Introduction
Premature ovarian failure (POF) is a condition that
causes amenorrhea and hypergonadotropic hypoestrogenism before the
age of 40 (1–5). POF affects 1% of women in the general
population, but its prevalence is steadily increasing (1–5).
Patients with POF demonstrate a number of characteristic symptoms,
as follows: i) Primary or secondary amenorrhea (2,6–8); ii) intermittent or chronic
hypoestrogenism (2,6); and iii) hypergonadotropism (7,8).
Furthermore, the age of patients at the time of onset is typically
under 40 years of age (3–6). In a number of reports describing
patients with POF, laparoscopy has revealed an absence of
developing follicles, and ovarian biopsies have demonstrated a
network of connective tissue interspersed with fibroblasts.
Previous studies have reported that the uterus and vaginal mucosa
in patients with POF undergo atrophy due to estrogen
understimulation as a result of inactive ovaries (4,9).
Currently, POF is irreversible and, although treatments are
available, drugs to treat POF are very limited; there are no
particularly effective treatments and drugs. Hormonal therapy and
in vitro fertilization methods are available to help
patients with POF conceive, but there is an urgent requirement for
improved treatment strategies (5–7,9).
Growth hormone (GH) is a pleiotropic hormone that
affects a broad spectrum of physiological functions, from
carbohydrate and lipid metabolism to immune response (10–16).
Several previous studies have used GH to treat autoimmune diseases
and POF. For example, Rojanathammanee et al (14) reported that GH alters the glutathione
S-transferase and mitochondrial thioredoxin systems in long-living
Ames dwarf mice. Villares et al (16) used GH to treat type 1 diabetes,
demonstrating that it prevented the development of diabetes by a
mechanism involving specific GH-mediated effects on islet β-cells,
Th17/Th1 plasticity, M1/M2 macrophage
differentiation and Treg cell function. Visser et
al (11) reported that serum
anti-Müllerian hormone levels correlated with karyotype, pubertal
development, luteinizing hormone and follicle-stimulating hormone
(FSH), and is detected at higher levels in patients with Turner
syndrome undergoing GH therapy. Furthermore, Hartmann et al
(12) evaluated the effects of oral
hormone replacement therapy (HRT) on body weight, insulin-like
growth factor 1 (IGF-1) levels and GH response to exogenous GnRH in
women with POF. This previous study revealed that women with POF
who were not treated with HRT had significantly higher IGF-1
levels, compared with women with POF treated with HRT, which
reduced during HRT; however, the body weight of these patients
remained stable (12). Numerous
studies are beginning to generate and characterize a number of
GH-encoding transgenes in mice, expressing factors such as ovine
GH, human GH and bovine GH (11–13,16).
Although GH has been used in preclinical trials to treat POF, the
mechanisms underlying its activity in the alleviation of POF are
poorly understood.
The Notch signaling pathway has critical roles in
the development and homeostasis of tissues by regulating cell fate,
proliferation, differentiation and apoptosis, and in stem cell
self-renewal (17,18). The Notch proteins are a family of
evolutionarily conserved receptors that regulate cell fate
(17–22). These Notch receptors are activated
following direct contact with their ligands, which are expressed on
adjacent cells (18,19). In mammals, there are four Notch
receptors (NOTCH1-NOTCH4) and five ligands (Jagged-1 and −2 and
delta-like protein 1, 3, and 4) (18,20).
NOTCH receptors have extracellular, transmembrane and intracellular
domains (18,19). Upon ligand binding, the NOTCH
intracellular domain (NICD) of the receptor is cleaved by
γ-secretase and translocates to the nucleus, where it associates
with the recombination signal-binding protein jκ (RBPjκ) (16,17,20).
RBPjκ is a key transcription factor in the canonical Notch
signaling pathway, and acts downstream of all four NOTCH receptors
(19–21). Within the nucleus, NICD forms a large
transcriptional activator complex with RBPjκ/CBF1 and Mastermind
(19–21). This transcriptional complex then
activates the transcription of target genes such as hairy/enhancer
of split (Hes) and Hes-associated with YRPW motif, two proteins of
the basic helix-loop-helix gene families (21,22). A
previous study has demonstrated that the Notch/Hes-1 signaling
pathway controls the proliferation of intestinal immature
progenitor cells (21). A number of
previous studies have indicated that Notch also works with other
transcription factors to regulate the expression of its target
genes such as cyclin D1, B cell lymphoma 2 and Survivin (16–19,21,22).
Furthermore, Notch-1 overexpression has been reported to inhibit
apoptosis in numerous types of human cancer, suggesting that it has
potential as a therapeutic target (19–22).
The current study aimed to determine whether
recombinant GH can be used as a treatment of POF, and to
investigate whether the therapeutic activity of GH is associated
with the activation of the Notch-1 signaling pathway.
Materials and methods
Generation of a mouse model of POF and
treatment with recombinant mouse growth hormone (rmGH)
Female C57BL/6 mice (n=72) at 4–5 weeks of age, ~20
g weight, were obtained from the Animal Research Center, Longhua
Hospital (Shanghai, China). The present study received ethical
approval from the Animal Ethics Committee of the Shanghai
University of Traditional Chinese Medicine, in compliance with the
Experimental Animal Regulations of the National Science and
Technology Commission, China. A total of 3–4 mice per cage were
maintained for 14 days in a temperature-controlled environment
under 12 h light-dark cycles with ad libitum access to food
and water as previously described (23). To induce POF, mice were administered
a single intraperitoneal injection of 70 mg/kg cyclophosphamide
(Sigma-Aldrich, St. Louis, MO, USA) at 7 weeks of age. The animals
were divided into control and experimental groups as follows: i) An
untreated control group (wild-type; WT) of 12 mice; ii) a negative
control group of 12 POF mice receiving a saline injection (100 µl);
four experimental groups of 12 POF mice, injected daily with 100 µl
of either iii) 0.4 (low-dose); iv) 0.8 (medium-dose); or v) 1.6
(high-dose) mg/kg rmGH (Sigma-Aldrich), dissolved in saline; vi) 12
POF mice were not administered any treatment. Injections of saline
or rmGH were provided a week after induction of POF, and additional
experiments were conducted 21 days after this treatment. At this
point, mice were sacrificed by cervical dislocation.
Enzyme-linked immunosorbent assay
(ELISA)
Mouse blood plasma (100 µl) was obtained by mouse
retro-orbital blood collection (23), centrifuged at 453 × g at 4°C for 10
min, and the supernatant was collected. The mouse estradiol
(E2) and FSH ELISA kit (cat. nos. 29764 and 29755;
Westang Bio, Shanghai, China) was used according to the
manufacturer's protocol, in order to determine the levels of
E2 or FSH in the mouse blood plasma. Briefly, 100 µl of
mouse E2 or FSH antigens standardized to 125–8,000 pg/ml
or 0.156–10 ng/ml, or diluted mouse plasma, were added to the
anti-E2 or FSH antibody-precoated microwells (as
appropriate, in the case of the antigens) and incubated for 60 min.
Following 3 washes, horseradish peroxidase-conjugated detection
antibodies were added, followed by the substrate solution (Westang
Bio). The absorbance of each well was measured at 450 nm using a
microplate reader (BioTek Synergy Mx; BioTek Instruments, Inc.,
Winooski, VT, USA).
Hematoxylin and eosin staining
Briefly, all ovarian tissue samples were washed 3
times with phosphate-buffered saline (PBS), fixed with 4%
paraformaldehyde (Sigma-Aldrich) for 30 min, dehydrated using a
graded series of ethanol, vitrified in xylene and embedded in
paraffin (both purchased from Sigma-Aldrich). Following this,
serial 6-µm thick sections (Leica RM2235 microtome; Leica
Microsystems, Wetzlar, Germany) were made and stained with
hematoxylin and eosin (Sigma-Aldrich).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA (200 ng/µl) was isolated from mouse ovary
tissue from each cell type using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. RNA samples were treated with DNase I (2.0
mU/µl; Sigma-Aldrich), quantified and reverse-transcribed into cDNA
(20 µl) using the ReverTra Ace-α reverse transcription kit (cat.
no. FKS-101; Toyobo Co., Ltd., Osaka, Japan). RT-qPCR was conducted
using a realplex4 real-time PCR detection system (Eppendorf,
Hamburg, Germany) with SYBR Green PCR Master mix (Toyobo Co.,
Ltd.). Primers used for cDNA amplification are listed in Table I. RT-qPCR amplification was performed
over 40 cycles of denaturation at 95°C for 15 sec, annealing at
58°C for 45 sec and final elongation at 72°C for 42 sec (initial
denaturation at 95°C for 5 min for 1 cycle, and final elongation at
72°C for 10 min for 1 cycle). Target cDNA levels were then measured
using the 2−ΔΔCq relative quantification method
(24). Comparative quantification
cycle (Cq) values were used to determine relative gene
expression, normalized to 18S rRNA. For each sample, Cq
values were normalized using the following formula: ΔCq
= Cq experimental genes - Cq 18S rRNA.
Relative expression levels were calculated using the formula:
ΔΔCq = ΔCq all groups - ΔCq
untreated control group.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer (5′-3′) |
|---|
| NOTCH1 |
|
| F |
CTCCAACTGTGACACCAACCCTG |
| R |
TGTAGCCCTGTAGACACTGGCACTC |
| CBF1 |
|
| F |
GTGGCACTGTTCAATCGCCTTCG |
| R |
CAGTCTGCCCGTAATGGATGTAG |
| Hes1 |
|
| F |
GAGAAGAGGCGAAGGGCAAGAATAAA |
| R |
CAACACGCTCGGGTCTGTGCTGA |
| Ccnd1 |
|
| F |
TGTGAGGAGCAGAAGTGCGAAGA |
| R |
GCCGGATAGAGTTGTCAGTGTAGATGC |
| 18S
rRNA |
|
| F |
AGGGGAGAGCGGGTAAGAGA |
| R |
GGACAGGACTAGGCGGAACA |
Immunohistochemistry
Immunohistochemical analysis was performed as
previously described (23,25). Briefly, all ovarian tissue samples
were washed 3 times with PBS, fixed with 4% paraformaldehyde for 30
min, dehydrated through a graded series of ethanol, vitrified in
xylene and embedded in paraffin. Subsequently, serial 6-µm thick
sections were made, rinsed with 3% phosphate buffer
(Sigma-Aldrich), and subjected to microwave heat repairing. The
samples were then incubated with primary antibodies for 45 min at
37°C, as follows: Anti-NOTCH-1 (1:1,000 dilution; mouse polyclonal;
cat. no. sc-6014); anti-CBF-1 (1:1,000 dilution; mouse polyclonal;
cat. no. sc-9417); anti-Hes-1 (1:100 dilution; mouse polyclonal;
cat. no. sc-13844); and anti-p53 (1:100 dilution; goat polyclonal;
sc-1313) (all Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Horseradish-peroxidase conjugated goat anti-rabbit immunoglobulin G
(1:1,000 dilution; sc-2768; Santa Cruz Biotechnology Inc.) was then
incubated with samples for 45 min at 37°C. Finally, ABC chromogenic
reagent (Sigma-Aldrich) was added to aid the visualization of the
secondary antibody, in addition to color detection. PBS (pH 7.4)
was used as a negative control in the place of primary antibody.
Five randomly selected fields of view (magnification, ×200; Olympus
BX43; Olympus Corporation, Tokyo, Japan) were observed for each
tissue section and analyzed using IPP software (version 4.0; Intel
Corporation, Santa Clara, CA, USA).
Statistical analysis
All data were analyzed using GraphPad Prism version
5.0 (GraphPad Software, Inc., La Jolla, CA, CA, USA). The data in
each experiment are reported as the mean ± standard error, where
applicable, and the differences were evaluated for statistical
significance with one-way analysis of variance. P<0.05 was
considered to represent a statistically significant difference.
Results
Medium doses of rmGH rescue ovarian
weight, hormone secretion and the number of normal follicles in POF
mice
At 14 days after injection, the weight of the
ovaries in each group was determined (Fig. 1A). A statistically significant
difference was not observed in ovarian weight between POF mice
(2.934±0.257 mg), POF mice treated with a low dose of rmGH
(3.939±0.321 mg) and POF mice treated with saline (3.014±0.365 mg)
14 days after injection. However, ovarian weight in medium-dose
(4.268±0.405 mg) and high-dose groups (6.545±0.551 mg) was
significantly increased when compared with that of the POF model
group (P<0.05; n=12 mice; Fig.
1A). An ELISA revealed no observably statistically significant
difference in plasma E2 levels between the POF model
group (64.58±7.93 pg/ml), the low-dose group (112.41±10.15 pg/ml)
and the negative control group treated with saline (72.94±9.32
pg/ml). Conversely, the plasma E2 levels increased with
time in medium-dose (242.12±15.39 pg/ml) and high-dose
(463.32±29.91 pg/ml) groups compared with that of the POF model
group (P<0.05; n=12 mice; Fig.
1B). An ELISA also indicated that plasma FSH levels did not
appear to vary significantly between the POF model group
(1,351.01±68.61 pg/ml), the low-dose group (778.41±97.48 pg/ml) and
the saline-treated group (1,308.02±81.14 pg/ml), but decreased
significantly with time in the medium-dose group (618.22±95.11
pg/ml) compared with the POF model group (P<0.05; n=12 mice;
Fig. 1B). Conversely, the plasma FSH
levels increased with time in the high-dose group (3,357.04±407.81
pg/ml) when compared with the POF model group (P<0.01; n=12
mice; Fig. 1B).
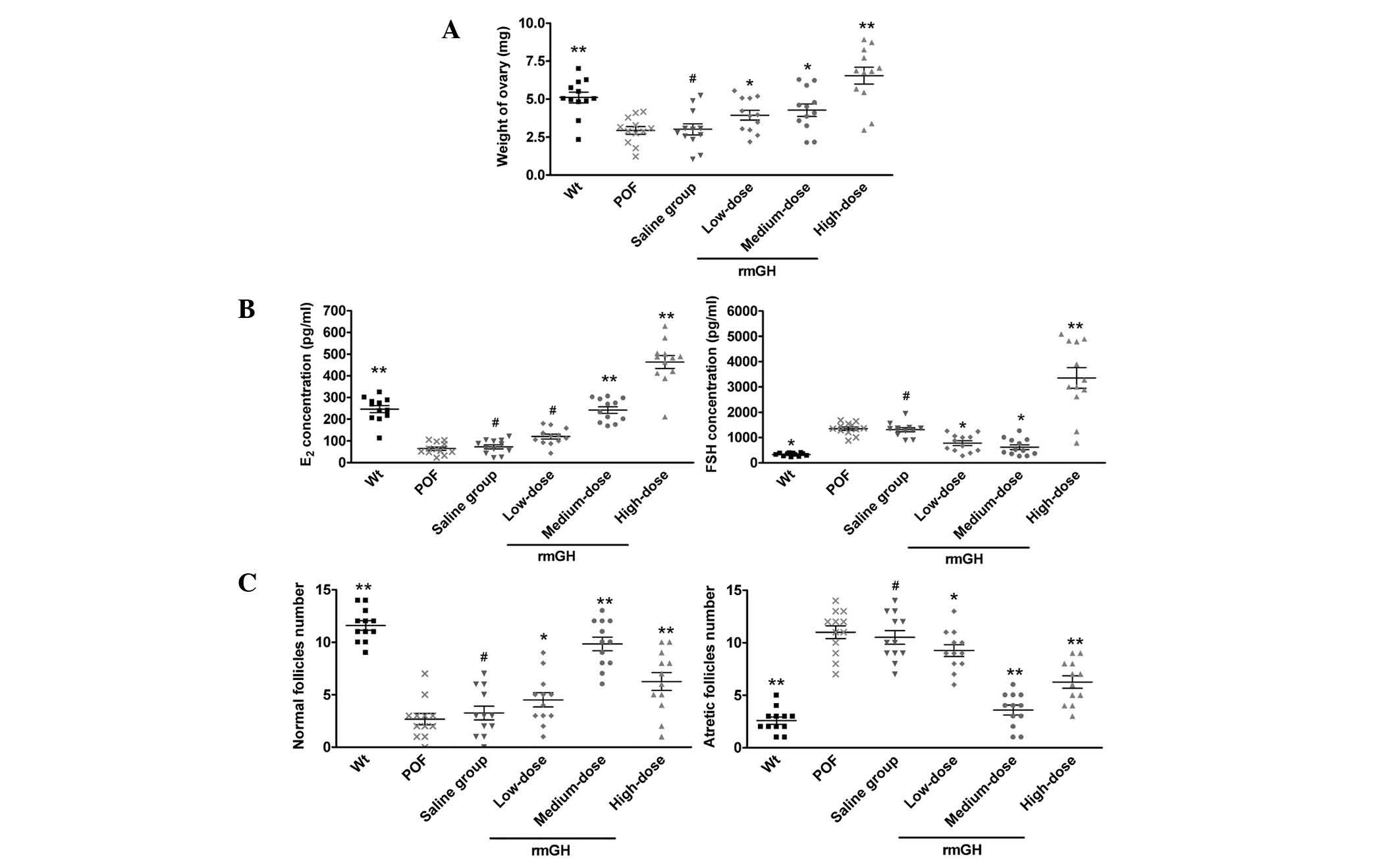 | Figure 1.Ovarian weight, plasma E2
and FSH levels and follicle count following treatment with rmGH or
saline, reported as means ± standard error. (A) Ovarian weight in
Wt mice was significantly higher compared with the POF model and
saline-treated mice, but no significant difference was identified
between medium- and high-dose rmGH-treated POF mice. (B) Plasma
E2 levels increased with time in medium-dose and
high-dose groups compared with the POF model group. Meanwhile,
plasma FSH levels did not appear to vary significantly between the
POF model group, the low-dose group and the saline-treated group,
but decreased significantly with time in the medium-dose group
compared with the POF model group. (C) Follicle counts revealed no
statistically significant difference in normal and atretic
follicles counts between the POF model and saline-treated groups.
Conversely, medium- and high-dose groups had a lower number of
atretic follicles than the POF model group. **P<0.01, vs. the
POF group; *P<0.05, vs. the POF group; #P>0.05,
vs. the POF group; n=12 mice/group. Wt, wild-type; POF, premature
ovarian failure model (untreated group); rmGH, recombinant mouse
growth hormone; E2, estradiol; FSH, follicle stimulating
hormone. |
Following this, the number of normal and atretic
ovarian follicles were counted in each group. A statistically
significant difference was not identified in the number of normal
follicles and atretic follicles between the POF model group (3±1
and 11±2, respectively), the low-dose group (5±2 and 9±1,
respectively) and the saline-treated group (3±2 and 11±2,
respectively). Conversely, the medium-dose (10±2 normal and 4±1
atretic follicles) and high-dose (6±2 normal and 6±2 atretic
follicles) groups had a lower number of atretic follicles than that
observed in the POF model group (P<0.05; n=12 mice; Fig. 1C).
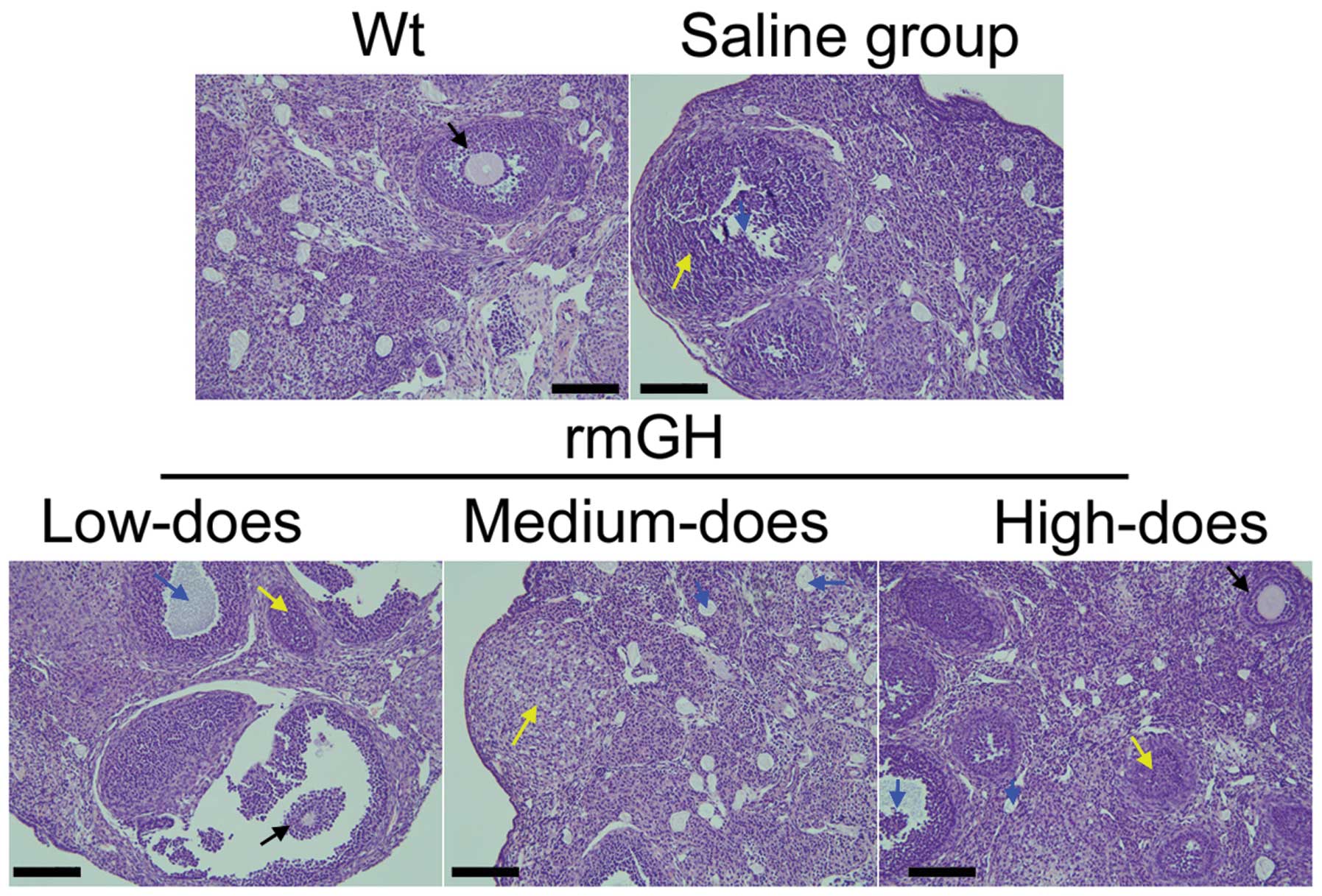
Hematoxylin and eosin staining revealed that the
ovaries of WT mice contained a large number of follicles at all
stages of development, ranging from immature to mature (Fig. 2). Conversely, the atrophied ovaries
of POF mice consisted primarily of interstitial cells in a fibrous
matrix, with a reduced number of follicles at each stage.
Furthermore, the ovaries of the POF mice contained an increased
number of collapsed oocytes and ovaries were smaller than those of
WT mice (Fig. 2). However, following
treatment with rmGH, mice exhibited a significant reduction in POF
pathology within their ovaries, attenuation of ovarian granulosa
cell injury, reduction in the number of atretic follicles and a
significant increase in the number of mature oocytes (Fig. 2).
Medium doses of rmGH activate the
Notch-1 signaling pathway in the ovarian granulosa cells of POF
mice
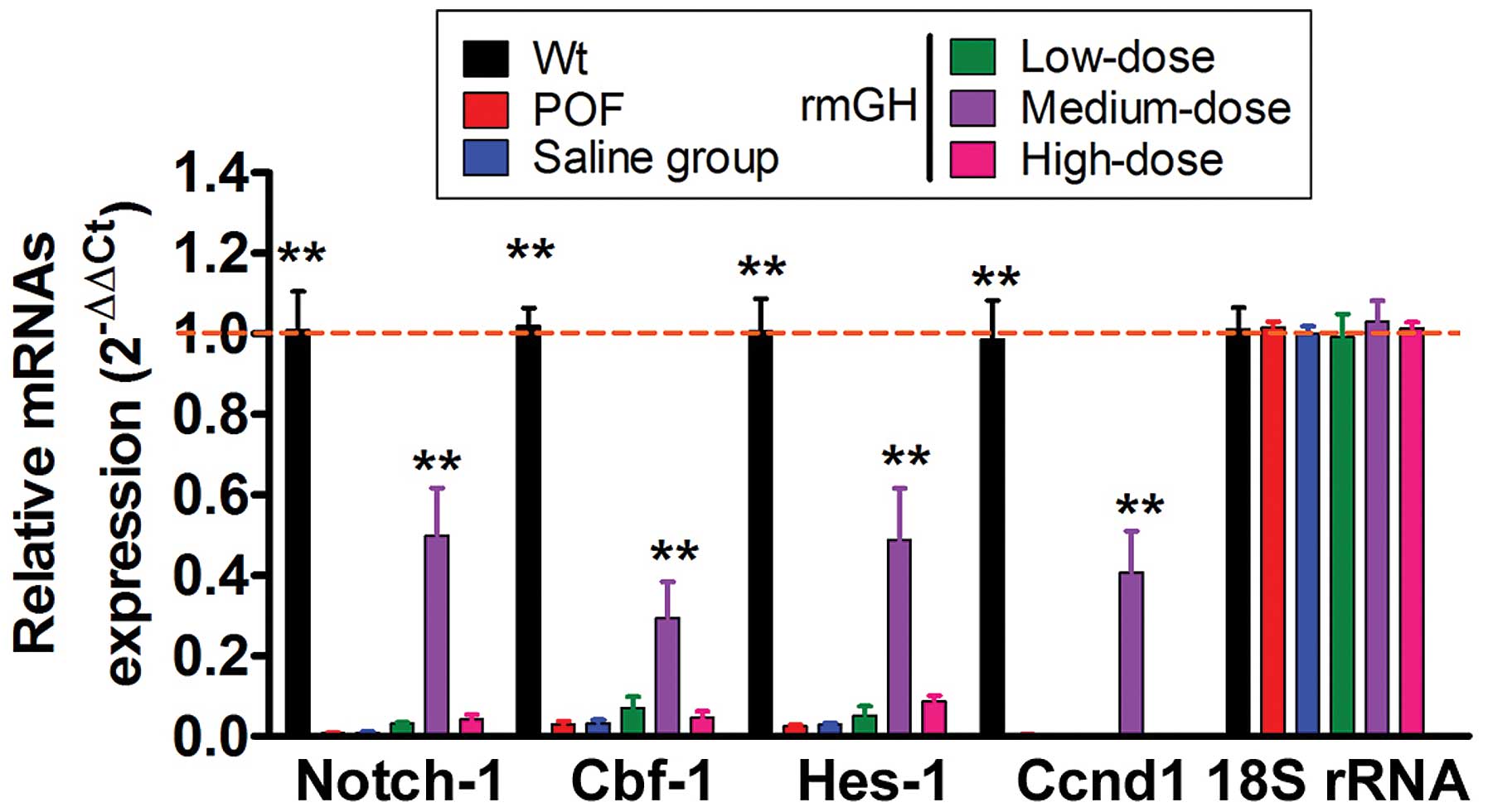
At 14 days after injection, the expression levels of
the genes involved in the Notch-1 signaling pathway in mouse
ovarian granulosa cells were analyzed by RT-qPCR and
immunohistochemistry. The RT-qPCR results revealed that the
expression levels of Notch1 signaling pathway genes (NOTCH1,
Cbf1 and Hes1) and cell proliferation factor
(Ccnd1; cyclin D1) had decreased in the POF model group
compared with the WT group (P<0.05; n=12 mice; Fig. 3). Furthermore, a statistically
significant difference was not identified in the expression levels
of the Notch-1 signaling pathway genes and the Ccnd1 between
the POF model group, the low-dose group, high-dose group and the
saline-treated group (P>0.05; n=12 mice; Fig. 3). However, the expression levels of
Notch-1 signaling pathway genes and Ccnd1 were significantly
elevated in the medium-dose group compared with the POF model group
(P<0.01; n=12 mice; Fig. 3).
Immunohistochemical staining also revealed positive staining for
Notch1 signaling pathway factors (Notch1, CBF1, and HES1) in the WT
group, medium-dose group and high-dose group, but not in the
low-dose or saline-treated groups (Fig.
4). However, p53 staining was positive in the low-dose and
saline-treated groups, but not in the WT, medium-dose or high-dose
groups (Fig. 4).
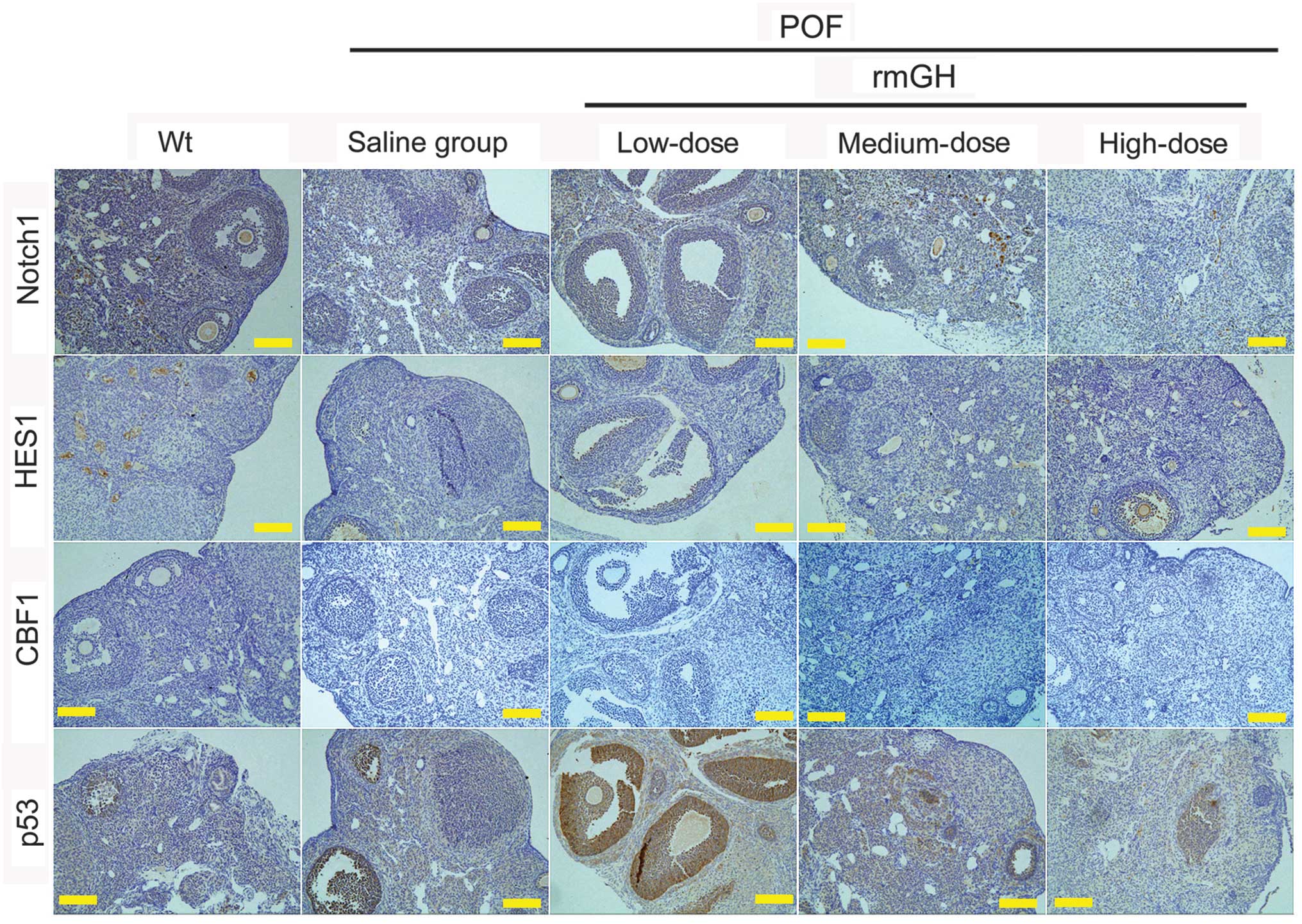 | Figure 4.Protein expression of Notch1
signaling pathway factors and p53 using immunohistochemistry.
Staining indicated positive or markedly positive staining for
Notch1 signaling pathway factors (Notch1, CBF1, and HES1), but not
for p53 in the Wt, medium-dose, and high-dose groups, as compared
with the low-dose and saline-treated groups. Magnification, ×200.
Scale bar=200 µm. POF, premature ovarian failure model; rmGH,
recombinant mouse growth hormone; Wt, wild-type; HES1,
hairy/enhancer of split. |
Discussion
POF is an impactful disease and, at present, there
is no cure or effective treatment available for POF. Women
diagnosed with POF are faced with significant physical and
emotional challenges, as this disease can lead to loss of
fertility, in addition to osteoporosis and other complications
(1–5). GH is a pleiotropic hormone that affects
a broad spectrum of physiological functions, from carbohydrate and
lipid metabolism to the immune response (10–16).
Several previous studies have reported the effective use of GH to
treat autoimmune diseases and POF (14–16).
However, the mechanism underlying GH activity in the treatment of
these diseases remains unclear. In the present study, three main
questions are addressed: i) Does rmGH improve hormone release and
oocyte maturation in mice exhibiting POF? ii) Is the effect of rmGH
on the regulation of hormone levels and oocyte maturation
dose-dependent? and iii) What therapeutic mechanism does rmGH
employ to control hormone release and oocyte maturation in POF
mice?
Although E2 and FSH hormone levels in POF
mice were significantly improved following treatment with medium
and high doses of rmGH treatment, a medium dose of rmGH was the
most effective, leading to significantly increased E2
levels and decreased FSH levels in the peripheral blood of POF
mice. High doses of rmGH also elevated E2 levels in
peripheral blood, although FSH levels significantly increased from
that observed in the medium-dose group. It may be speculated that
high doses of GH stimulate the body to release hormones in a
non-specific manner, causing this increase in FSH. GH was also
reported to stimulate oocyte maturation and reduce the number of
atretic follicles in a dose-dependent manner; these effects were
only observed in POF mice treated with medium and high doses of
rmGH. Oocyte maturation is closely associated with several factors,
including the ovarian microenvironment, the extent of development
of the ovarian corpus luteum, the activity of ovarian granulosa
cells, hormone release and endocrine regulation (1–5). The
results of the current study confirm that GH may effectively treat
multiple features of POF in mice displaying disease symptoms.
The novel evidence that GH regulates the expression
of Notch-1 signaling pathway genes in ovarian tissue from POF mice
in order to induce regeneration and repair was also presented. The
Notch-1 signaling pathway is known to regulate cell proliferation
and tumorigenesis but, to the best of our knowledge, it has not
previously been associated with POF. In the current study, Notch-1
signaling pathway genes were expressed at different levels when POF
mice were treated with varying concentrations of GH. For example, a
medium dose of rmGH caused the greatest activation of Notch-1
signaling pathway genes in the ovarian tissue of POF mice. The
hypothesized role of the Notch pathway genes based on the current
model suggests that the direct effect of Notch-1 signaling pathway
activation is the proliferation of ovarian cells to restore
function, and thus, repair and replace damaged ovarian cells in the
POF mouse model. It is therefore hypothesized that GH was activated
through the expression of the Notch-1 pathway in ovarian cells to
repair ovarian function.
In conclusion, the results of the present study
suggest that GH promotes ovarian tissue repair and regeneration,
estrogen release and oocyte maturation by activating the expression
of Notch-1 signaling pathway factors in the ovarian tissue of mice
exhibiting POF.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81273794), the
National Natural Science Foundation of China (grant no. 81202811),
and the China Postdoctoral Science Foundation (grant nos.
2014M550250 and 2015T80455).
References
|
1
|
Bandyopadhyay S, Chakrabarti J, Banerjee
S, Pal AK, Goswami SK, Chakravarty BN and Kabir SN: Galactose
toxicity in the rat as a model for premature ovarian failure: An
experimental approach readdressed. Hum Reprod. 18:2031–2038. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beck-Peccoz P and Persani L: Premature
ovarian failure. Orphanet J Rare Dis. 1:92006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Persani L, Rossetti R and Cacciatore C:
Genes involved in human premature ovarian failure. J Mol
Endocrinol. 45:257–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McGuire MM, Bowden W, Engel NJ, Ahn HW,
Kovanci E and Rajkovic A: Genomic analysis using high-resolution
single-nucleotide polymorphism arrays reveals novel microdeletions
associated with premature ovarian failure. Fertil Steril.
95:1595–1600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vujović S, Ivović M, Tancić-Gajić M,
Marina L, Barać M, Arizanorić Z, Nenezić A, Ivaniserić M, Micić J,
Sajić S and Micić D: Premature ovarian failure. Srp Arh Celok Lek.
140:806–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qin Y, Sun M, You L, Wei D, Sun J, Liang
X, Zhang B, Jiang H, Xu J and Chen ZJ: ESR1, HK3 and BRSK1 gene
variants are associated with both age at natural menopause and
premature ovarian failure. Orphanet J Rare Dis. 7:52012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rebar RW: Premature ovarian 'failure' in
the adolescent. Ann N Y Acad Sci. 1135:138–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalantari H, Madani T, Zari Moradi S,
Mansouri Z, Almadani N, Gourabi H and Mohseni Meybodi A:
Cytogenetic analysis of 179 Iranian women with premature ovarian
failure. Gynecol Endocrinol. 29:588–591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duncan M, Cummings L and Chada K: Germ
cell deficient (gcd) mouse as a model of premature ovarian failure.
Biol Reprod. 49:221–227. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cecchi CR, Higuti E, Oliveira NA, Lima ER,
Jakobsen M, Dagnaes-Hansen F, Gissel H, Aagaard L, Jensen TG, Jorge
AA, et al: A novel homologous model for gene therapy of dwarfism by
non-viral transfer of the mouse growth hormone gene into
immunocompetent dwarf mice. Curr Gene Ther. 14:44–51. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Visser JA, Hokken-Koelega AC, Zandwijken
GR, Limacher A, Ranke MB and Flück CE: Anti-Müllerian hormone
levels in girls and adolescents with Turner syndrome are related to
karyotype, pubertal development and growth hormone treatment. Hum
Reprod. 28:1899–1907. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hartmann BW, Kirchengast S, Albrecht A,
Huber JC and Söregi G: Effect of hormone replacement therapy on
growth hormone stimulation in women with premature ovarian failure.
Fertil Steril. 68:103–107. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kopchick JJ, List EO, Kelder B, Gosney ES
and Berryman DE: Evaluation of growth hormone (GH) action in mice:
Discovery of GH receptor antagonists and clinical indications. Mol
Cell Endocrinol. 386:34–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rojanathammanee L, Rakoczy S and
Brown-Borg HM: Growth hormone alters the glutathione S-transferase
and mitochondrial thioredoxin systems in long-living Ames dwarf
mice. J Gerontol A Biol Sci Med Sci. 69:1199–1211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chesnokova V, Zhou C, Ben-Shlomo A, Zonis
S, Tani Y, Ren SG and Melmed S: Growth hormone is a cellular
senescence target in pituitary and nonpituitary cells. Proc Natl
Acad Sci USA. 110:E3331–E3339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Villares R, Kakabadse D, Juarranz Y,
Gomariz RP, Martínez AC and Mellado M: Growth hormone prevents the
development of autoimmune diabetes. Proc Natl Acad Sci USA.
110:E4619–E4627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Subramaniam D, Ponnurangam S, Ramamoorthy
P, Standing D, Battafarano RJ, Anant S and Sharma P: Curcumin
induces cell death in esophageal cancer cells through modulating
Notch signaling. PLoS One. 7:e305902012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao L, Kan EM, Kaur C, Dheen ST, Hao A, Lu
J and Ling EA: Notch-1 signaling regulates microglia activation via
NF-κB pathway after hypoxic exposure in vivo and in vitro. PLoS
One. 8:e784392013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji X, Wang Z, Geamanu A, Goja A, Sarkar FH
and Gupta SV: Delta-tocotrienol suppresses notch-1 pathway by
upregulating miR-34a in nonsmall cell lung cancer cells. Int J
Cancer. 131:2668–2677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Hilton MJ, Anolik JH, Welle SL,
Zhao C, Yao Z, Li X, Wang Z, Boyce BF and Xing L: NOTCH inhibits
osteoblast formation in inflammatory arthritis via noncanonical
NF-κB. J Clin Invest. 124:3200–3214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen G, Qiu Y, Sun L, Yu M, Wang W, Xiao
W, Yang Y, Liu Y, Yang S, Teitelbaum DH, et al: The
Jagged-2/Notch-1/Hes-1 pathway is involved in intestinal epithelium
regeneration after intestinal ischemia-reperfusion injury. PLoS
One. 8:e762742013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Azmi AS, Ahmad A, Banerjee S, Wang
S, Sarkar FH and Mohammad RM: TW-37, a small-molecule inhibitor of
Bcl-2, inhibits cell growth and induces apoptosis in pancreatic
cancer: Involvement of Notch-1 signaling pathway. Cancer Res.
69:2757–2765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu T, Huang Y, Zhang J, Qin W, Chi H,
Chen J, Yu Z and Chen C: Transplantation of human menstrual blood
stem cells to treat premature ovarian failure in mouse model. Stem
Cells Dev. 23:1548–1557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen DZ, Xin SL, Chen C and Liu T: Effect
of atorvastatin on expression of TLR4 and NF-κB p65 in
atherosclerotic rabbits. Asian Pac J Trop Med. 6:493–496. 2013.
View Article : Google Scholar : PubMed/NCBI
|