Introduction
Polymorphonuclear neutrophils (PMN) have an
important role in host defense and various inflammatory diseases
(1). Increased number, enhanced
chemotaxis and superoxide anion generation of PMN have been
observed in animal and clinical research with obstructive jaundice
(OJ), which is closely associated with exaggerated systemic
inflammation (2–6). Several recent reports have focused on
the fate of peripheral PMN and their significance in inflammatory
diseases or surgical stress (7–11).
Inhibited peripheral PMN apoptosis has been demonstrated in burns
(7), severe trauma (8), systemic inflammatory response syndrome
(SIRS) (9) and acute pancreatitis
(10). The inhibition of PMN
apoptosis may result in prolonged PMN life span in the circulation,
leading to excessive inflammation (12). If the PMN function is infinitely
exaggerated, it may lead to SIRS or even progress to multiple organ
dysfunction syndrome (13).
The precise mechanisms underlying PMN apoptosis
during OJ are poorly understood. Peripheral PMN have the shortest
half-life among leukocytes (14).
Senescent PMN undergo apoptosis, which is the principal signaling
pathway responsible for cell destruction (15). It is thought that PMN apoptosis
involves three signaling pathways (16): The endogenous pathway, the exogenous
pathway and the endocytoplasmic reticulum pathway. These three
signaling pathways communicate and interact with one another in
physiological conditions, which has an important role in regulating
PMN function and cell dissolution (17). Among the three signaling pathways,
the passage from cytochrome c (Cytc) to caspase cascade
activation in the mitochondria is an important step that regulates
cell death (18). Only 10 min are
required to complete this process, which is termed the key ‘10 min
event’ (18). Therefore, the
mitochondrial signaling pathway has an important role in
controlling PMN apoptosis.
Recent studies reported that the outcome of acute
inflammation in OJ is closely associated with the function of
peripheral PMN (2–6). Previous studies demonstrated that
delayed PMN apoptosis can cause excessive inflammation, and that
the potential PMN apoptosis suppression mechanism may result in a
decrease in caspase-3 activity (19,20). It
has been reported that caspase cascade activation is involved in
the cell apoptotic signaling pathway, which has an impact on the
initiation and outcome of inflammation (20). However, the endogenous mitochondrial
apoptosis signaling pathway regulating cellular life-span and
affecting PMN apoptosis in OJ has yet to be elucidated.
The present study used bile duct-ligated (BDL) rats
to mimic OJ in order to investigate the changes in peripheral PMN
apoptosis, the regulatory mechanism of the endogenous apoptotic
signaling pathway, and the effect on PMN apoptosis following
treatment with Cytc, as well as to attempt to elucidate the
mechanisms underlying PMN apoptosis dysfunction in OJ.
Materials and methods
Animals
A total of 110 male Sprague-Dawley rats (weight,
200–250 g; age, 6–8 weeks) were purchased from the Medical
Experimental Animal Center of Guangdong Province (Guangzhou,
China), of which 104 survived. Food and water were provided ad
libitum. The animals were housed in a laminar-flow specific
pathogen-free atmosphere. A temperature of 22±1°C and 12 h
light/dark cycle were maintained. All the experimental protocols
were approved by the Animal Ethics Committee of the First
Affiliated Hospital of Shenzhen University (Shenzhen, China).
Experimental design
The rats were randomly divided into four groups, of
which the following numbers of rats survived: A control group
(n=8), a sham group (n=32), a BDL group (n=32), and a BDL + Cytc
group (n=32). The control group represented untreated rats at the
beginning of the experiment. The remaining three groups were
divided into rats at four time points: 12, 24, 48 and 72 h
following the BDL surgical procedure, with 8 rats at each time
point group.
Prior to undergoing surgery the rats were subjected
to a fast for 12 h with ad libitum access to water. All
surgical procedures were performed under 10% chloral hydrate
(0.4–0.5 ml/100 g; Sigma-Aldrich, St. Louis, MO, USA)
intraperitoneal anesthesia with aseptic surgical techniques.
Briefly, the common bile duct of the rats in the sham group was
dissected away from the surrounding tissue but not ligated. In the
BDL group and BDL + Cytc group, the surgical and postoperative care
were the same as for the sham group. The rats in these two groups
underwent BDL using double 4–0 silk sutures. Rats in the BDL + Cytc
group were also injected intravenously with 20 mg/kg Cytc
(Sigma-Aldrich) following BDL. The rats in the control group
received no surgical procedure or treatment.
Sample collection
At the various time points prior to (in control
rats) or following surgery (in experimental animals), all surviving
animals were anesthetized with 10% chloral hydrate (0.4–0.5 ml/100
g), and the abdomen was opened prior to collection of blood samples
from the inferior vein cava for biochemical analysis and PMN
isolation. The rats were then sacrificed using cervical
dislocation. The blood samples from each rat were sent to the
Central Laboratory at the First Affiliated Hospital of Shenzhen
University for analysis.
Liver function and blood routine
examination
A total of 1 ml of blood sample for each rat was
centrifuged (600 × g, 10 min, 4°C). The upper plasma was collected
and assayed for total bilirubin (TBIL) and alanine aminotransferase
(ALT) levels using a multi-channel automatic biochemical analyzer
(Hitachi 7600; Hitachi, Ltd., Tokyo, Japan). A further 0.5 ml of
blood from each rat was used to count the number of blood cells
using a three classification blood cell analyzer (ABX Hemastar-3;
Horiba ABX SAS, Montpellier, France).
Isolation of peripheral PMN
In order to isolate peripheral PMN, 5 ml blood
samples were obtained from each rat. PMN were harvested using
gradient centrifugation and hypotonic erythrocyte lysis, as
described previously (21). The
cells were centrifuged at 900 × g for 10 min with cold RPMI-1640
medium (GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 1% fetal bovine serum (Zhejiang Tianhang Biotechnology Co.,
Ltd., Huzhou, China). Giemsa staining (Amresco, LLC, Solon, OH,
USA) and trypan blue (Sigma-Aldrich) exclusion test determined a
purity of >95% and a viability of >98% of the PMN,
respectively, in each sample.
PMN apoptosis
An Annexin V-fluorescein isothiocyanate (FITC)
Apoptosis Detection kit (cat. no. FAK011.100; Neobioscience,
Shenzhen, China) was used to detect early and late apoptotic
activity following PMN isolation. According to the standard
protocol, the binding buffer was diluted at 1:4 with sterile
deionized water. A total of 50 µl PMN suspension was resuspended
with 150 µl binding buffer, and 5 µl Annexin V-FITC was added and
gently mixed. Following an interval of 3 min, 10 µl propidium
iodide (PI; 20 µg/ml) was added, incubated in the dark for 10 min
following gentle mixing at room temperature, and then added to 250
µl binding buffer prior to further mixing. The cells were detected
and analyzed using a Fluorescence Activated Cell Sorter (FACS;
Beckman Coulter Epics XL; Beckman Coulter, Inc., Brea, CA, USA). A
total of 5×103 cells were counted, and
Annexin-V+/PI− cells were indicative of
apoptosis. The percentage of Annexin-V+/PI−
cells was used to determine the apoptotic rate.
Measurement of PMN mitochondrial
membrane potential (ΔΨm)
A total of 10 µl PMN suspension was washed twice
with phosphate-buffered saline (PBS). The supernatant was discarded
and 20 µl rhodamine-123 added (Rho-123; 10 µg/ml; Sigma-Aldrich),
incubated for 20 min at 37°C, then washed twice in PBS for 5 min.
The cells were detected and analyzed using the FACS. A total of
5×103 cells were counted and the above procedures were
repeated three times. Finally, the fluorescence intensity of the
cells was detected by FACS using Rho-123, and the mean fluorescence
intensity (MFI) was calculated.
Western blot analysis
Mitochondrial or cytosolic proteins from the PMN
cells were extracted using a Cell Mitochondrial Isolation kit
(Beyotime Institute of Biotechnology, Haimen, China), according to
the manufacturer's protocol. They were then denatured in 5X sodium
dodecyl sulfate (SDS)-loading buffer (1,000 mmol/l Tris-HCL, 200
mmol/l dithiothreitol, 10% SDS, 1% bromophenol blue and 50%
glycerol; all components sourced from Sigma-Aldrich) at 100℃ for 10
min. Equal protein concentrations, determined using a bicinchoninic
acid assay (Beyotime Institute of Biotechnology), were separated on
12% SDS-polyacrylamide gels (40 µg/lane) to detect Cytc in the
subcellular fractions. Proteins were transferred to nitrocellulose
membranes (Pierce Biotechnology, Inc., Rockford, IL, USA) and these
were blocked in 5% skim milk solution for 1 h at room temperature.
Membranes were then incubated with rabbit primary antibodies at a
dilution of 1:1,000, as follows, overnight at 4°C: Anti-Cytc (cat.
no. 4272; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-Cytc oxidase IV (a mitochondrial marker; cat. no. 4844; Cell
Signaling Technology, Inc.) and tubulin (cat. no. 2128; Cell
Signaling Technology, Inc.), to control for equal sample loading in
each subcellular fraction. Membranes were washed in Tris-buffered
saline with Tween 20, then incubated at room temperature for 1 h
with horseradish peroxidase-conjugated goat anti-rabbit IgG
(dilution, 1:2,000; BioVision, Inc., Milpitas, CA, USA). The
membranes were then subjected to enhanced chemiluminescence (ECL)
using an ECL detection kit (Pierce Biotechnology, Inc.), and
quantified using Gel Pro Analyzer v. 4.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Transmission electron microscopy
Following PMN suspension centrifugation (600 × g, 10
min, 4°C), 2.5% glutaraldehyde (Ted Pella, Inc., Redding, CA, USA)
was added to the precipitate and stored at 4℃ overnight. The PMN
were then dehydrated, saturated, embedded in Epon-812 epoxy resin
(Ted Pella, Inc.), fixed using 1% osmic acid (Ted Pella, Inc.),
dehydrated using an ethanol gradient (50, 70, 90 and 100%),
polymerized, sectioned with an EM UC7 ultramicrotome (Leica
Microsystems GmbH, Wetzlar, Germany) and dyed using uranium
acetate-lead citrate (Ted Pella, Inc.). Subsequently the sections
were dried and images were captured using a transmission electron
microscope (TEM; JEM-1400; JEOL, Ltd., Tokyo, Japan).
Statistical analysis
The data are presented as means ± standard
deviation. SPSS 17.0 (SPSS, Inc, Chicago, IL, USA) was used for to
carry out the statistical analyses. Multiple comparisons were
performed using One-way analysis of variance with Bonferonni's
post-hoc test. P<0.05 was considered to indicate a statistically
significant value.
Results
Characteristics of the experimental
models
The serum biochemical measurement values for the
various groups are shown in Table I.
ALT and TBIL levels in both the BDL and BDL + Cytc groups were
significantly higher compared with those in the control group and
the corresponding time point for the sham group (P<0.01). TBIL
levels in the BDL and in the BDL + Cytc groups were significantly
increased 12 h following the surgical procedure and remained ≥1.24
mg/dl throughout the subsequent experimental time points. The sham
surgical procedure had no significant effect on TBIL levels. ALT
levels in the BDL and in the BDL + Cytc groups were significantly
increased compared with the control and sham groups 12 h after the
surgical procedure, but decreased gradually in a time-dependent
manner, reaching a minimum of 49.95 U/l throughout the subsequently
observed time points.
 | Table I.Serum ALT and TBIL biochemical levels
at each time point. |
Table I.
Serum ALT and TBIL biochemical levels
at each time point.
| Plasma
component | Groups | 0 | 12 h | 24 h | 48 h | 72 h |
|---|
| ALT, U/l | Control |
31.05±8.66 | – | – | – | – |
|
| Sham | – |
35.10±8.65 |
42.19±7.21 |
24.64±6.36 |
25.65±6.61 |
|
| BDL | – |
62.75±10.82a |
57.25±6.67a |
52.38±10.56a |
49.95±8.08a |
|
| BDL + Cytc | – |
67.90±19.00a |
60.13±11.46a |
54.25±6.32a |
51.75±7.74a |
| TBIL, mg/dl | Control |
0.14±0.06 | – | – | – | – |
|
| Sham | – |
0.10±0.05 |
0.08±0.05 |
0.08±0.03 |
0.09±0.04 |
|
| BDL | – |
1.28±0.16a |
4.04±0.88a |
5.42±0.94a |
7.14±1.60a |
|
| BDL + Cytc | – |
1.24±0.15a |
3.61±0.99a |
5.03±0.90a |
6.69±1.48a |
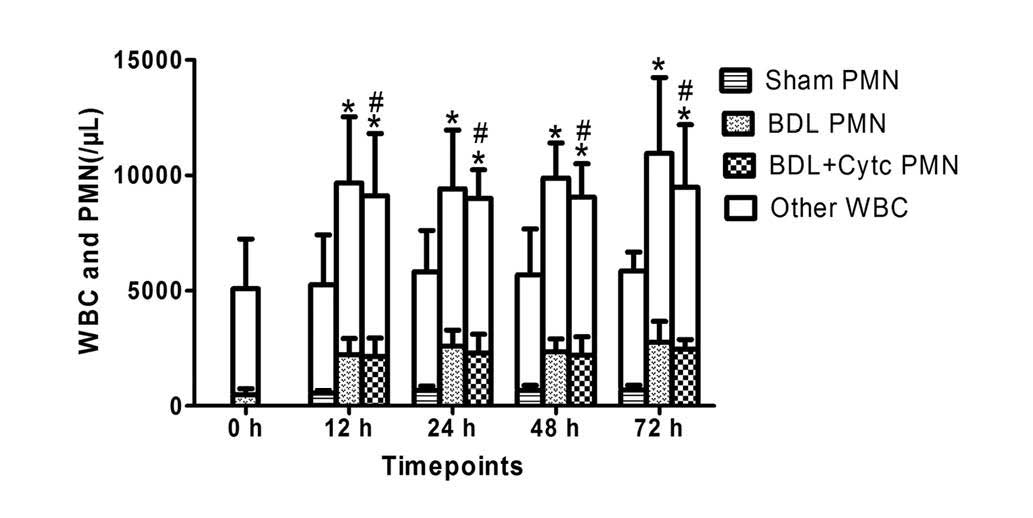
Time-dependent changes in the number
of white blood cells (WBC) and PMN following treatment with BDL and
the Cytc
The WBC and PMN counts in each group, determined
from blood sample collection, are presented in Table II. The WBC and PMN counts increased
significantly 12 h following BDL, and continued to increase at 24,
48 and 72 h following BDL. The WBC and PMN counts revealed similar
changes in the BDL + Cytc group, which were observed to be
non-significantly different compared with the BDL rats at each time
point (P>0.05). The sham surgical procedure had no significant
effect on WBC and PMN count (Fig.
1).
 | Table II.Time-dependent changes in the number
of WBC and PMN at each time point. |
Table II.
Time-dependent changes in the number
of WBC and PMN at each time point.
| Blood cells | Groups | 0 | 12 h | 24 h | 48 h | 72 h |
|---|
| WBC, n/µl | Control |
5,081±2,150 | – | – | – | – |
|
| Sham | – |
5,250±2,162 |
5,811±1,799 |
5,679±1,996 |
5,838±832 |
|
| BDL | – |
9,661±2,871a |
9,411±2,549a |
9,876±1,517a |
10,949±3,288a |
|
| BDL + Cytc | – |
9,098±2,713a,b |
8,986±1,250a,b |
9,045±1,452a,b |
9,481±2,706a,b |
| PMN, n/µl | Control |
488±258 | – | – | – | – |
|
| Sham | – |
570±88 |
683±176 |
673±212 |
688±207 |
|
| BDL | – |
2,220±709a |
2,590±692a |
2,353±556a |
2,761±911a |
|
| BDL + Cytc | – |
2,151±793a,b |
2,300±817a,b |
2,208±799a,b |
2,460±420a,b |
| PMN/WBC, % | Control |
9.55±2.75 | – | – | – | – |
|
| Sham | – |
13.51±7.51 |
12.14±2.21 |
13.88±9.00 |
12.20±4.74 |
|
| BDL | – |
23.31±4.14a |
27.67±3.51a |
23.90±4.60a |
26.00±6.37b |
|
| BDL + Cytc |
|
23.80±4.46a,b |
25.07±6.91a,b |
24.63±8.58a,b |
27.61±8.82a,b |
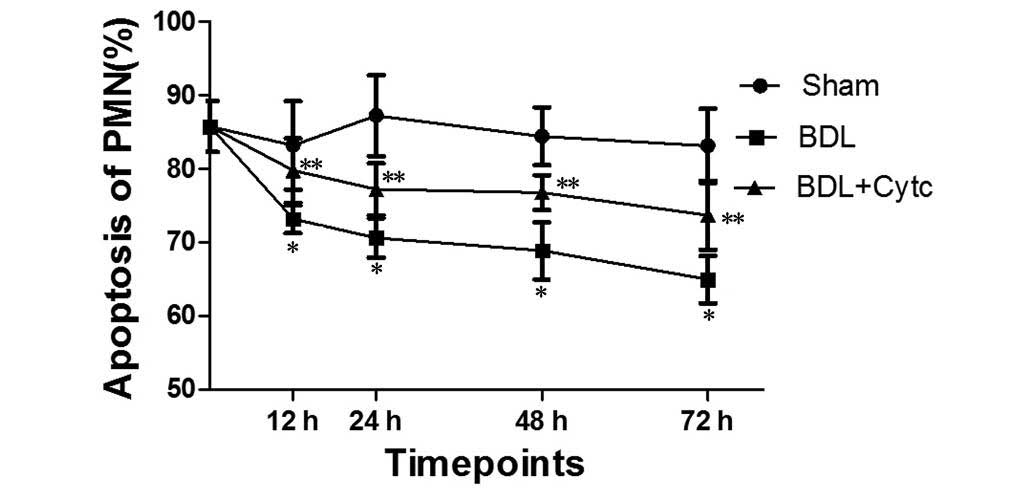
Time-dependent changes in PMN
apoptosis following BDL and treatment with Cytc
Sequential changes in PMN apoptosis at each time
point are shown in Fig. 2.
Significantly decreased PMN apoptotic levels were observed as early
as 12 h following BDL, and these levels decreased further from
73.13±1.89% at 12 h to 64.93±3.24% at 72 h (Fig. 2). The values then remained unchanged
until the end of the observation period. Compared with control
group rats (85.70±3.43%) and the sham rats at the respective time
points, the PMN apoptosis rate of the BDL group was significantly
decreased (P<0.01). Compared with the corresponding time points
for the BDL group, the BDL + Cytc group showed a significantly
increased PMN apoptosis rate (P<0.01).
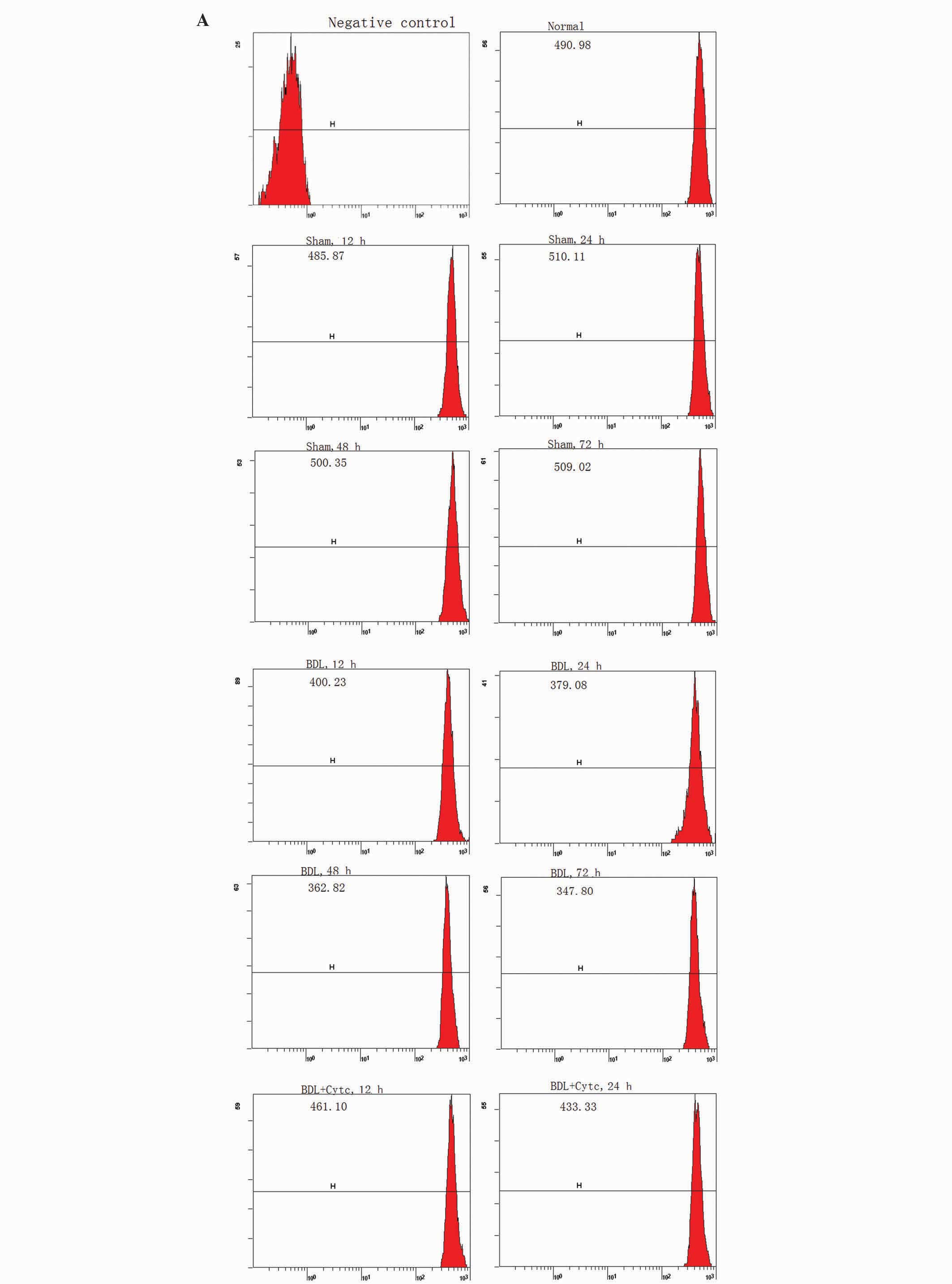
Time-dependent changes in PMN ΔΨm
following BDL and the Cytc administration on the mitochondrial
apoptotic signaling pathway
Mitochondria have life-supporting functions and are
able to regulate apoptosis (22–24).
Cytc has a key role in the mitochondria, and participates in the
assembly of a multimolecular complex known as the apoptosome, which
is a core element of the apoptotic signaling pathway (22,23,25).
ΔΨm, which prevents apoptosis proteins (Cytc and so on) from being
released from the mitochondria into the cytoplasm or nucleus, was
detected using Rho-123 staining and FACS, and expressed as the MFI
(22,23). When the Rho-123 MFI value is lower,
the ΔΨm level is higher (26).
As shown in Fig. 3,
Rho-123 MFI in PMN mitochondria was markedly decreased as early as
12 h following BDL, from 398.51±21.26 at 12 h to 343.60±22.23 at 72
h, which then remained unchanged until the end of the observation
period. Compared with the control group rats (492.07±47.21) and the
respective sham rat time points, the BDL group exhibited
significant changes in MFI (P<0.01). Compared with the
corresponding time point for the BDL group, the MFI in the BDL +
Cytc group gradually increased (P<0.01).
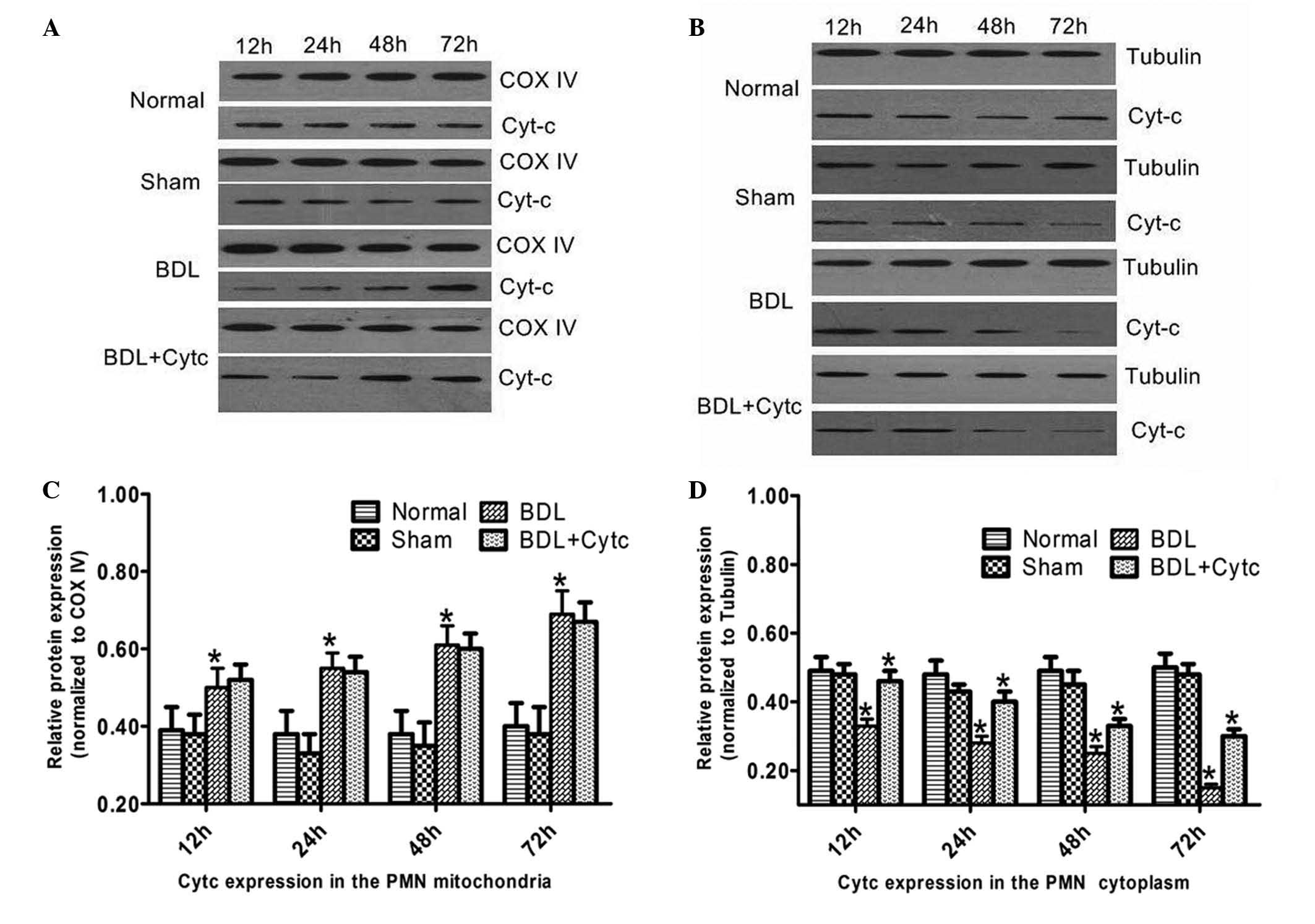
Time-dependent changes in Cytc
expression levels in the mitochondria and cytoplasm
Cytc expression levels in both the mitochondria and
cytoplasm of the PMN are shown in Fig.
4. Cytc expression levels in the mitochondria increased in a
time-dependent manner 12–72 h following BDL, and were markedly
higher compared with the control group and the corresponding time
point for the sham group. There were no differences in Cytc
expression levels between the BDL and BDL + Cytc groups in the
mitochondria (Fig. 4A,C). Cytc
expression levels in the cytoplasm gradually decreased 12–72 h
following BDL, and were markedly lower compared with that of the
control group and the sham group at the corresponding time points.
Cytc expression levels in the cytoplasm for the BDL + Cytc group
gradually exceeded that of the BDL group at each time point
(Fig. 4B and D).
Morphological changes in PMN
mitochondria following BDL and Cytc administration
The morphological changes in the PMN mitochondria
were observed under TEM. As shown in Fig. 5, typical apoptotic changes in PMN,
characterized by cell and nucleus shrinkage, retraction of
pseudopodes, plasma membrane blebbing, chromatin condensation and
nuclear fragmentation, were found in BDL rats, and markedly in BDL
+ Cytc rats. Furthermore, damaged structures, cracked cristae and
vacuolization phenomena in the mitochondria were marginal in the
BDL group, although more extensive in the BDL + Cytc group 12–72 h
following the surgical procedure.
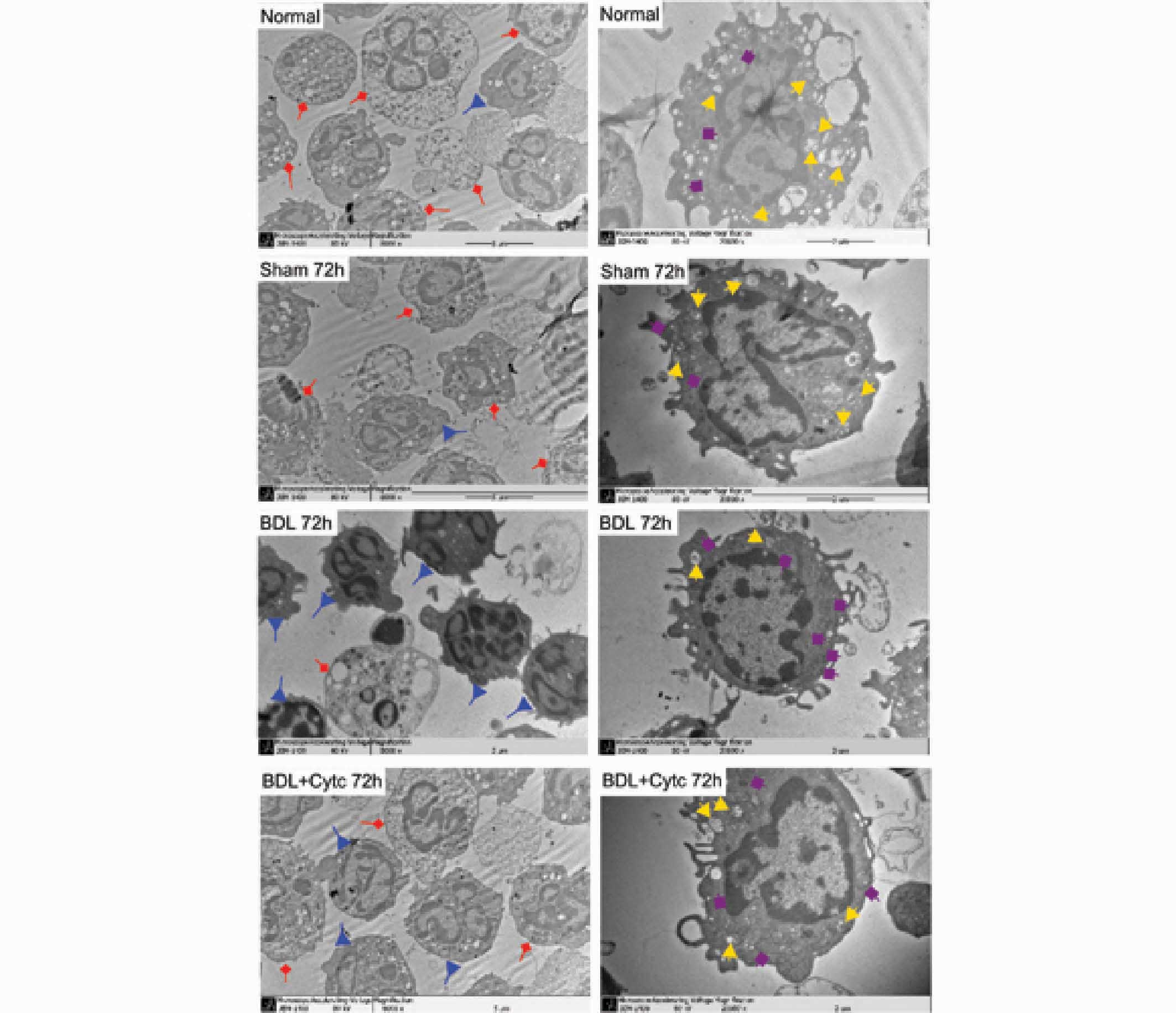 | Figure 5.Morphological changes of
mitochondrial PMN. Left panel, morphological changes of the PMN as
determined by TEM (magnification, ×8,000). Fewer apoptotic PMN and
more normal PMN were observed at 72 h in the BDL group, but
differing results were found in the control group and sham and BDL
+ Cytc groups at the corresponding time points, which displayed
more apoptotic PMN and fewer normal PMN. Right panel, morphological
changes of the PMN as determined by TEM (magnification, ×20,000).
Fewer damaged mitochondria and more normal mitochondria can be
observed in the PMN of the BDL group at 72 h, but differing results
were found in the control group and sham and BDL + Cytc groups at
the corresponding time points, which displayed more extensively
damaged structures, cracked cristae and vacuolization of the
mitochondria. Apoptotic PMN are represented in red; normal PMN are
represented in blue; damaged mitochondria are represented in
yellow; and normal mitochondria are represented in purple. PMN,
polymorphonuclear neutrophils; TEM, transmission electron
microscopy; BDL, bile duct ligation; Cytc, cytochrome c. |
Discussion
Peripheral PMN is a type of acute inflammatory cell,
the number and function of which impact inflammatory processes and
their reversal, during the development of inflammatory diseases
(27). Senescent PMN in the
peripheral blood or infiltrating tissue undergo apoptosis.
Apoptotic PMN are phagocytosed by macrophages without the release
of proinflammatory mediators, leading to limited tissue injury and
the end of inflammatory processes (12,16).
Inhibited peripheral PMN apoptosis has been demonstrated in burns
(7), severe trauma (8), SIRS (9)
and acute pancreatitis (10). The
present study demonstrated that peripheral PMN apoptosis in rats
was markedly inhibited 12 h following BDL and decreased in a
time-dependent manner. The results also indicated that the number
of PMN and WBC in BDL rats increased significantly, as compared
with the control group rats and sham rats at each corresponding
time point. Inhibited PMN apoptosis may result in increased numbers
of PMN in the blood circulation, which benefits the host defense
against systemic bacterial invasion, causing the uncontrolled
release of toxic metabolites and leading to amplified systemic
inflammation and organ injury (10,28). It
has been reported that cytoplasmic microinjection of Cytc promotes
cell apoptosis activation (29).
Accordingly, the present study used Cytc injection (20 mg/kg) in
the tail vein of rats subjected to BDL to investigate the
activating effect of Cytc on PMN apoptosis. The results
demonstrated that the number of PMN decreased and PMN apoptosis
increased. Compared with the corresponding time point for the BDL
group, rats intravenously treated with Cytc following BDL exhibited
a significant increase in the PMN apoptosis rate at 12, 24, 48 and
72 h. It was therefore hypothesized that intravenous Cytc
administration in rats subjected to BDL has an important role in
inducing PMN apoptosis. The precise mechanism underlying this
process requires further clarification.
Cell apoptosis is a complex process that has been
the subject of numerous studies (12,16,17).
Apoptosis is a form of programmed cell death, characterized by cell
and nucleus shrinkage, retraction of pseudopodes, plasma membrane
blebbing, chromatin condensation and nuclear fragmentation
(30). This process is tightly
controlled by gene regulation, receptor recognition and signal
transduction (16). However, the
potential mechanisms underlying PMN apoptosis have been extensively
debated. It is thought that several factors have roles in this
process and affect apoptosis development (22,31–34).
Matsuda et al (11) showed
that cytokine-modulated inhibition of PMN apoptosis at a local site
augments PMN functions and induces excessive inflammatory response.
The anti-apoptotic B cell lymphoma-2 (Bcl-2) family members
Bcl-X-linked, A1 and myeloid cell leukemia-1, have been
demonstrated to inhibit PMN apoptosis and promote survival by
antagonizing the pro-apoptotic proteins (35). However, Fas ligation or ligation of
the tumor necrosis-related apoptosis-inducing ligand receptor on
PMN may also trigger apoptosis (36–38).
Involved in either apoptosis or inflammation, caspases in PMN can
be activated by intrinsically regulated apoptosis as well as death
receptor-mediated apoptosis (22,24).
Despite a limited role of PMN mitochondria in cellular metabolism,
certain investigations have suggested that these organelles may be
involved in PMN cell death (39,40). The
results of the present study demonstrated that the mitochondria,
although limited in number, do participate in PMN apoptosis in BDL
rats. These organelles exhibit low mitochondrial enzymatic activity
and do not synthesize a large amount of ATP, but preserve their ΔΨm
and contain proapoptotic proteins, which trigger PMN apoptosis if
released into the cytosol (41). The
mitochondria of the PMN were observed using TEM, demonstrating the
presence of marginal morphological characteristic changes in BDL
rats, and marked changes in BDL + Cytc rats, which exhibited
damaged structures, cracked cristae and vacuolization phenomenon in
the mitochondria.
Cytc is thought to be one of the most important
pro-apoptotic proteins, and Cytc levels are markedly reduced in
normal cell cytoplasm and markedly increased in apoptotic cells
(22). ΔΨm was detected using
Rho-123 staining and FACS, and expressed as MFI to investigate the
effect of Cytc on the mitochondrial apoptotic signaling pathway
following BDL. The MFI value in BDL rats decreased from
415.66±30.77 at 12 h to 388.51±31.66 at 72 h, a value that was
significantly different compared with that of control group rats
and sham rats at the corresponding time points. These results
suggest that the ΔΨm levels in PMN mitochondria increase gradually
following BDL. Therefore, Cytc stored in the mitochondria did not
move into the cytosol, inhibiting PMN apoptosis.
In an attempt to clarify the role of mitochondria in
apoptosis, Cytc expression was investigated using western blotting.
Cytc expression gradually increased in the mitochondria and
decreased in the cytoplasm from 12 to 72 h in the BDL group, and
these expression levels were significantly different from those of
the control group and corresponding time points of the sham group.
In the BDL + Cytc group Cytc was intravenously administered to the
rats following BDL, demonstrating that the ΔΨm of the PMN
mitochondria gradually decreased and the Cytc expression levels in
the cytoplasm increased. These results were significantly different
from those of the BDL group at the corresponding time points.
However, Cytc expression levels in the mitochondria remained
elevated in the BDL + Cytc group from 12 to 72 h, and these
expression levels were not significantly different from those of
the BDL group at the corresponding time points. The possible
mechanisms underlying this phenomenon are as follows: i) The PMN
may have spontaneously lost some of the Cytc proteins during the
BDL process. ii) The ΔΨm in the mitochondria is thought to be the
conductor of the mitochondrial permeability transition pore. When
the ΔΨm decreases the pores open and Cytc proteins are released
from the mitochondria into the cytoplasm (41). This suggests that Cytc expression
levels gradually increase in the cytoplasm of the BDL + Cytc group
from 12 to 72 h. iii) Intravenous Cytc administration may directly
infiltrate into the PMN cytoplasm, and has no significant effect on
Cytc expression in the mitochondria. It is difficult to determine
whether intravenous Cytc administration affects ΔΨm in the
mitochondria, and if so the exact amount of Cytc proteins that
infiltrate into the cytoplasm via the intravenous route. Further
studies are required in order to elucidate this mechanism.
In summary, the results of the present study
suggested that PMN apoptosis is inhibited in BDL rats and the
mitochondrial apoptotic signaling pathway participates in the
apoptosis process. PMN mitochondria usually maintain their ΔΨm
within normal range. High ΔΨm in the mitochondria and decreased
Cytc expression levels in the cytoplasm may result in PMN apoptosis
inhibition in BDL rats. Intravenous Cytc administration may help to
compensate for the lack of Cytc proteins in the cytoplasm, inducing
PMN apoptosis and reversal of inflammatory processes following BDL.
These results provide an important theoretical basis for
inflammatory complications during OJ.
Acknowledgements
The present study was supported by Science and
Technology Planning Project of Guangdong Province (grant nos.
2012B031800349 and 2014A020212701), Medical Scientific Research
Foundation of Guangdong Province (grant no. B2014326), Science and
Technology Planning Project of Shenzhen (grant no.
JCYJ20130401112153105), Medical Scientific Research Foundation of
Shenzhen (grant no. 201401015).
References
|
1
|
Smith JA: Neutrophils, host defense, and
inflammation: A double-edged sword. J Leuko Biol. 56:672–686.
1994.PubMed/NCBI
|
|
2
|
Tsuji K, Kubota Y, Yamamoto S, Yanagitani
K, Amoh Y, Takaoka M, Ogura M, Kin H and Inoue K: Increased
neutrophil chemotaxis in obstructive jaundice: An in vitro
experiment in rats. J Gastroenterol Hepatol. 14:457–463. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takaoka M, Kubota Y, Tsuji K, Yamamoto S,
Ogura M, Yanagitani K, Shimatani M, Shibatani N and Inoue K: Human
neutrophil functions in obstructive jaundice.
Hepatogastroenterology. 48:71–75. 2001.PubMed/NCBI
|
|
4
|
Shibatani N, Yamamoto S, Kubota Y, Tsuji
K, Takaoka M, Amoh Y, Matsushita M, Shimatani M, Imai Y and Inoue
K: Neutrophil chemotaxis in bile duct-obstructed rats, and effect
of internal biliary drainage. Hepatogastroenterology. 49:918–923.
2002.PubMed/NCBI
|
|
5
|
Shimatani M, Tsuji K, Aze Y, Yamamoto S,
Shibatani N, Imai Y, Takamido S, Kubota Y and Okazaki K: Effects of
obstructive jaundice on neutrophil production and acquisition of
chemotactic activity in the bone marrow. J Gastroenterol Hepatol.
20:117–125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Badger SA, Jones C, McCaigue M, Clements
BW, Parks RW, Diamond T, McCallion K and Taylor MA: Cytokine
response to portal endotoxaemia and neutrophil stimulation in
obstructive jaundice. Eur J Gastroenterol Hepatol. 24:25–32. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chitnis D, Dickerson C, Munster AM and
Winchurch RA: Inhibition of apoptosis in polymorphonuclear
neutrophils from burn patients. J Leuko Biol. 59:835–839.
1996.PubMed/NCBI
|
|
8
|
Ertel W, Keel M, Infanger M, Ungethum U,
Steckholzer U and Trentz O: Circulating mediators in serum of
injured patients with septic complications inhibit neutrophil
apoptosis through up-regulation of protein-tyrosine
phosphorylation. J Trauma. 44:767–775; discussion 775–776. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fanning NF, Kell MR, Shorten GD, Kirwan
WO, Bouchier-Hayes D, Cotter TG and Redmond HP: Circulating
granulocyte macrophage colony-stimulating factor in plasma of
patients with the systemic inflammatory response syndrome delays
neutrophil apoptosis through inhibition of spontaneous reactive
oxygen species generation. Shock. 11:167–174. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiu DF, Chen JC, Chen HM, Ng CJ, Shyr MH
and Chen MF: Results of treating severe acute pancreatitis with
gabexate is associated with neutrophil apoptosis activity.
Hepatogastroenterology. 50:553–558. 2003.PubMed/NCBI
|
|
11
|
Matsuda T, Saito H, Fukatsu K, Han I,
Inoue T, Furukawa S, Ikeda S and Hidemura A: Cytokine-modulated
inhibition of neutrophil apoptosis at local site augments exudative
neutrophil functions and reflects inflammatory response after
surgery. Surgery. 129:76–85. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simon HU: Neutrophil apoptosis pathways
and their modifications in inflammation. Immunol Rev. 193:101–110.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maier RV: Pathogenesis of multiple organ
dysfunction syndrome-endotoxin, inflammatory cells, and their
mediators: Cytokines and reactive oxygen species. Surg Infect
(Larchmt). 1:197–204; discussion 204–205. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Colotta F, Re F, Polentarutti N, Sozzani S
and Mantovani A: Modulation of granulocyte survival and programmed
cell death by cytokines and bacterial products. Blood.
80:2012–2020. 1992.PubMed/NCBI
|
|
15
|
Esmann L, Idel C, Sarkar A, Hellberg L,
Behnen M, Möller S, van Zandbergen G, Klinger M, Köhl J, Bussmeyer
U, et al: Phagocytosis of apoptotic cells by neutrophil
granulocytes: Diminished proinflammatory neutrophil functions in
the presence of apoptotic cells. J Immunol. 184:391–400. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Twomey C and McCarthy JV: Pathways of
apoptosis and importance in development. J Cell Mol Med. 9:345–359.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schwartz JT, Barker JH, Kaufman J, Fayram
DC, McCracken JM and Allen LA: Francisella tularensis inhibits the
intrinsic and extrinsic pathways to delay constitutive apoptosis
and prolong human neutrophil lifespan. J Immunol. 188:3351–3363.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Green DR: Apoptotic pathways: Ten minutes
to dead. Cell. 121:671–674. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ottonello L, Frumento G, Arduino N,
Bertolotto M, Dapino P, Mancini M and Dallegri F: Differential
regulation of spontaneous and immune complex-induced neutrophil
apoptosis by proinflammatory cytokines. Role of oxidants, Bax and
caspase-3. J Leukoc Biol. 72:125–132. 2002.PubMed/NCBI
|
|
20
|
Le'Negrate G, Rostagno P, Auberger P,
Rossi B and Hofman P: Downregulation of caspases and Fas ligand
expression, and increased lifespan of neutrophils after
transmigration across intestinal epithelium. Cell Death Differ.
10:153–162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nauseef WM: Isolation of human neutrophils
from venous blood. Methods Mol Biol. 412:15–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scorrano L: Opening the doors to
cytochrome c: Changes in mitochondrial shape and apoptosis.
Int J Biochem Cell Biol. 41:1875–1883. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gustafsson AB and Gottlieb RA: Heart
mitochondria: Gates of life and death. Cardiovasc Res. 77:334–343.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Xing D, Zhou F and Chen Q:
Mitochondrial autophagy protects against heat shock-induced
apoptosis through reducing cytosolic cytochrome c release
and downstream caspase-3 activation. Biochem Biophys Res Commun.
395:190–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mei Y, Yong J, Liu H, Shi Y, Meinkoth J,
Dreyfuss G and Yang X: tRNA binds to cytochrome c and inhibits
caspase activation. Mol Cell. 37:668–678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kurtoglu M and Lampidis TJ: From
delocalized lipophilic cations to hypoxia: Blocking tumor cell
mitochondrial function leads to therapeutic gain with glycolytic
inhibitors. Mol Nutr Food Res. 53:68–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fox S, Leitch AE, Duffin R, Haslett C and
Rossi AG: Neutrophil apoptosis: Relevance to the innate immune
response and inflammatory disease. J Innate Immun. 2:216–227. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taneja R, Parodo J, Jia SH, Kapus A,
Rotstein OD and Marshall JC: Delayed neutrophil apoptosis in sepsis
is associated with maintenance of mitochondrial transmembrane
potential and reduced caspase-9 activity. Crit Care Med.
32:1460–1499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kole AJ, Knight ER and Deshmukh M:
Activation of apoptosis by cytoplasmic microinjection of cytochrome
c. J Vis Exp pii. 27732011.
|
|
30
|
Kroemer G, Dallaporta B and Resche-Rigon
M: The mitochondrial death/life regulator in apoptosis and
necrosis. Annu Rev Physiol. 60:619–642. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lundqvist-Gustafsson H, Norrman S, Nilsson
J and Wilsson A: Involvement of p38-mitogen protein kinase in
Staphylococcus aureus-induced neutrophil apoptosis. J Leukoc Biol.
70:642–648. 2001.PubMed/NCBI
|
|
32
|
Altavilla D, Saitta A, Squadrito G,
Galeano M, Venuti SF, Guarini S, Bazzani C, Bertolini A, Caputi AP
and Squadrito F: Evidence for a role of nuclear factor-kappaB in
acute hypovolemic hemorrhagic shock. Surgery. 131:50–58. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sousa LP, Lopes F, Silva DM, Tavares LP,
Vieira AT, Rezende BM, Carmo AF, Russo RC, Garcia CC, Bonjardim CA,
et al: PDE4 inhibition drives resolution of neutrophilic
inflammation by inducing apoptosis in a PKA-PI3K/Akt-dependent and
NF-kappaB-independent manner. J Leukoc Biol. 87:895–904. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fernandes CA, Fievez L, Ucakar B, Neyrinck
AM, Fillee C, Huaux F, Delzenne NM, Bureau F and Vanbever R:
Nicotinamide enhances apoptosis of G(M)-CSF-treated neutrophils and
attenuates endotoxin-induced airway inflammation in mice. Am J
Physiol Lung Cell Mol Physiol. 300:L354–L361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Paunel-Görgülü A, Zörnig M, Lögters T,
Altrichter J, Rabenhorst U, Cinatl J, Windolf J and Scholz M: Mcl-1
mediated impairment of the intrinsic apoptosis pathway in
circulating neutrophils from critically ill patients can be
overcome by Fas stimulation. J Immunol. 183:6198–6206. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nwakoby IE, Reddy K, Patel P, Shah N,
Sharma S, Bhaskaran M, Gibbons N, Kapasi AA and Singhal PC:
Fas-mediated apoptosis of neutrophils in sera of patients with
infection. Infect Immun. 69:3343–3349. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dunican AL, Lenemoth SJ, Grutkoski P,
Ayala A and Simms HH: TNF alpha-induced suppression of PMN
apoptosis is mediated through interleukin-8 production. Shock.
14:284–288; discussion 288–289. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Geering B and Simon HU: A novel signaling
pathway in TNFα-induced neutrophil apoptosis. Cell Cycle.
10:2821–2822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Maianski NA, Mul FP, van Buul JD, Roos D
and Kuijpers TW: Granulocyte colony-stimulating factor inhibits the
mitochondria-dependent activation of caspase-3 in neutrophils.
Blood. 99:672–679. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maianski NA, Roos D and Kuijpers TW: Tumor
necrosis factor alpha induces a caspase-independent death pathway
in human neutrophils. Blood. 101:1987–1995. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maianski NA, Geissler J, Srinivasula SM,
Alnemri ES, Roos D and Kuijpers TW: Functional characterization of
mitochondria in neutrophils: A role restricted to apoptosis. Cell
Death Differ. 11:143–153. 2004. View Article : Google Scholar : PubMed/NCBI
|