Introduction
Gastric cancer (GC) is the most common malignant
tumor of the digestive system and its incidence and mortality rank
forth and second, respectively, among common malignancies worldwide
(1). GC has the highest incidence
and mortality rates among digestive tract malignant tumors in China
(2–5). The incidence of GC varies greatly
between regions due to variations in population susceptibility and
exposure to GC risk factors (6). The
risk factors for GC include Helicobacter pylori infection
(7), poor living habits (including
smoking, alcohol consumption and excessive consumption of smoked,
salted and pickled foods) (8,9), chronic
atrophic gastritis and gastric ulcers (9,10–17). GC
includes various pathological types, among which gastric
adenocarcinoma accounts for 90% of total cases (18). Gastric adenocarcinoma was therefore
investigated in the present study. Due to the characteristics of
occult onset, high degree of malignancy, rapid development and
susceptibility to metastasis, the early diagnosis rates for GC are
<10% and the prognosis of advanced GC is poor (19). Although progress has been made in the
diagnosis and treatment of GC, surgical resection remains the most
effective therapeutic strategy to cure GC at present (20). Numerous factors, stages and genes are
involved in the occurrence, development and metastasis of GC
(21,22); therefore, investigation of the gene
mutations and alterations in factors that underlie this process
will help determine the biological behavior of GC and evaluate the
prognosis of patients with GC. It is important to screen effective
biomarkers for early diagnosis, treatment and prognostic evaluation
of GC.
Tumor necrosis factor-α (TNF-α)-induced proteins
(TNFAIP) are involved in numerous biological processes via their
ability to activate nuclear factor-κB (23,24).
TNFAIP8 belongs to the TNFAIP family that also includes TNFAIP8
ligands 1, 2 and 3 (25). TNFAIP8
contains a death domain (25) and
has a role in the regulation of processes including cell
proliferation, inflammation and apoptosis (26,27).
Previous studies demonstrated that the expression of TNFAIP8 in
numerous human solid tumors is correlated with tumor occurrence,
development, invasion, metastasis and prognosis (28–36).
Overexpression of TNFAIP8 in tumor cells can enhance cell
proliferation and promote tumor growth, and the cancerous
characteristics of the tumor cells are significantly reduced
following TNFAIP8 gene knocked out (28,37). To
the best of our knowledge, there has yet to have been any
investigation into the association between TNFAIP8 expression and
the clinical pathological features of gastric adenocarcinoma, and
the association between TNFAIP8 expression and gastric
adenocarcinoma prognosis has yet to be fully elucidated.
In the present study, TNFAIP8 expression was
detected in metastatic lymph node tissue samples and normal tissue
adjacent to cancerous gastric cancer tissues, and the association
between TNFAIP8 expression, clinical pathological features and
gastric adenocarcinoma prognosis was assessed.
Materials and methods
Tissue specimens
Paraffin embedded tissue specimens were provided by
the Department of Pathology of the Affiliated Hospital of Shandong
Provincial Academy of Medical Sciences (Jinan, China). Tissue
specimens were collected from 106 patients with gastric
adenocarcinoma who were hospitalized between August 2008 and July
2013 at the Affiliated Hospital of Shandong Provincial Academy of
Medical Sciences following surgical treatment. In situ
gastric adenocarcinoma tissue samples and normal tissue samples
adjacent to the tumor (>5 cm from cancer tissue margin; control)
were collected from 106 patients with gastric adenocarcinoma. Among
these 106 patients, 60 cases were also diagnosed with pathological
lymph node metastasis, and tissue samples from the metastatic lymph
nodes were also collected from these patients. Of the 106 patients,
70 were male and 36 were female, with ages of 26–83 years (mean
age, 62 years). Study participants fulfilled the inclusion criteria
of patients with gastric adenocarcinoma who had not received
radiotherapy or chemotherapy prior to surgery. All cases of gastric
adenocarcinoma were confirmed by pathological diagnosis (38), and their clinical data and follow-up
data were complete. Patients were followed-up by telephone or via
correspondence. The follow-up period ended in August 2013, and the
median follow-up period was 21 months (3–58 months). Patients who
succumbed to other diseases or did attend the follow-up were
excluded from the study. Clinical and pathological indexes included
histological grade (38), TNM stage,
tumor size, lymph vessel invasion, depth of tumor invasion, lymph
node metastasis, carbohydrate antigen 72–4 (CA72-4) levels and the
survival time of the patients. CA72-4 levels were measured using an
Roche 2010 automatic electrochemiluminescence immunoassay analyzer
(Roche Diagnostics, Basel, Switzerland). The survival time was
calculated from the date of the surgical procedure to the last
follow-up date or until to the patient succumbed to the disease due
to recurrence or metastasis. Detailed clinical information is
listed in Table I. TNM stage was
classified according to the 2010 edition of the AJCC Cancer Staging
Manual (39). The patients did not
receive any systemic radiotherapy or chemotherapy prior to surgical
intervention. Written and informed consent was obtained from the
patients. The study protocol was approved by the Ethics Committee
of Qilu Hospital, Shandong University (Jinan, China).
 | Table I.Correlation of TNFAIP8 expression
with clinical and pathological characteristics of gastric
adenocarcinoma patients. |
Table I.
Correlation of TNFAIP8 expression
with clinical and pathological characteristics of gastric
adenocarcinoma patients.
|
|
| TNFAIP8
expression |
|
|
|---|
|
|
|
|
|
|
|---|
|
| Cases | Positive | Negative |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Variable | n | n | % | n | % | χ2 | P-value |
|---|
| Total cases | 106 | 50 | 47.2 | 56 | 52.8 |
|
|
| Gender |
|
|
|
|
| 1.500 | 0.221 |
|
Male | 70 | 36 | 51.4 | 34 | 48.6 |
|
|
|
Female | 36 | 14 | 38.9 | 22 | 61.1 |
|
|
| Age (years) |
|
|
|
|
|
|
|
|
≥60 | 74 | 34 | 45.9 | 40 | 54.1 | 0.147 | 0.701 |
|
<60 | 32 | 16 | 50.0 | 16 | 50.0 |
|
|
| Pathological
grading |
|
|
|
|
| 4.413 | 0.110 |
| Well
differentiated | 14 | 3 | 21.4 | 11 | 78.6 |
|
|
|
Moderately differentiated | 24 | 13 | 54.2 | 11 | 45.8 |
|
|
| Poorly
differentiated | 68 | 34 | 50.0 | 34 | 50.0 |
|
|
| Tumor size (maximum
diameter) |
|
|
|
|
| 0.482 | 0.488 |
| ≤5.0
cm | 31 | 14 | 45.2 | 17 | 54.8 |
|
|
| >5.0
cm | 75 | 37 | 49.3 | 38 | 51.7 |
|
|
| Vascular
invasion |
|
|
|
|
| 9.974 | 0.002 |
|
Yes | 66 | 39 | 59.1 | 27 | 40.9 |
|
|
| No | 40 | 11 | 27.5 | 29 | 72.5 |
|
|
| CA72-4 |
|
|
|
|
| 22.718 | <0.001 |
| ≤6.9
KU/l | 47 | 10 | 21.2 | 37 | 78.7 |
|
|
| >6.9
KU/l | 59 | 40 | 67.8 | 19 | 32.2 |
|
|
| Lymphatic node
metastasis |
|
|
|
|
| 9.133 | 0.003 |
| No | 60 | 36 | 60.0 | 24 | 40.0 |
|
|
|
Yes | 46 | 14 | 30.4 | 32 | 69.6 |
|
|
| TNM stage |
|
|
|
|
| 15.222 | <0.001 |
| I and
II | 27 | 4 | 14.8 | 23 | 85.2 |
|
|
|
III | 41 | 24 | 58.5 | 17 | 41.5 |
|
|
| IV | 38 | 22 | 57.9 | 16 | 42.1 |
|
|
| Tumor stage |
|
|
|
|
| 13.151 | 0.001 |
|
T1-T2 | 28 | 5 | 17.9 | 23 | 82.1 |
|
|
| T3 | 44 | 25 | 56.8 | 19 | 43.2 |
|
|
| T4 | 34 | 20 | 58.8 | 14 | 41.2 |
|
|
Immunohistochemical staining
Immunohistochemical ultra sensitive 2-Step plus
Poly-horseradish peroxidase Anti-Mouse/Rabbit IgG Detection System
kit (PV-9000) with 3,3′-diamino-benzidine (DAB) was purchased from
Beijing Zhongshan Jinqiao Biological Technology Co., Ltd.,
(Beijing, China). Immunohistochemical staining was performed
according to the manufacturer's protocol. Briefly, the tissue
specimens (1–2 cm) were fixed with 4% paraformaldehyde
(Sigma-Aldrich, St. Louis, MO, USA) and embedded in paraffin
(Shanghai Huayong Shila Ltd., Shanghai, China). The
paraffin-embedded tissue samples were cut into 4-µm-thick sections
using a Leica RM2126 microtome (Leica Microsystems GmbH, Wetzlar,
Germany). The tissue sections were deparaffinized and rehydrated in
xylene (Sigma-Aldrich) and a graded alcohol series. Antigen
retrieval was achieved by incubation with boiled citric acid buffer
(10 mM citric acid and 0.05% Tween 20; pH 6) for 15 min and
endogenous peroxidase was blocked with 3% hydrogen peroxide in
methanol (Shenzhou Huamei Science and Technology Co., Ltd.,
Beijing, China) for 30 min at room temperature. Non-specific
binding was blocked by incubation with goat serum (12168A03;
Zhongshan Golden Bridge Biological Technology Inc., Beijing,
China). Primary rabbit anti-human TNFAIP8 monoclonal antibody
(1:100; ab64988; Abcam, Cambridge, MA, USA) was added to the tissue
sections and incubated at 37°C for 1 h. Phosphate-buffered saline
(PBS) was used instead of the primary antibody as a blank control.
Tissue sections were subsequently incubated with biotin-labeled
goat anti-rabbit secondary antibodies (1:200) for 30 min at room
temperature following washing with PBS. Horseradish
peroxidase-labeled streptavidin was added and incubated for 30 min
at 37°C. Immunoreactivity was visualized using DAB at room
temperature at 1 h and terminated with distilled water until
brown-colored particles appeared in the cytoplasm and non-specific
coloration was detected in the surrounding tissue. Tissue sections
were subsequently counterstained with hematoxylin, differentiated
with hydrochloric acid ethanol, dehydrated with gradient alcohol
and xylene, and mounted with neutral gum (all Sigma-Aldrich).
Sections were observed under an Olympus BX51 optical microscope
(Olympus Corporation, Tokyo, Japan).
Determination of immunohistochemical
staining results
Each sample was observed in five high magnification
fields (magnification, ×400) and the cells exhibiting
yellow-colored particles in the cytoplasm were considered to be
positive for TNFAIP8 protein expression. The staining intensity was
observed and the percentage of positive cells was calculated. Based
on the staining intensity, the immunohistochemical staining results
were scored as follows: Score 0, no positive staining (−); score 1,
pale yellow staining (+); score 2, yellow staining (++); and score
3, dark brown staining (+++). Based on the percentage of positive
staining, the immunohistochemical staining results were scored as
follows: Score 0, 0% positive staining; score 1, 1–25% positive
staining; score 2, 26–50% positive staining; and score 3, 51–100%
positive staining. The degree of staining was calculated by two
independent pathologists by combining the percentage of positive
staining and the intensity of staining. A third pathologist was
consulted until a consensus was reached if discrepancies occurred.
Scores ≤2 were considered to indicate low TNFAIP8 expression, and
scores >2 were considered to indicate high TNFAIP8 expression.
All tissue sections were carefully examined twice in order to
ensure the reproducibility of the results.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 19.0; SPSS, Inc, Chicago, IL, USA). χ2
tests were performed to analyze the association between TNFAIP8
expression and clinical pathological data. The Kaplan-Meier method
(40) was performed to analyze
survival rates, and a Log-rank test was conducted in order to
compare the difference between survival rates. Single-factorial
survival analysis and COX proportional-hazard regression model
analysis were subsequently performed to analyze the independent
prognostic factor and multi-factors for survival, respectively.
Data were presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant result.
Results
Expression of TNFAIP8 in gastric
adenocarcinoma tissue samples, normal adjacent tissue samples and
lymph node metastatic tissue samples
In order to investigate the expression of TNFAIP8 in
the gastric adenocarcinoma and adjacent normal tissue samples of
106 patients, as well as the expression of TNFAIP8 in the
metastatic lymph nodes of 60 of these patients, immunohistochemical
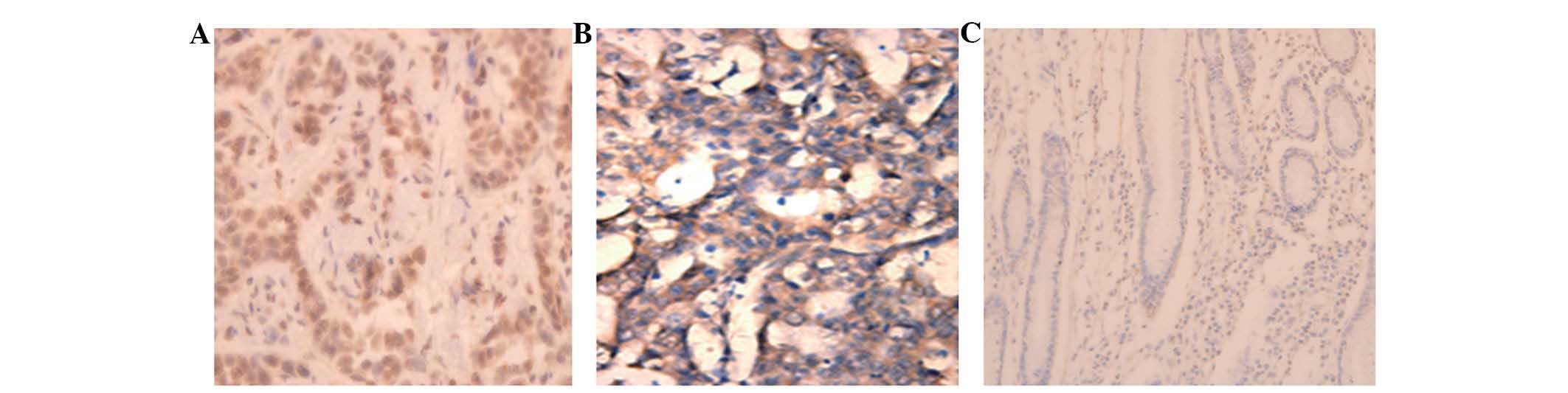
analysis was performed. Light yellow or brown-colored granules were
present in the cytoplasm of gastric adenocarcinoma cells, but not
in the interstitial tissue (Fig.
1A). In addition, brown-colored granules were present in the
metastatic lymph nodes, indicating high TNFAIP8 expression
(Fig. 1B). No or weak TNFAIP8
expression was detected in the adjacent normal gastric mucosa
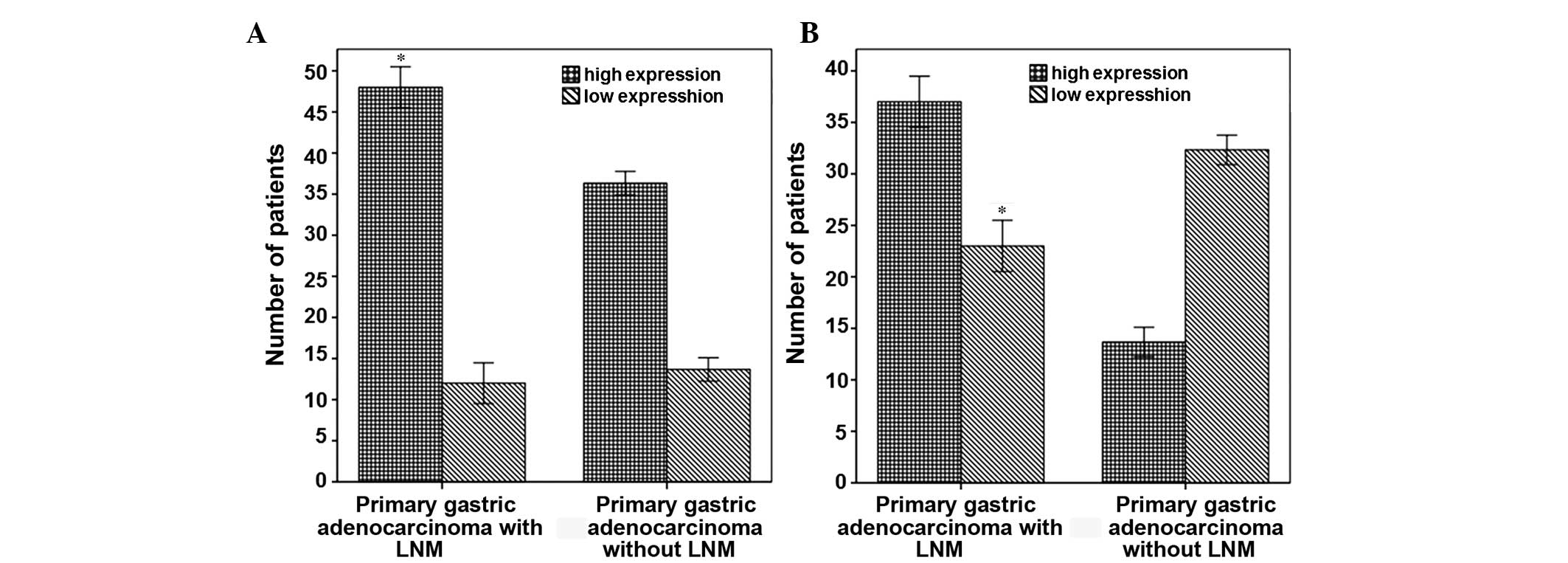
(Fig. 1C). A total of 47.2% (50/106)
of the in situ gastric adenocarcinoma tumor cases presented
with high TNFAIP8 expression, as compared with 81.7% (49/60) in the
metastatic lymph node tissue cases. Among the 60 patients with
lymph node metastasis, the number of cases with high TNFAIP8
expression in the gastric adenocarcinoma in situ tumor
tissue samples accounted for 60% (36/60), and the number of cases
with high TNFAIP8 expression in the metastatic lymph nodes
accounted for 80.6% (49/60). These results were statistically
significant (P<0.05; Fig. 2A). Of
the 46 cases of gastric adenocarcinoma without lymph node
metastasis, only 14 cases exhibited high TNFAIP8 expression in the
in situ gastric adenocarcinoma tumor tissue samples. This
indicated that the number of patients with high TNFAIP8 expression
in the in situ gastric adenocarcinoma tissue samples was
significantly higher in patients with lymph node metastasis (60%,
36/60), as compared with patients without lymph node metastasis
(30.4%, 14/46; P<0.05; Fig. 2B).
These results suggest that TNFAIP8 expression is closely correlated
with lymph node metastasis in gastric adenocarcinoma.
Correlation between TNFAIP8 expression
and clinical and pathological characteristics
The correlation between TNFAIP8 expression and
clinical and pathological characteristics of the gastric cancer
patients were analyzed. The 106 patients were grouped according to
age, gender, tumor size, lymph node metastasis and vascular
invasion (Table I). In the patients
with high TNFAIP8 expression in the gastric adenocarcinoma tissue
samples, 78% (39/50) exhibited vascular invasion, 72% (36/50) had
lymph node metastasis, 76% (38/50) had TNM stage IV tumors and 90%
(45/50) had T3 or T4 tumors. These results indicated that TNFAIP8
expression was correlated with progression and
metastasis-associated factors such as TNM staging (P<0.001),
tumor grade (P=0.001), vascular invasion (P=0.002) and lymph node
metastasis (P=0.003). In addition, TNFAIP8 expression in patients
with gastric adenocarcinoma was associated with high serum CA72-4
levels (P<0.001). However, TNFAIP8 expression had no significant
correlation with age, gender, histological grading or tumor area.
These results suggest that TNFAIP8 expression is closely correlated
with local invasion and metastasis of gastric adenocarcinoma.
Association between TNFAIP8 expression
and the prognosis of patients with gastric adenocarcinoma
To reveal the association between TNFAIP8 expression
and the prognosis of the patients, the survival data of 98/106
patients with gastric adenocarcinoma with a follow-up period of
3–58 months were obtained. The postoperative survival rates of the
patients with gastric adenocarcinoma and high or low TNFAIP8
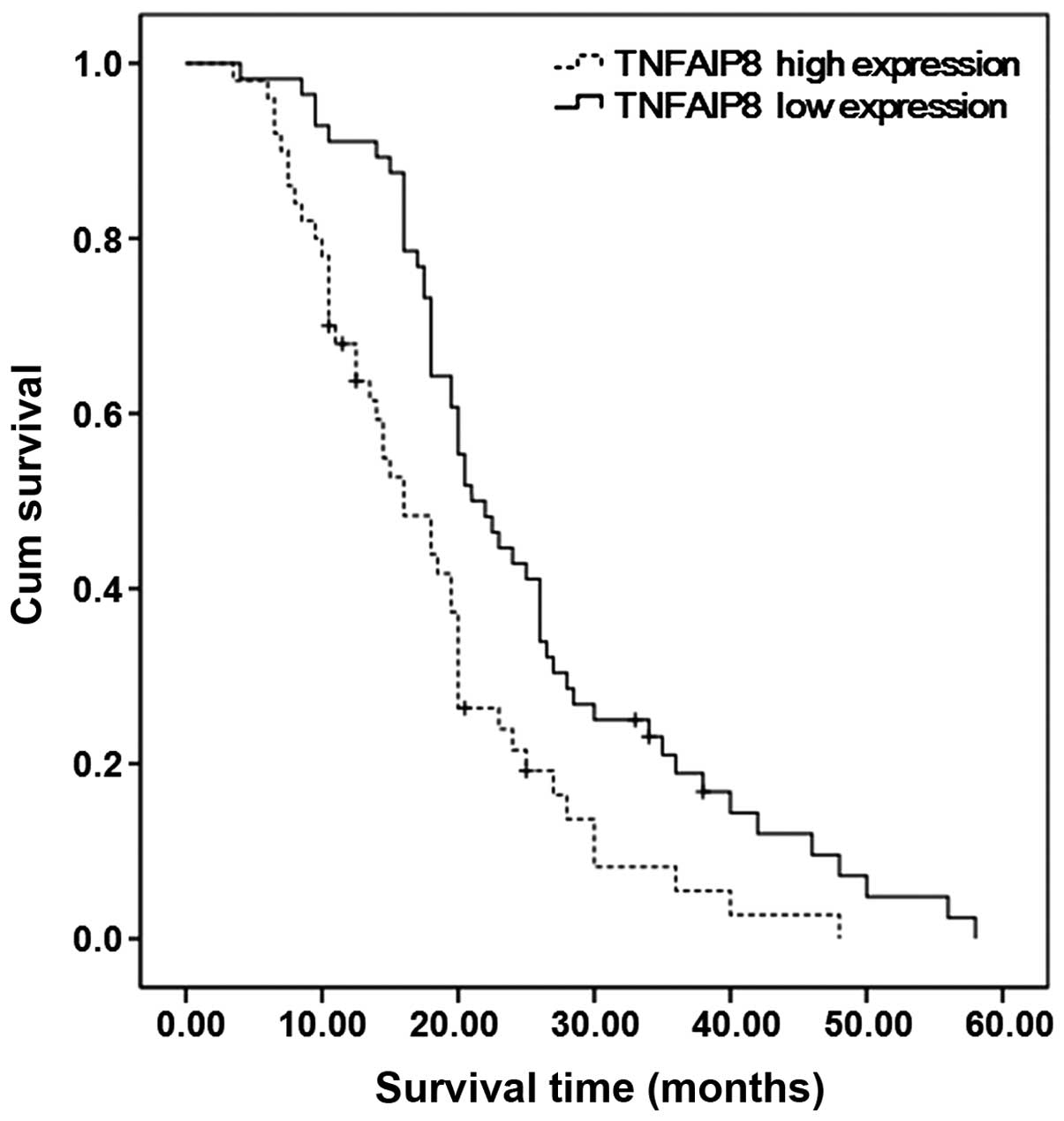
expression were compared. The median survival time of patients with
high TNFAIP8 expression (mean, 16 months) was shorter compared with
patients with low TNFAIP8 expression (mean, 21 months). A Log-rank
test demonstrated that the overall survival rates of the two groups
were significantly different (Fig.
3; P=0.002). Single-factorial survival analysis demonstrated
that, in addition to the expression levels of TNFAIP8, lymph node
metastasis (P=0.003), CA72-4 levels (P<0.001), TNM staging
(P<0.001) and tumor classification (P<0.001) were important
prognostic factors (Table II). The
prognostic factors of gastric adenocarcinoma such as TNFAIP8
expression levels, lymph node metastasis, CA72-4 levels and TNM
staging were included in the COX multivariate analysis. In
addition, factors of age and gender were included as covariates in
the model. The results of multivariate the COX proportional hazard
model analysis demonstrated that with the exception of TNM staging,
TNFAIP8 expression levels were the only independent prognostic
marker for gastric adenocarcinoma survival (relative risk, 1.736;
P=0.029; Table III). These results
indicate that TNFAIP8 is an independent prognostic factor for
gastric adenocarcinoma.
 | Table II.Single-factorial survival
analysis. |
Table II.
Single-factorial survival
analysis.
| Variable | χ2 | P-value |
|---|
| Gender | 0.395 | 0.530 |
| Age | 0.079 | 0.779 |
| Tumor size | 2.154 | 0.142 |
| Vascular
invasion | 1.009 | 0.315 |
| CA72-4 | 15.794 | <0.001 |
| Lymphatic node
metastasis | 9.117 | 0.003 |
| TNM stage | 55.954 | <0.001 |
| Invasive depth | 47.830 | <0.001 |
| TNFAIP8
expression | 9.918 | 0.002 |
 | Table III.Multivariate survival analysis. |
Table III.
Multivariate survival analysis.
|
| 95% CI for RR |
|---|
|
|
|
|---|
| Variable | β | Wald | P-value | RR | Lower | Upper |
|---|
| Age | 0.233 | 0.958 | 0.328 | 1.262 | 0.792 | 2.012 |
| Gender | −0.592 | 4.417 | 0.063 | 0.565 | 0.345 | 0.925 |
| Lymphatic node
metastasis | −0.480 | 2.530 | 0.112 | 0.619 | 0.343 | 1.118 |
| CA72-4 | −0.587 | 3.728 | 0.054 | 0.556 | 0.306 | 1.009 |
| TNM stage |
| 35.621 | <0.001 |
|
|
|
| TNM (I, II vs.
IV) | −2.173 | 29.807 | <0.001 | 0.114 | 0.052 | 0.248 |
| TNM (III vs.
IV) | −1.792 | 26.792 | <0.001 | 0.167 | 0.085 | 0.328 |
| TNFAIP8
expression |
0.552 | 4.751 | 0.029 | 1.736 | 1.057 | 2.851 |
Discussion
TNFAIP8 (also called SCC-S2/GG2-1/MDC3.13) was first
identified by Patel et al (23) in 1997 in a human head and neck
squamous cell carcinoma cell line. It is the first member of the
TNFAIP8 family (23). TNFAIP8 has
important roles in promoting cell proliferation and inhibiting
apoptosis (28,37). Overexpression of TNFAIP8 can promote
DNA synthesis, cell proliferation and inhibit the activities of
apoptosis enzymes of caspase 8 and caspase 3 (41). High expression levels of TNFAIP8 have
been reported in tumor cells and decreasing the expression of
TNFAIP8 can reduce the tumorigenicity of tumor cells (42). Therefore, TNFAIP8 has an important
role in cell survival and malignant growth-associated signaling
pathways. Numerous recent studies demonstrated that TNFAIP8 was
closely associated with the occurrence and development of several
types of tumor, including renal cell carcinoma (28), colon cancer (29), prostate cancer (30), esophageal squamous cell carcinoma
(31), cervical cancer (32), non-small cell lung cancer (33), breast cancer (28), pancreatic cancer (34), epithelial ovarian carcinoma (35) and endometrial carcinoma (36).
In the present study, TNFAIP8 expression in gastric
adenocarcinoma, surrounding normal tissues and lymph node
metastatic tissues were detected by immunohistochemistry. In
addition, the correlation between TNFAIP8 expression and
clinicopathological factors (including CA72-4) and prognosis were
analyzed. The results of the present investigation demonstrated
that the rates of high TNFAIP8 expression in metastatic lymph nodes
was increased compared with in situ tumor tissue samples,
and this result was concordant with a previous study (43). The rates of high TNFAIP8 expression
in the in situ tumor tissue samples in patients with lymph
node metastasis were significantly higher compared with the in
situ tissue samples of patients without metastasis. Further
analysis demonstrated that TNFAIP8 expression was associated with
TNM stage, tumor grade, vascular invasion and lymph node
metastasis. These results indicated that TNFAIP8 expression was
correlated with the progression and metastasis of gastric
adenocarcinoma, suggesting that it may have an important role in
tumor invasion and metastasis. In addition, the data suggested that
TNFAIP8 expression was correlated with serum CA72-4 levels.
Previous studies have demonstrated that the serum levels of CA72-4
can be used for early diagnosis and prognosis evaluation of gastric
adenocarcinoma (44–47). Therefore, we suggest that the
combined detection of TNFAIP8 and CA72-4 in serum will be helpful
for the accurate prediction of the prognosis of patients with
gastric adenocarcinoma. Further studies are required in order to
test this hypothesis.
Studies have shown that TNFAIP8 expression is
negatively correlated with prognosis in numerous types of tumors,
including prostate cancer (30),
esophageal squamous cell carcinoma (31), cervical cancer (32), non-small cell lung cancer (33) and epithelial ovarian cancer (35). In the current study, the survival
results demonstrated that the median survival time of gastric
adenocarcinoma patients with high TNFAIP8 expression patients was
shorter than patients with low TNFAIP8 expression, and the overall
survival rate of TNFAIP8-positive patients was relatively low.
Through single-factorial survival analysis, the results indicated
that in addition to TNFAIP8 expression, lymph node metastasis, TNM
stage and the levels of serum CA72-4 all had important prognostic
value. However, age, gender, histological differentiation and tumor
size had no significant prognostic value. Survival and subsequent
multivariate analysis demonstrated that TNFAIP8 expression levels
were an independent prognostic factor of patients with gastric
adenocarcinoma, indicating that TNFAIP8 may be used as a novel
prognostic factor for gastric adenocarcinoma.
In summary, the results of the present study
demonstrated that high expression levels of TNFAIP8 in gastric
adenocarcinoma were associated with gastric adenocarcinoma
progression and metastasis. The expression of TNFAIP8 was an
independent prognostic indicator in gastric adenocarcinoma. In
addition, high expression levels of TNFAIP8 indicated high
metastasis and poor prognosis. Further studies are required in
order to investigate the possible mechanism underlying the effects
of TNFAIP8 on metastasis and prognosis of gastric
adenocarcinoma.
Acknowledgements
The authors of the present study would like to thank
Dr Xiuwen Wang for his suggestions, and are grateful for the
technical assistance from the Department of Pathology, Affiliated
Hospital of Shandong Academy of Medical Sciences.
References
|
1
|
American Cancer Society: Global cancer
facts and figures (2nd). Atlanta, USA: 19–20. 2011.
|
|
2
|
Compare D, Rocco A and Nardone G: Risk
factors in gastric cancer. Eur Rev Med Pharmacol Sci. 14:302–308.
2010.PubMed/NCBI
|
|
3
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen J, Rocken C, Malfertheiner P and
Ebert MP: Recent advances in molecular diagnosis and therapy of
gastric cancer. Dig Dis. 22:380–385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blaser MJ and Atherton JC: Helicobacter
pylori persistence: Biology and disease. J Clin Invest.
113:321–333. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Sun LP, Xing CZ, Xu Q, He CY, Li
P, Gong YH, Liu YP and Yuan Y: Interaction between GSTP1 Val allele
and H. pylori infection, smoking and alcohol consumption and
risk of gastric cancer among the Chinese population. PLoS One.
7:e471782012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim J, Cho YA, Choi IJ, Lee YS, Kim SY,
Shin A, Cho SJ, Kook MC, Nam JH, Ryu KW, et al: Effects of
interleukin-10 polymorphisms, Helicobacter pylori infection
and smoking on the risk if noncardia gastric cancer. PLoS One.
7:e296432012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukuda H, Saito D, Hayashi S, Hisai H, Ono
H, Yoshida S, Oguro Y, Noda T, Sato T, Katoh M, et al:
Helicobacter pylori infection, serum pepsinogen level and
gastric cancer: A case-control study in Japan. Jpn J Cancer Res.
86:64–71. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohata H, Kitauchi S, Yoshimura N, Mugitani
K, Iwane M, Nakamura H, Yoshikawa A, Yanaoka K, Arii K, Tamai H, et
al: Progression of chronic atrophic gastritis associated with
Helicobacter pylori infection increases risk of gastric
cancer. Int J Cancer. 109:138–143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oishi Y, Kiyohara Y, Kubo M, Tanaka K,
Tanizaki Y, Ninomiya T, Doi Y, Shikata K, Yonemoto K, Shirota T, et
al: The serum pepsinogen test as a predictor of gastric cancer: The
Hisayama study. Am J Epidemiol. 163:629–637. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren JS, Kamangar F, Qiao YL, Taylor PR,
Liang H, Dawsey SM, Liu B, Fan JH and Abnet CC: Serum pepsinogens
and risk of gastric and oesophageal cancers in the general
population nutrition intervention trial cohort. Gut. 58:636–642.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Watabe H, Mitsushima T, Yamaji Y, Okamoto
M, Wada R, Kokubo T, Doi H, Yoshida H, Kawabe T and Omata M:
Predicting the development of gastric cancer from combining
Helicobacter pylori antibodies and serum pepsinogen status:
A prospective endoscopic cohort study. Gut. 54:764–768. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye W, Held M, Lagergren J, Engstrand L,
Blot WJ, McLaughlin JK and Nyrén O: Helicobacter pylori
infection and gastric atrophy: Risk of adenocarcinoma and
squamous-cell carcinoma of the esophagus and adenocarcinoma of the
gastric cardia. J Natl Cancer Inst. 96:388–396. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
You WC, Blot WJ, Zhang L, Kneller RW, Li
JY, Jin ML, Chang YS, Zeng XR, Zhao L, Fraumeni JF Jr, et al: Serum
pepsinogens in relation to precancerous gastric lesions in a
population at high risk for gastric cancer. Cancer Epidemiol
Biomarkers Prev. 2:113–117. 1993.PubMed/NCBI
|
|
17
|
Correa P: A human model of gastric
carcinogenesis. Cancer Res. 48:3554–3560. 1988.PubMed/NCBI
|
|
18
|
Zhang M, Wang X, Li W and Cui Y: miR-107
and miR-25 simultaneously target LATS2 and regulate proliferation
and invasion of gastric adenocarcinoma (GAC) cells. Biochem Biophys
Res Commun. 460:806–812. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song YX, Zhou X, Wang ZN, Gao P, Li AL,
Liang JW, Zhu JL, Xu YY and Xu HM: The association between
individual SNPs or haplotypes of matrix metalloproteinase 1 and
gastric cancer susceptibility, progression and prognosis. PLoS One.
7:e380022012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ajani JA, Barthel BJ and Bekall-Saab T:
NCCN Clinical practice guidelines in oncology, gastric cancer.
v.1.2011. Washington: National Comprehensive Cancer Network 26.
2011.
|
|
21
|
Saif MW, Makrilia N, Zalonis A, Merikas M
and Syrigos K: Gastric cancer in the elderly: An overview. Eur J
Surg Oncol. 36:709–717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patel S, Wang FH, Whiteside TL and Kasid
U: Identification of seven differentially displayed transcripts in
human primary and matched metastatic head and neck squamous cell
carcinoma cell lines: Implications in metastasis and/or radiation
response. Oral Oncol. 33:197–203. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar D, Whiteside TL and Kasid U:
Identification of a novel tumor necrosis factor-alpha-inducible
gene, SCC-S2, containing the consensus sequence of a death effector
domain of fas-associated death domain-like interleukin-1
beta-converting enzyme-inhibitory protein. J Biol Chem.
275:2973–2978. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
You Z, Quyang H, Lopatin D, Polver PJ and
Wang CY: Nuclear factor-kappa B-inducible death effector
domain-containing protein suppresses tumor necrosis factor-mediated
apoptosis by inhibiting caspase-8 activity. J Biol Chem.
276:26398–26404. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Freundt EC, Bidere N and Lenardo MJ: A
different TIPE of immune homeostasis. Cell. 133:401–402. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun H, Gong S, Carmody RJ, Hilliard A, Li
L, Sun J, Kong L, Xu L, Hilliard B, Hu S, et al: TIPE2, a negative
regulator of innate and adaptive immunity that maintains immune
homeostasis. Cell. 133:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang C, Chakravarty D, Sakabe I, Mewani
RR, Boudreau HE, Kumar D, Ahmad I and Kasid UN: Role of SCC-S2 in
experimental metastasis and modulation of VEGFR-2, MMP-1 and MMP-9
expression. Mol Ther. 13:947–955. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miao Z, Zhao T, Wang Z, Xu Y, Song Y, Wu J
and Xu H: SCC-S2 is overexpressed in colon cancers and regulates
cell proliferation. Tumour Biol. 33:2099–2106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang C, Kallakury BV, Ross JS, Mewani RR,
Sheehan CE, Sakabe I, Luta G, Kumar D, Yadavalli S, Starr J, et al:
The significance of TNFAIP8 in prostate cancer response to
radiation and docetaxel and disease recurrence. Int J Cancer.
133:31–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hadisaputri YE, Miyazaki T, Suzuki S,
Yokobori T, Kobayashi T, Tanaka N, Inose T, Sohda M and Kuwano H:
TNFAIP8 overexpression: Clinical relevance to Esophageal squamous
cell carcinoma. Ann Surg Oncol. 19(Suppl 3): S589–S596. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi TY, Cheng X, Yu KD, Sun MH, Shao ZM,
Wang MY, Zhu ML, He J, Li QX, Chen XJ, et al: Functional variants
in TNFAIP8 associated with cervical cancer susceptibility and
clinical outcomes. Carcinogenesis. 34:770–778. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong QZ, Zhao Y, Liu Y, Wang Y, Zhang PX,
Jiang GY, Dong XJ, Cui QZ and Wang EH: Overexpression of SCC-S2
correlates with lymph node metastasis and poor prognosis in
patients with non-small-cell lung cancer. Cancer Sci.
101:1562–1569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu K, Qin CK, Wang ZY, Liu SX, Cui XP and
Zhang DY: Expression of tumor necrosis factor-alpha-induced protein
8 in pancreas tissues and its correlation with epithelial growth
factor receptor levels. Asian Pac J Cancer Prev. 13:847–850. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu T, Gao H, Chen X, Lou G, Gu L, Yang M,
Xia B and Yin H: TNFAIP8 as a predictor of metastasis and a novel
prognostic biomarker in patients with epithelial ovarian cancer. Br
J Cancer. 109:1685–1692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu T, Gao H, Yang M, Zhao T, Liu Y and
Lou G: Correlation of TNFAIP8 overexpression with the
proliferation, metastasis and disease-free survival in endometrial
cancer. Tumour Biol. 35:5805–5814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar D, Gokhale P, Broustas C,
Chakravarty D, Ahmad I and Kasid U: Expression of SCC-S2, an
antiapoptotic molecule, correlates with enhanced proliferation and
tumorigenicity of MDA-MB 435 cells. Oncogene. 23:612–616. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hamilton SR and Aaltonen LA: Pathology and
genetics of tumours of the digestive system. World Health
Organization Classification of Tumours (Lyon, France). IARC Press.
38–52. 2000.
|
|
39
|
Stephen EB, David BR, Carolyn CC, April
GF, Frederick LG and Andy T: AJCC cancer staging manual (7th).
Lippincott-Raven. Philadelphia, PA: 2010:117–127. 2010.
|
|
40
|
Klein JP and Moeschberger ML: Survival
analysis: Techniques for censored and truncated data. Springer. New
York: 83–109. 2003.
|
|
41
|
Romanuik TL, Ueda T, Le N, Haile S, Yong
TM, Thomson T, Vessella RL and Sadar MD: Novel biomarkers for
prostate cancer including noncoding transcripts. Am J Pathol.
175:2264–2276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jacobs JJ, Lehe C, Cammans KD, Yoneda K,
Das PK and Elliott GR: An automated method for the quantification
of immunostained human Langerhans cells. J Immunol Method.
247:73–82. 2001. View Article : Google Scholar
|
|
43
|
Wen Q, Liao HF, Yuan SB, Qiu XF, Zhuang GH
and Liu ZC: Expression and clinical significance of TNFAIP8 in
gastric tissues. Mian Yi Xue Za Zhi. 02:181–184. 2012.(In
Chinese).
|
|
44
|
Byrne DJ, Browning MC and Cuschieri A:
CA72-4: A new tumor marker for gastric cancer. Br J Surg.
77:1010–1013. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hamazoe R, Maeta M, Matsui T, Shibata S,
Shiota S and Kaibara N: CA72-4 compared with carcinoembryonic
antigen as a tumor marker for gastric cancer. Eur J Cancer.
28A:1351–1354. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ikeguchi M, Katano K, Saitou H, Tsujitani
S, Maeta M and Kaibara N: Pre-operative serum levels of CA72-4 in
patients with gastric adenocarcinoma. Hepatogastroenterology.
44:866–871. 1997.PubMed/NCBI
|
|
47
|
Sougioultzis S, Syrios J, Xynos ID,
Bovaretos N, Kosmas C, Sarantonis J, Dokou A, Tzivras D, Zografos
G, Felekouras E, et al: Palliative gastrectomy and other factors
affecting overall survival in stage IV gastric adenocarcinoma
patients receiving chemotherapy: A retrospective analysis. Eur J
Surg Oncol. 37:312–318. 2011. View Article : Google Scholar : PubMed/NCBI
|