namely the mini-CHOP regimen, was developed by Windebank et
al (23) and Gross et al
(24) and is associated with reduced
drug toxicity and better tolerability.
PTLD following solid organ transplantation is
understood to be immunoreactive to CD20 in >90% of cases, and
DLBCL affects ≤75% of adult PTLD cases with monomorphic histology
(25). The chimeric antibody
rituximab, which specifically targets the CD20 antigen, has been
incorporated in the treatment of PTLD in a number of case reports
and retrospective studies (26–28). The
use of rituximab may allow a reduction in chemotherapy dose and
result in reduced cytoxicity, particularly in pediatric patients.
In 5 out of 6 pediatric PTLD patients, rituximab combined with
dose-reduced chemotherapy resulted in a complete response within an
8- to 29-month follow-up period, with limited toxicity (29). Gupta et al (13) reported that direct rituximab and
reduced dose chemotherapy following the failure of reduced
immunosuppression appeared to be effective as an alternative
treatment of option for B-cell PTLD. Kusuki et al (30) reported that the rituximab and
combination chemotherapy regimen was effective in inducing and
maintaining remission of PTLD. The present study is the first case
report to evaluate the efficacy and safety of the R-mini-CHOP
regimen in an adult PTLD patient following solid organ
transplantation.
PTLD patients, in particular those suffering from
the late-onset subtype, typically have an unfavorable prognosis,
and approximately one-third of post-heart transplantation patients
succumb to PTLD (31,32). The survival probability of pediatric
early-onset PTLD heart recipients varies among previous reports
(31,32), although remains low in adult
late-onset patients. In a case study of 56 pediatric heart
recipients with PTLD, the probability of survival was 75% at 1
year, 68% at 3 years and 67% at 5 years after diagnosis (32). The patient in the current study
exhibited clinical remission free of cytoxicity and allograft
rejection at 5 years following R-mini-CHOP therapy for PTLD.
In conclusion, the patient in the present study
presented EBV-negative, late-onset PTLD following heart
transplantation. Reduced immunosuppression and the R-mini-CHOP
regimen resulted in long-term clinical remission in the patient.
This treatment regimen exhibited good tolerability and a good
safety profile with respect to cytoxicity and allograft rejection.
These results support the clinical practice of the management of
B-cell PTLD in adult late-onset patients.
References
|
1
|
Gao SZ, Chaparro SV, Perlroth M, Montoya
JG, Miller JL, DiMiceli S, Hastie T, Oyer PE and Schroeder J:
Post-transplantation lymphoproliferative disease in heart and
heart-lung transplant recipients: 30-year experience at Stanford
University. J Heart Lung Transplant. 22:505–514. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taylor AL, Marcus R and Bradley JA:
Post-transplant lymphoproliferative disorders (PTLD) after solid
organ transplantation. Crit Rev Oncol Hematol. 56:155–167. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wasson S, Zafar MN, Best J and Reddy HK:
Post-transplantation lymphoproliferative disorder in heart and
kidney transplant patients: A single-center experience. J
Cardiovasc Pharmacol Ther. 11:77–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khedmat H, Alavian S and Taheri S:
Significance of Epstein-Barr virus infection in the outcome of
renal transplant patients with lymphoproliferative disorders. Ann
Transplant. 15:40–44. 2010.PubMed/NCBI
|
|
5
|
Poirel HA, Bernheim A, Schneider A, Meddeb
M, Choquet S, Leblond V, Charlotte F, Davi F, Canioni D, Macintyre
E, et al: Characteristic pattern of chromosomal imbalances in
posttransplantation lymphoproliferative disorders: Correlation with
histopathological subcategories and EBV status. Transplantation.
80:176–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsai DE, Hardy CL, Tomaszewski JE, Kotloff
RM, Oltoff KM, Somer BG, Schuster SJ, Porter DL, Montone KT and
Stadtmauer EA: Reduction in immunosuppression as initial therapy
for posttransplant lymphoproliferative disorder: Analysis of
prognostic variables and long-term follow-up of 42 adult patients.
Transplantation. 71:1076–1088. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herman J, Vandenberghe P, van den Heuvel
I, Van Cleemput J, Winnepenninckx V and Van Damme-Lombaerts R:
Successful treatment with rituximab of lymphoproliferative disorder
in a child after cardiac transplantation. J Heart Lung Transplant.
21:1304–1309. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Campo E, Swerdlow SH, Harris NL, Pileri S,
Stein H and Jaffe ES: The 2008 WHO classification of lymphoid
neoplasms and beyond: Evolving concepts and practical applications.
Blood. 117:5019–5032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barnard CN: The first transplantation of a
human heart. Presse Med. 75:2815–2816. 1967.(In French). PubMed/NCBI
|
|
10
|
Marker SC, Ascher NL, Kalis JM, Simmons
RL, Najarian JS and Balfour HH Jr: Epstein-Barr virus antibody
responses and clinical illness in renal transplant recipients.
Surgery. 85:433–440. 1979.PubMed/NCBI
|
|
11
|
Kinch A, Baecklund E, Backlin C, Ekman T,
Molin D, Tufveson G, Fernberg P, Sundström C, Pauksens K and Enblad
G: A population-based study of 135 lymphomas after solid organ
transplantation: The role of Epstein-Barr virus, hepatitis C and
diffuse large B-cell lymphoma subtype in clinical presentation and
survival. Acta Oncol. 53:669–679. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yousem SA, Randhawa P, Locker J, Paradis
IL, Dauber JA, Griffith BP and Nalesnik MA: Posttransplant
lymphoproliferative disorders in heart-lung transplant recipients:
Primary presentation in the allograft. Hum Pathol. 20:361–369.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gupta S, Fricker FJ, González-Peralta RP,
Slayton WB, Schuler PM and Dharnidharka VR: Post-transplant
lymphoproliferative disorder in children: Recent outcomes and
response to dual rituximab/low-dose chemotherapy combination.
Pediatr Transplant. 14:896–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weissmann DJ, Ferry JA, Harris NL, Louis
DN, Delmonico F and Spiro I: Posttransplantation
lymphoproliferative disorders in solid organ recipients are
predominantly aggressive tumors of host origin. Am J Clin Pathol.
103:748–755. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schubert S, Abdul-Khaliq H, Lehmkuhl HB,
Yegitbasi M, Reinke P, Kebelmann-Betzig C, Hauptmann K,
Gross-Wieltsch U, Hetzer R and Berger F: Diagnosis and treatment of
post-transplantation lymphoproliferative disorder in pediatric
heart transplant patients. Pediatr Transplant. 13:54–62. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shan D, Ledbetter JA and Press OW:
Apoptosis of malignant human B cells by ligation of CD20 with
monoclonal antibodies. Blood. 91:1644–1652. 1998.PubMed/NCBI
|
|
17
|
Trofe J, Buell JF, Beebe TM, Hanaway MJ,
First MR, Alloway RR, Gross TG, Succop P and Woodle ES: Analysis of
factors that influence survival with post-transplant
lymphoproliferative disorder in renal transplant recipients: The
Israel Penn International Transplant Tumor Registry experience. Am
J Transplant. 5:775–780. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khedmat H and Taheri S: Early versus late
outset of lymphoproliferative disorders post-heart and lung
transplantation: The PTLD. Int Survey. Hematol Oncol Stem Cell
Ther. 4:10–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Webber SA, Naftel DC, Fricker FJ,
Olesnevich P, Blume ED, Addonizio L, Kirklin JK and Canter CE:
Pediatric Heart Transplant Study: Lymphoproliferative disorders
after paediatric heart transplantation: A multi-institutional
study. Lancet. 367:233–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Au WY, Lie AK, Kwong YL, Shek TW, Hawkins
BR, Lai KN, Tang SC, Lo CM, Fan ST, Liu CL, et al:
Post-transplantation lymphoproliferative disease in Chinese: The
Queen Mary Hospital experience in Hong Kong. Leuk Lymphoma.
43:1403–1407. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garrett TJ, Chadburn A, Barr ML, Drusin
RE, Chen JM, Schulman LL, Smith CR, Reison DS, Rose EA and Michler
RE: Posttransplantation lymphoproliferative disorders treated with
cyclophosphamide-doxorubicin-vincristine-prednisone chemotherapy.
Cancer. 72:2782–2785. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choquet S, Trappe R, Leblond V, Jäger U,
Davi F and Oertel S: CHOP-21 for the treatment of post-transplant
lymphoproliferative disorders (PTLD) following solid organ
transplantation. Haematologica. 92:273–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Windebank K, Walwyn T, Kirk R, Parry G,
Hasan A, Bown N and Wilkins B: Post cardiac transplantation
lymphoproliferative disorder presenting as t(8;14) Burkitt
leukaemia/lymphoma treated with low intensity chemotherapy and
rituximab. Pediatr Blood Cancer. 53:392–396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gross TG, Hinrichs SH, Winner J, Greiner
TC, Kaufman SS, Sammut PH and Langnas AN: Treatment of
post-transplant lymphoproliferative disease (PTLD) following solid
organ transplantation with low-dose chemotherapy. Ann Oncol.
9:339–340. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harris N, Swerdlow SH, Frizzera G and
Knowles DM: Post-transplant lymphoproliferative disorders. IARC
Press. Cheenra: 2001.
|
|
26
|
Milpied N, Vasseur B, Parquet N, Garnier
JL, Antoine C, Quartier P, Carret AS, Bouscary D, Faye A, Bourbigot
B, et al: Humanized anti-CD20 monoclonal antibody (Rituximab) in
post transplant B-lymphoproliferative disorder: A retrospective
analysis on 32 patients. Ann Oncol. 11(Suppl 1): 113–116. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oertel SH, Anagnostopoulos I, Bechstein
WO, Liehr H and Riess HB: Treatment of posttransplant
lymphoproliferative disorder with the anti-CD20 monoclonal antibody
rituximab alone in an adult after liver transplantation: A new drug
in therapy of patients with posttransplant lymphoproliferative
disorder after solid organ transplantation? Transplantation.
69:430–432. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Verschuuren EA, Stevens SJ, van Imhoff GW,
Middeldorp JM, de Boer C, Koëter G, The TH and van Der Bij W:
Treatment of posttransplant lymphoproliferative disease with
rituximab: The remission, the relapse and the complication.
Transplantation. 73:100–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Orjuela M, Gross TG, Cheung YK, Alobeid B,
Morris E and Cairo MS: A pilot study of chemoimmunotherapy
(cyclophosphamide, prednisone and rituximab) in patients with
post-transplant lymphoproliferative disorder following solid organ
transplantation. Clin Cancer Res. 9:3945S–3952S. 2003.PubMed/NCBI
|
|
30
|
Kusuki S, Hashii Y, Fukushima N, Takizawa
S, Tokimasa S, Kogaki S, Ohta H, Tsuda E, Nakagawa A and Ozono K:
Pediatric post-transplant diffuse large B cell lymphoma after
cardiac transplantation. Int J Hematol. 89:209–213. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boyle GJ, Michaels MG, Webber SA, Knisely
AS, Kurland G, Cipriani LA, Griffith BP and Fricker FJ:
Posttransplantation lymphoproliferative disorders in pediatric
thoracic organ recipients. J Pediatr. 131:309–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cohen AH, Sweet SC, Mendeloff E, Mallory
GB Jr, Huddleston CB, Kraus M, Kelly M, Hayashi R and DeBaun MR:
High incidence of posttransplant lymphoproliferative disease in
pediatric patients with cystic fibrosis. Am J Respir Crit Care Med.
161:1252–1255. 2000. View Article : Google Scholar : PubMed/NCBI
|