Introduction
Contrast-induced nephropathy (CIN) is an acute
kidney injury following administration of iodinated contrast media,
and is currently the third most common type of hospital-acquired
renal failure (1). CIN often appears
in patients who underwent coronary angiography (CAG), and may
result in renal function deterioration and in certain cases, death.
CIN following CAG often causes long-term decline in renal function
(2). CIN is defined as an increase
in serum creatinine concentration >26.5 µmol/l or >25% of its
baseline creatinine level within 3 days after contrast medium
administration (3). Typically after
a peak value of serum creatinine within the fifth day, in which
granular casts and moderate proteinuria may appear, serum
creatinine levels return to the baseline level within 7–10 days
(4).
However, it has also been observed at Shanghai Jiao
Tong University Affiliated Sixth People's Hospital (Shanghai,
China) that certain patients that do not exhibit CIN develop
irreversible deterioration of renal function within 1–6 months
following CAG. This is informally called ‘delayed contrast-induced
nephropathy’ or ‘delayed kidney injury (DKI) after CAG’, and it has
been hypothesized by several cardiologists at the Shanghai Jiao
Tong University Affiliated Sixth People's Hospital to be due to
iodinated contrast media administration. However, it is unknown
whether delayed CIN really exists, or whether it is associated with
CAG or induced by iodinated contrast media.
In the present study, it was hypothesized that DKI
after CAG may be caused by numerous factors, but that contrast did
not contribute. In order to investigate the pathogenesis of DKI, a
retrospective study was conducted in patients receiving CAG and
coronary stenting to investigate whether DKI exists and to
understand its etiology and mechanism of action.
Materials and methods
Subjects
Patients receiving CAG and coronary stenting between
January 1, 2008 and December 31, 2009 at the Shanghai Jiao Tong
University Affiliated Sixth People's Hospital were enrolled in the
present study. The inclusion criteria were as follows: Age, ≥18
years old; receiving CAG and percutaneous coronary intervention.
The present study was approved by the Ethics Committee of Shanghai
Jiao Tong University Affiliated Sixth People's Hospital and adhered
to the Declaration of Helsinki (5).
Written informed consent was obtained from all of the
participants.
The exclusion criteria were as follows: Diagnosis of
CIN; electrophysiological examination and percutaneous transluminal
septal myocardial ablation conducted; baseline estimated glomerular
filtration rate (eGFR) <30 ml/min; occurrence of malignant
tumors, renal artery stenosis and urological obstruction illnesses;
recurrence of myocardial infarction during follow-up; renal toxic
medicine intake (except aspirin); prerenal acute kidney injury
(AKI); and AKI secondary to primary kidney diseases.
Diagnostic criteria and methods
The CIN diagnostic criteria included the following:
Cases with a serum creatinine increase (after CAG) ≥25% within
24–72 h compared with baseline values; or an absolute increase
>44.2 µmol/l (6). The risk scores
were taken into account in accordance with the risk score described
by Mehran (7). The CAG-associated
DKI diagnostic criteria were as follows: Any serum creatinine value
increase ≥26.5 µmol/l or >50% of the baseline value, 1–6 months
following CAG. The baseline creatinine was the serum creatinine
level at 1 month after CAG. The eGFR was calculated in accordance
with the simplified Cockcroft-Gault (CG) formula as follows:
CG-eGFR: Creatinine clearance = (140-age) × weight × 0.85 (if
female)/(72 × serum creatinine) (8).
The hospital and follow-up medical records of enrolled patients
were collected and the different cases were divided into groups,
those cases diagnosed with DKI (the DKI group) and those cases
without DKI (the non-DKI group). The Mehran scores of the DKI group
were calculated and common clinical characteristics were screened
(7).
Statistical analysis
SPSS software, version 13.0 was employed for data
analysis. Student's t-test was used to analyze the measurement data
while the χ2 test was used for categorical comparison.
The risk factors associated with DKI, including age, gender, body
weight, hospital stay, heart function grade, hemoglobin, baseline
serum creatinine, fasting plasma glucose, eGFR, contrast dosage,
isotonic contrast, urine protein, diabetes, angiotensin converting
enzyme (ACE) inhibitors/angiotensin II receptor blockers (ARBs),
aspirin, diuretics and hydration therapy, were analyzed by logistic
regression. P<0.05 was considered to indicate a statistically
significant difference.
Results
Basic information of DKI and non-DKI
subjects
A total of 769 patients were receiving CAG in the
present study, and there were 333 cases excluded according to the
exclusion criteria, including 47 cases with definite CIN and 29
drop-out cases. In total, there were 436 valid cases with intact
follow-up data enrolled in the present study. In addition, the eGFR
levels for all the subjects prior to CAG were >30 ml/min. The
incidence of DKI was 7.1% (31/436) and the average time after CAG
that DKI occurred was 16.5±4.0 weeks. The percentage of heart
failure and cerebrovascular disease were significantly higher in
DKI patients than that in the non-DKI group (P=0.009 and 0.041,
respectively). However, no differences in proteinuria, diabetes,
hypertension or hyperlipidemia were observed between the groups
(Table I). Furthermore, the cases
were divided into the non-DKI and DKI group, and the results
presented significant differences in hemoglobin (Hb), hematocrit
and serum creatinine levels (P=0.039, 0.043 and 0.033,
respectively; Table II), between
the two groups but no differences in white blood cell, alanine
transaminase and low-density lipoprotein.
 | Table I.Basic information of patients with
DKI. |
Table I.
Basic information of patients with
DKI.
| Characteristic | Non-DKI (n=405) | DKI (n=31) |
|---|
| Age, years | 65.3±11.9 |
69.1±12.4a |
| Male, N (%) | 273 (67.4) | 22 (80.0) |
| Proteinuria, N
(%) |
|
|
|
Absent | 247 (61.0) | 16 (51.6) |
|
Microalbuminuria | 131 (32.3) | 12 (38.7) |
|
Proteinuria | 27 (6.7) | 3 (9.7) |
| Comorbities, N
(%) |
|
|
| Diabetes
mellitus | 122 (30.1) | 14 (45.2) |
|
Hypertension | 276 (68.1) | 21 (67.7) |
|
Hyperlipidemia | 319 (78.8) | 20 (64.5) |
| Heart
failure | 22 (5.4) | 7 (22.6)a |
| CVD | 34 (8.4) | 6 (19.4)a |
| PVD | 33 (8.1) | 5 (16.1) |
 | Table II.Biochemical parameters in patients
with DKI. |
Table II.
Biochemical parameters in patients
with DKI.
| Parameter | Non-DKI | DKI |
|---|
| White blood cell,
109/l | 6.4±1.2 | 6.6±1.5 |
| Hemoglobin, g/l | 133.8±18.6 |
121.2±17.3a |
| Hematocrit, % | 38.6±5.4 | 35.1±5.3a |
| Alanine transaminase,
U/l | 35.2±13.6 | 37.4±12.9 |
| Serum creatinine,
µmol/l | 91.7±37.6 |
110.9±43.2a |
| Tc, mmol/l | 4.6±1.1 | 4.7±1.5 |
| Low-density
lipoprotein, g/l | 3.1±0.9 | 3.2±1.1 |
| eGFR, ml/min | 71.9±28.6 |
66.4±30.2a |
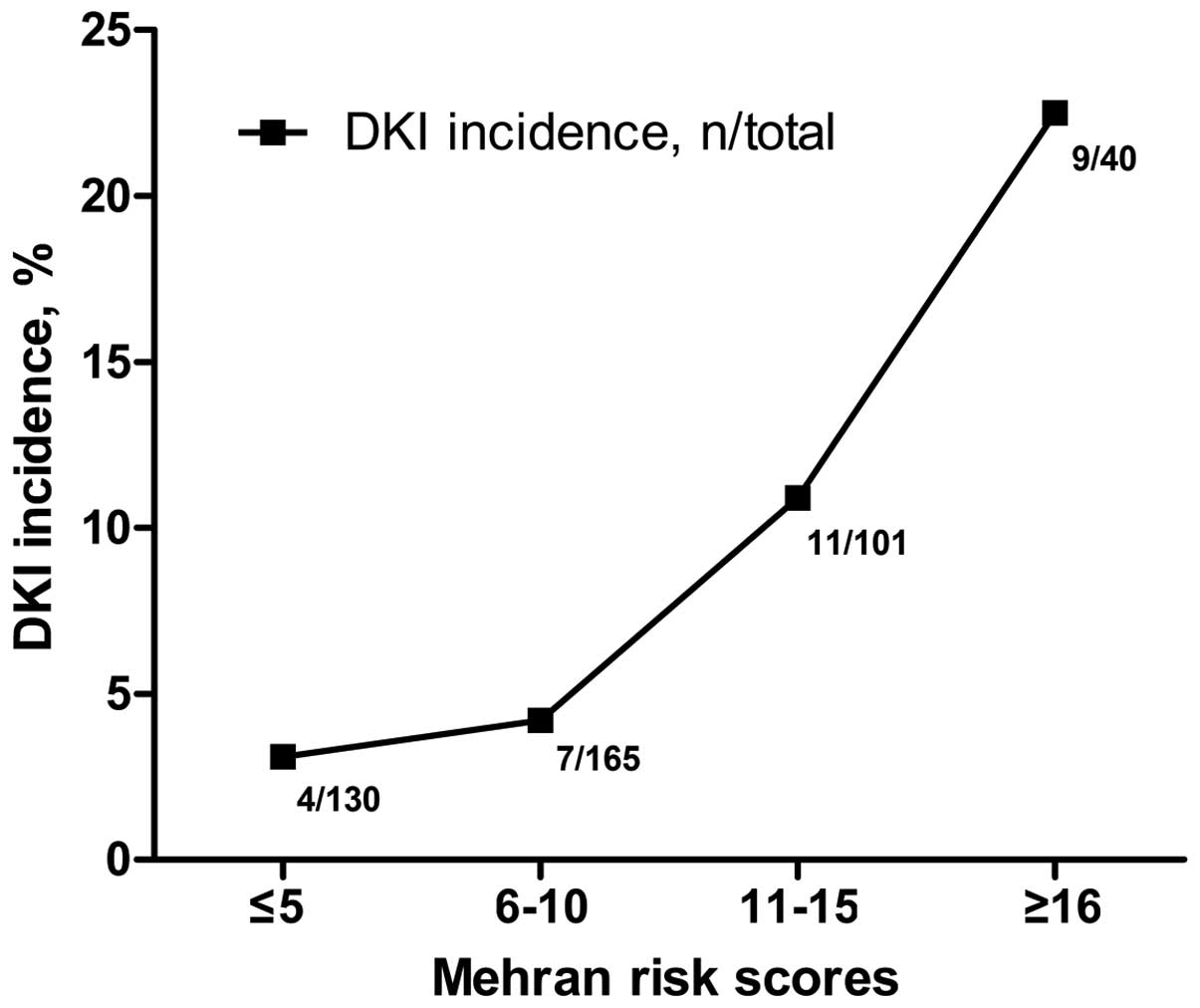
DKI incidence increased with Mehran
risk scores
The results demonstrated that the incidence rate of
DKI increased with Mehran risk scores (Fig. 1), with a DKI incidence of 22.5%
(9/40) in patients with scores ≥16.
DKI may be attributed to high-dosage
aspirin intake
As presented in Table
III, a total of 29% (9/31) and 5.7% (23/405) of patients in the
DKI and non-DKI groups, respectively, were treated with 300 mg
aspirin + clopidogrel following CAG, presenting a significant
difference of P<0.01. However, no difference was observed in the
number of cases treated with ACE inhibitors/ARBs, diuretics,
statins and other anti-platelet drugs (all P>0.05).
 | Table III.Medicine intake of the DKI patients
during the follow-up. |
Table III.
Medicine intake of the DKI patients
during the follow-up.
| Medicine | Non-DKI (n, %)
n=405 | DKI (n, %) n=31 |
|---|
| ACEI | 181 (44.7) | 16 (51.6) |
| ARB | 99 (24.4) | 5 (16.2) |
| Diuretics | 241 (59.5) | 20 (64.5) |
| Statins | 258 (63.7) | 22 (71.0) |
| Anti-platelet
drugs |
|
|
| Asp 100
mg | 15 (3.7) | 1 (3.2) |
|
Clop | 36 (8.9) | 1 (3.2) |
|
War | 10 (24.7) | 1 (3.2) |
| Asp 100
mg + clop | 243 (60.0) | 14 (51.6) |
|
Cilostazol + clop | 47 (11.6) | 3 (9.7) |
| Asp100
mg + clop + war | 31 (7.6) | 2 (6.5) |
| Asp 300
mg + clop | 23 (5.7) | 9 (29)a |
Multivariate logistic regression analysis was
performed to identify the risk factors for DKI following CAG
(Table IV). The analyzed factors
included age, gender, body weight, hospital stay, heart function
grade, hemoglobin, baseline serum creatinine, fasting plasma
glucose, eGFR, contrast dosage, isotonic contrast, urine protein,
diabetes, ACE inhibitors/ARBs, aspirin, diuretics and hydration
therapy. The results demonstrated that anemia (hemoglobin <110
g/l), heart failure and 300 mg aspirin intake were risk factors for
DKI (P<0.05), whereas the contrast amount, isotonic contrast,
diabetes, ACE inhibitors/ARBs, eGFR and other factors were not
associated with DKI (P>0.05).
 | Table IV.Results of univariate and
multivariate analysis for DKI. |
Table IV.
Results of univariate and
multivariate analysis for DKI.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Symptom or
medication | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Heart failure | 5.078 | 1.973–13.067 |
<0.001a | 1.846 | 1.080–2.611 | 0.039b |
| Hemoglobin (<110
g/l) | 3.083 | 1.240–7.663 | 0.011a | 3.599 | 2.078–5.119 | 0.003b |
| Baseline serum
creatinine (>106 µmol/l) | 5.070 | 2.067–12.436 |
<0.001a | 1.266 | 0.596–1.935 | 0.656 |
| eGFR (<60
ml/min) | 2.797 | 1.069–7.316 | 0.029a | 1.598 | 0.260–2.937 | 0.098 |
| Aspirin 300 mg +
clopidogrel | 6.794 | 2.812–16.419 |
<0.001a | 5.692 | 1.962–9.421 |
<0.001b |
| Diuretics | 2.625 | 1.206–5.715 | 0.012a | 1.690 | 1.307–2.072 | 0.226 |
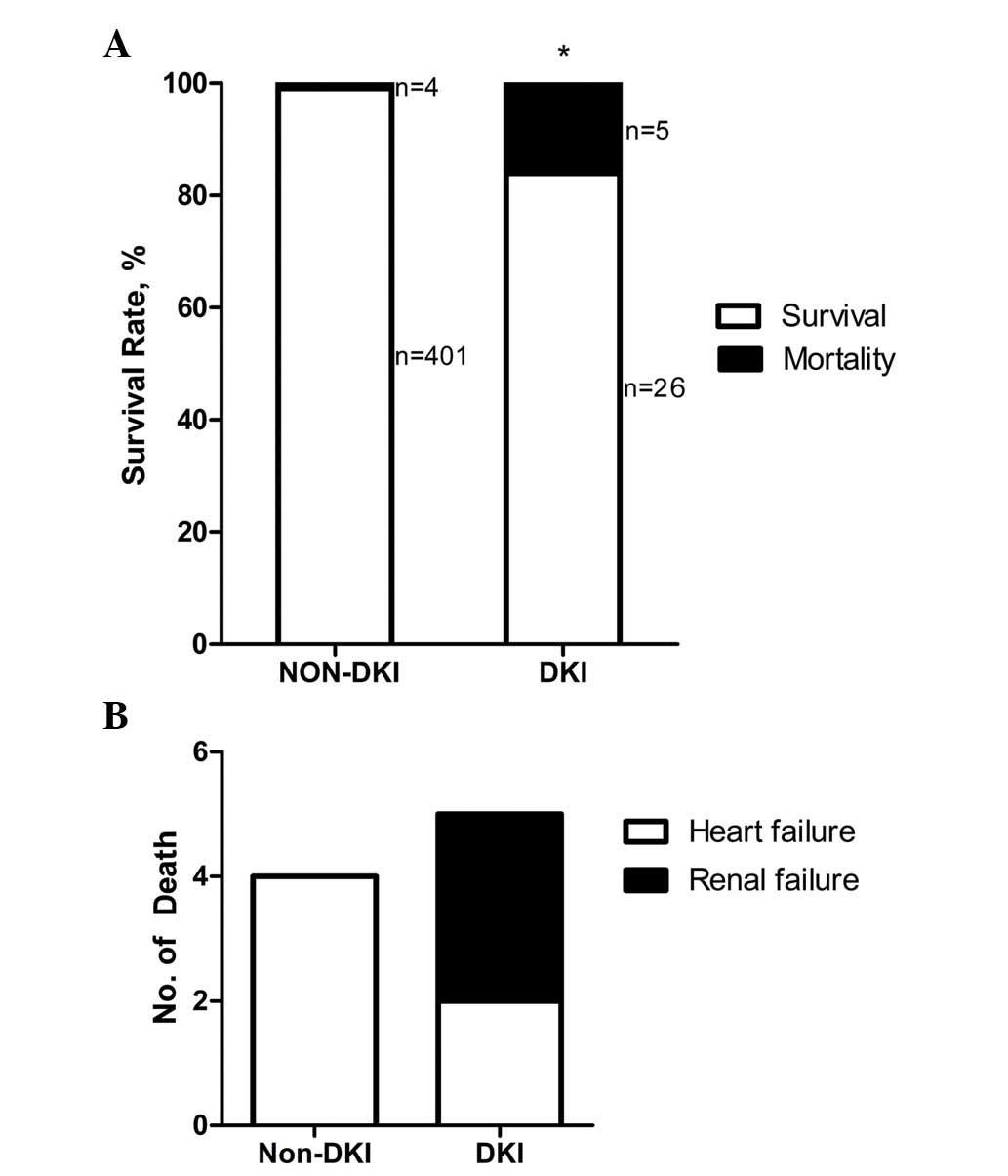
Poor prognosis for patients with DKI
following CAG compared with non-DKI patients
As presented in Fig.
2A, 26 patients with DKI, out of a total of 31, survived until
the follow-up of 6 months; the mortality rate was 16.1%. In
addition, there were 402 cases without DKI, out of a total of 405,
that survived, and the mortality rate in this group was 1.0%
(P<0.01). Causes of mortality following DKI included heart and
renal failure (Fig. 2B).
Discussion
Patients with coronary heart disease often present a
high-risk of CIN, therefore studies on the prevention of CIN
following CAG is of note (9–13). By contrast, coronary
artery-associated illnesses such as acute coronary syndrome,
myocardial infarction and ischemic cardiomyopathy may lead to heart
failure and decreased renal blood flow and kidney injury (14,15). The
present study hypothesized that DKI following CAG may not be a
result of contrast itself, but other associated factors. In order
to examine this hypothesis, 436 cases that underwent CAG were
enrolled. The results demonstrated that 7.1% patients (31/436)
developed DKI according to the agreed diagnostic criteria. Thus,
DKI existed in patients after CAG. DKIs occurring 1 month or later
after coronary angiography had not been previously reported. All of
the enrolled cases had already excluded CIN, therefore the renal
toxicity of the contrast may not be able to account for the kidney
injury within 1–6 months after CAG. Two questions remain, including
whether DKI is associated with CAG or coronary heart diseases; and
what pathogenesis underlies DKI. In the present study, the possible
risk factors were assessed.
The Mehran score is a useful tool for predicting CIN
risk, indicating the anti-stress capability of the kidney (7,14).
Therefore, the Mehran score was introduced to assess the risks for
DKI in the present study. Through stratification by the Mehran
score, it was revealed that the incidence of DKI increased with the
Mehran risk scores. In the present cohort, 22.5% of patients with
Mehran scores ≥16 were diagnosed with DKI. The univariate analysis
revealed that the serum levels of creatinine in the DKI group were
higher than those in the non-DKI group, while Hb and eGFR levels
were lower. Patients with DKI were characterized as older, and with
a higher incidence of heart failure and cerebrovascular diseases.
The analysis of oral drugs after CAG during the follow-up
demonstrated that aspirin was used more frequently in the DKI
group. The multivariate logistic regression analysis suggested that
anemia, heart failure and 300 mg aspirin intake were risk factors
for DKI, while eGFR, diabetes, hypertension, ACE inhibitors/ARBs
and statins were not.
Numerous drugs are used in patients that have
undergone coronary stenting in order to cure several combined
disorders; these drugs include anti-hypertensive drugs, statins and
anti-platelet drugs. It is established that several hypotensors,
statins and anti-platelet drugs cause renal toxicity, particularly
in aged patients with declined renal function. There are several
anti-platelet treatments used to prevent stent thrombosis and
reduce future ischemic events, aspirin being one important and
common anti-platelet therapy (16).
As one of the traditional non-steroid, anti-inflammatory drugs,
aspirin intake may result in renal failure or aggravate renal
dysfunction (17,18). In addition, the use of anti-platelets
is a routine therapy in the treatment of CKD in order to prevent
the recurrence of stroke or cardiovascular complications, since CKD
increases the risk of incident stroke, heart failure and myocardial
infarction (19).
Furthermore, heart failure is important in the
decline of renal function in CKD patients, which often leads to
death or other complications, such as pulmonary infection (20–23).
Nowadays, it is considered that CIN is a short-term syndrome based
on the pathophysiology, laboratory research and clinical studies
(24–27). It is currently accepted that only a
creatinine level increase within 7 days after CAG should be
considered CIN (6). Thus,
contrast-induced delayed kidney injury is actually not caused by
contrast agents directly. Furthermore, the data of the present
study demonstrated that 300 mg aspirin and heart failure may
account for DKI after CAG in a Chinese population. Finally, it is
speculated that DKI will attract increasingly more attention in
future studies. As a result, it is recommended that using large
doses of aspirin as a long-term anti-platelet therapy be avoided in
Chinese patients, and for high-risk patients, the long-term renal
function follow-up results should not be ignored.
In conclusion, delayed CIN after CAG is not a
logical term, while delayed kidney injury after CAG appears to be
more appropriate when used to describe the renal injury occurring 1
month after CAG. The data of the present study suggested that 300
mg aspirin and deteriorated heart function may contribute to the
pathogenesis of DKI following CAG, and that iodinated contrast
media is not a factor. However, the small cohort size of the
current study is a limiting factor, and for this reason a follow-up
study with a larger cohort is required in order to investigate
delayed renal injury after CAG, in addition to a subsequent
prospective study in order to understand the etiology of DKI after
CAG.
Acknowledgements
The current study was sponsored by the National
Natural Science Foundation of China (grant no. 81570603), the
New-100 Talent Plan of Shanghai Jiao Tong University School of
Medicine (grant no. 2012) and the Shanghai Talents Development Fund
(grant no. 2013). We would also like to thank Dr Gary C. Mouradian
Jr. from the Medical College of Wisconsin for his English editing
of the manuscript.
Glossary
Abbreviations
Abbreviations:
|
CIN
|
contrast-induced nephropathy
|
|
CAG
|
coronary angiography
|
|
DKI
|
delayed kidney injury
|
|
eGFR
|
estimated glomerular filtration
rate
|
|
NSAIDs
|
non-steroid anti-inflammatory
drugs
|
|
ACEI/ARB
|
angiotensin converting enzyme
inhibitors/angiotensin II receptor blockers
|
|
CKD
|
chronic kidney disease
|
References
|
1
|
Koo HM, Doh FM, Ko KI, Kim CH, Lee MJ, Oh
HJ, Han SH, Kim BS, Yoo TH, Kang SW and Choi KH: Diastolic
dysfunction is associated with an increased risk of
contrast-induced nephropathy: A retrospective cohort study. BMC
Nephrol. 14:1462013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
James MT, Ghali WA, Tonelli M, Faris P,
Knudtson ML, Pannu N, Klarenbach SW, Manns BJ and Hemmelgarn BR:
Acute kidney injury following coronary angiography is associated
with a long-term decline in kidney function. Kidney Int.
78:803–809. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kelly AM, Dwamena B, Cronin P, Bernstein
SJ and Carlos RC: Meta-analysis: Effectiveness of drugs for
preventing contrast-induced nephropathy. Ann Intern Med.
148:284–294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCullough PA: Contrast-induced acute
kidney injury. J Am Coll Cardiol. 51:1419–1428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
World Medical Association: World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. J Postgrad Med. 48:206–208.
2002.PubMed/NCBI
|
|
6
|
Solomon R: Contrast-induced acute kidney
injury (CIAKI). Radiol Clin North Am. 47:783–788. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mehran R, Aymong ED, Nikolsky E, Lasic Z,
Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, et al:
A simple risk score for prediction of contrast-induced nephropathy
after percutaneous coronary intervention: development and initial
validation. J Am Coll Cardiol. 44:1393–1399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gault MH, Longerich LL, Harnett JD and
Wesolowski C: Predicting glomerular function from adjusted serum
creatinine. Nephron. 62:249–256. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toprak O: Conflicting and new risk factors
for contrast induced nephropathy. J Urol. 178:2277–2283. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Davidson C, Stacul F, McCullough PA,
Tumlin J, Adam A, Lameire N and Becker CR: CIN Consensus Working
Panel: Contrast medium use. Am J Cardiol. 98:42K–58K. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reed M, Meier P, Tamhane UU, Welch KB,
Moscucci M and Gurm HS: The relative renal safety of iodixanol
compared with low-osmolar contrast media: A meta-analysis of
randomized controlled trials. JACC Cardiovasc Interv. 2:645–654.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mueller C, Buerkle G, Buettner HJ,
Petersen J, Perruchoud AP, Eriksson U, Marsch S and Roskamm H:
Prevention of contrast media-associated nephropathy: Randomized
comparison of 2 hydration regimens in 1620 patients undergoing
coronary angioplasty. Arch Intern Med. 162:329–336. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hogan SE, L'Allier P, Chetcuti S, Grossman
PM, Nallamothu BK, Duvernoy C, Bates E, Moscucci M and Gurm HS:
Current role of sodium bicarbonate-based preprocedural hydration
for the prevention of contrast-induced acute kidney injury: A
meta-analysis. Am Heart J. 156:414–421. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mehran R and Nikolsky E: Contrast-induced
nephropathy: Definition, epidemiology and patients at risk. Kidney
Int Suppl. S11–S15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morcos SK: Prevention of contrast media
nephrotoxicity - the story so far. Clin Radiol. 59:381–389. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanaka A, Ishii H, Sakakibara M, Okumura
S, Jinno Y, Okada K, Suzuki S, Inoue Y and Murohara T: Temporary
adjunctive cilostazol vs. clopidogrel loading for ST-segment
elevation acute myocardial infarction. Am J Cardiovasc Drugs.
14:131–136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SJ and Bang OY: Antiplatelet therapy
for preventing stroke in patients with chronic kidney disease.
Contrib Nephrol. 179:119–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harirforoosh S, Asghar W and Jamali F:
Adverse effects of nonsteroidal antiinflammatory drugs: An update
of gastrointestinal, cardiovascular and renal complications. J
Pharm Pharm Sci. 16:821–847. 2013.PubMed/NCBI
|
|
19
|
Harmon JP and Zimmerman DL and Zimmerman
DL: Anticoagulant and antiplatelet therapy in patients with chronic
kidney disease: Risks versus benefits review. Curr Opin Nephrol
Hypertens. 22:624–628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Braam B, Joles JA, Danishwar AH and
Gaillard CA: Cardiorenal syndrome-current understanding and future
perspectives. Nat Rev Nephrol. 10:48–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shamseddin MK and Parfrey PS: Mechanisms
of the cardiorenal syndromes. Nat Rev Nephrol. 5:641–649. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anand IS: Cardiorenal syndrome: A
cardiologist's perspective of pathophysiology. Clin J Am Soc
Nephrol. 8:1800–1807. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clementi A, Virzi GM, Goh CY, Cruz DN,
Granata A, Vescovo G and Ronco C: Cardiorenal syndrome type 4: A
review. Cardiorenal Med. 3:63–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haller C and Hizoh I: The cytotoxicity of
iodinated radiocontrast agents on renal cells in vitro. Invest
Radiol. 39:149–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heyman SN, Reichman J and Brezis M:
Pathophysiology of radiocontrast nephropathy: A role for medullary
hypoxia. Invest Radiol. 34:685–691. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liss P, Nygren A, Erikson U and Ulfendahl
HR: Injection of low and iso-osmolar contrast medium decreases
oxygen tension in the renal medulla. Kidney Int. 53:698–702. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deek H, Newton P, Sheerin N, Noureddine S
and Davidson PM: Contrast media induced nephropathy: A literature
review of the available evidence and recommendations for practice.
Aust Crit Care. 27:166–171. 2014. View Article : Google Scholar : PubMed/NCBI
|