Introduction
The radiotherapy and chemotherapy are the primary
treatment options for the majority of types of cancer (1). However, a variety of side effects are
associated with chemotherapeutic drugs, including
immunosuppression, discomfort of the gastrointestinal tract,
vomiting and inappetence, which may substantially impact patient
health and quality of life (2). The
majority of the anticancer drugs currently used in chemotherapy are
cytotoxic to normal cells, leading to unwanted side effects
(3). Therapeutically effective doses
of numerous types of anticancer drug may produce irreversible
changes in normal tissues (4).
Therefore, there is a required for cancer treatments which are able
to reduce the harmful side effects of anticancer drugs in normal
tissues. Combining herbal medicinal herbs with chemotherapy may
improve quality of life, tumor response and performance status, as
well as reduce the toxicity of chemotherapy (5). At present, certain medicinal herbs have
already attracted a attention due to their low toxicity and
purported curative effects.
Doxorubicin (Adriamycin®) is a widely
used anthraquinone anticancer drug, with significant dose-limiting
cardiac toxicity (6–8). In combination with other anticancer
drugs, it is used as first line therapy in malignant lymphoma,
sarcomas, cancer of the breast, lung, bladder and various other
cancer types (9). 5-Fluorouracil
(5-FU), a thymidylate synthetase enzyme inhibitor, is metabolized
intracellularly to 5-FU deoxynucleotide (5F-dUMP), which inhibits
deoxythymidylic acid synthetase, then prevents deoxyuridylic acid
(dUMP) from methylating to deoxythymidine monophosphate (dTMP),
which affects DNA synthesis (10).
5-FU is effective against cancers of the digestive system
(esophageal, stomach, intestinal cancer, carcinoma of pancreas,
liver cancer) and breast cancer (11). Furthermore, 5-FU may be effective in
treating cancer of the cervix, ovaries, bladder, head and neck, in
addition to chorioepithelioma (11).
Clinically doxorubicin and 5-FU (AF) are often combined; however,
their use is limited by cardiac and liver toxicity (12).
Abnormal Savda Munziq (ASMq) is an Uighur medicinal
herbal preparation that is widely used in the Xinjiang region of
China (13). ASMq consists of ten
medicinal herbs: Adiantum capillus-veneris L. Alhagi
pseudalhagi (Bieb.) Desv., Anchusa italica Retz.,
Cordia dichotoma G. Forst., Euphorbia maculata L.,
Foeniculum vulgare Mill., Glycyrrhiza glabra L.,
Lavandula angustifolia Mill., Melissa officinalis L.,
and Ziziphus jujuba Mill (14). ASMq has been applied to the
prevention and treatment of numerous chronic diseases, including
cancer, hypertension, diabetes mellitus and memory dysfunction
(15). ASMq has been used as a
traditional remedy for the prevention or treatment of digestive
cancer (16).
Previous pharmacological and clinical studies have
demonstrated that ASMq exhibits antioxidant effects, reducing
oxidative damage by free radicals (17), anti-DNA oxidative damage, and
inhibiting radiation-induced damage in mice (18–21). In
addition, ASMq has been shown to modulate cellular and humoral
immunity in a combined stress mouse model, protecting mitochondria
and DNA against damage induced by OH in a cell-free system
(17,22). Furthermore, ASMq is able to inhibit
cancer cell proliferation and viability in vitro (15,23,24), and
has exhibited anti-tumor properties in vitro (15,22–24) and
in rats (25). However, its
potential protective effects against toxicity induced by
doxorubicin and 5-FU have not been systematically evaluated.
Therefore, the aim of the present study was to investigate the
protective effect of ASMq against doxorubicin- and 5-FU-induced
toxicity in mice.
Materials and methods
Chemicals and reagents
ASMq was provided by Qikang Habo pharmaceutical Co.,
Ltd. (Xinjiang, China). Doxorubicin hydrochloride for injection
(adriamycin) was purchased from Pfizer Italia Srl Co., Ltd. (Rome,
Italy). 5-FU was purchased from JinYao Amino Acid Co., Ltd. (batch
no. 0912302; Tianjin, China). Kits for determining serum aspartate
aminotransferase (AST) and alanine aminotransferase (ALT)
activities, and total protein (TP), were obtained from the Xiamen
Jiaxing Biotechnology Co., Ltd. (Xiamen, China; cat. nos. C0010-2,
C009-2 and A045-2, respectively). Assay kits used for determination
of malondialdehyde (MDA) content, superoxide dismutase (SOD)
activity and glutathione peroxidase (GSH-Px) activity were obtained
from the Nanjing Jiancheng Institute of Biological Engineering
Institute (Nanjing, China; cat. nos. 201304010, 201004010 and
201304010, respectively).
Animals and treatment
A total of 50 Kunming mice (age, 4–6 weeks; weight,
20±2 g), including 25 male and 25 female mice, were supplied by the
Experimental Animal Centre of Xinjiang Medical University (Urumqi,
China). The present study was approved by the Ethics Committee of
the Xinjiang Medical University. Mice were housed in plastic cages
at room temperature (22±1°C) under a 12-h light/dark cycle and
provided with rodent chow and water ad libitum. A total of
50 mice were randomly divided into five groups (n=10 per group): i)
Normal control, orally received saline 0.2 ml/10 g for 14 days,
then intraperitoneally injected with 0.4 ml/10 g body weight saline
(normal group); ii) doxorubicin + 5-FU toxicity control mice were
intraperitoneally injected with doxorubicin (2.5 mg/kg) and 5-FU
(10 mg/kg) once in two days (doxorubicin + 5-FU group); and iii-v)
three groups of animals were treated with the 2, 4 or 8 g/kg ASMq
per day (ASMq.L, ASMq.M and ASMq.H groups, respectively) for 14
days, then intraperitoneally administered doxorubicin (2.5 mg/kg)
and 5-FU (10 mg/kg) once in two days (in 0.4 ml/10 g saline). Mice
were weighed and sacrificed by cervical dislocation following
intraperitoneal injection with 10% chloral hydrate (4 ml/kg;
Tianjin Fucheng Chemical Reagents Factory, Tianjin, China). Blood
and kidney, spleen, liver and heart tissue samples were collected.
Serum was separated for the hematological and biochemical assays.
Spleen and body weights were also measured, and the heart, liver,
kidney and spleen index was calculated as organ weight divided by
body weight. Subsequently, heart, liver, kidney and spleen tissues
were fixed in 10% buffered formalin (Sichuan Xilong Chemical
Industry Co., Ltd., Chengdu, China) for histopathological analysis.
The experiments were performed in accordance with local
institutional and governmental regulations on the use of
experimental animals.
Biochemical determinations
Serum biochemical markers of hepatic injury ALT, AST
and TP were assayed using commercial kits. The activities of AST
and ALT are expressed as an international unit (U/l).
Measurement of SOD, MDA and GSH-Px
levels in heart homogenate
Heart samples were homogenized in Tris-HCl buffer (5
mM, containing 2 mM EDTA; pH 7.4) resulting in 10% (w/v) liver
homogenate. Homogenates were then centrifuged at 191 × g for 10 min
at 4°C and the supernatants were used immediately for the
determination of antioxidant status. Activities of SOD and GSH-Px,
as well as the level of MDA, as an index of the extent of lipid
peroxidation in liver tissue, were determined using commercial
kits, according to the manufacturer's instructions. All samples
were assayed in triplicate. The content of MDA is expressed in
nmol, whereas SOD and GSH-Px activities are expressed as U/mg
protein. The protein content of the homogenates was determined
using a standard commercial kit (Nanjing Jiancheng Institute of
Biological Engineering Institute; cat. no. 20100420).
Histological investigation
After removal, samples of heart and liver tissue
were fixed in 10% buffered formalin. Samples were embedded in
paraffin and at least four 4–5 µm sections were produced from each
heart and liver and stained with hematoxylin and eosin (Beijing
SUOLAIBAO Technology, Co., Ltd., Beijing, China). Two changes (2
min xylene treatment each) were performed, and finally tissue
sections were mounted with DPX. The slides were observed for
histopathological changes and microphotographs were captured using
a BX50 microscope system (Olympus Corporation, Tokyo, Japan).
Statistical analysis
All data are presented as the mean ± standard error.
Statistical analyses were performed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). Differences between groups were assessed
using Student's t-test and general linear model univariate analysis
of variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of ASMq on heart, liver,
kidney and spleen indices
The body weight and heart, liver, kidney and spleen
weights were examined on the day of sacrifice, and organ indices
were calculated using the ratio of body weight to organ weight
(Tables I and II). Doxorubicin + 5-FU treatment
significantly reduced mouse body weight compared with the normal
group (P<0.05). In addition, treatment with low-, intermediate-,
and high-dose ASMq did not significantly increase the heart, liver
or kidney weights, as compared with the doxorubicin + 5-FU
treatment group (P>0.05). However, there was a significant
difference in the spleen and liver indices of the ASMq-treated
groups, as compared with the doxorubicin + 5-FU treatment group
(P<0.05), thus suggesting that ASMq showed a little therapeutic
effects to spleen and liver shrinking during doxorubicin and 5-FU
treatment. ASMq can restore the liver and spleen indices
proportionately, but it decreases kidney and heart indexes compared
with doxorubicin and 5-FU treatment, its reason still unknown
(Tables I and II).
 | Table I.Effect of ASMq on body and organ
weights of in mice (x̅±S). |
Table I.
Effect of ASMq on body and organ
weights of in mice (x̅±S).
| Group | Left kidney
(g) | Heart (g) | Spleen (g) | Liver (g) | Body (g) |
|---|
| Normal | 0.218±0.061 | 0.188±0.037 | 0.113±0.021 | 1.718±0.325 |
34.3±3.17 |
| A + 5-FU |
0.107±0.015a |
0.095±0.019a |
0.018±0.006a |
0.673±0.104a |
17.61±0.85a |
| ASMq.H | 0.108±0.013 | 0.097±0.021 | 0.026±0.010 |
0.853±0.109b |
19.22±1.66b |
| ASMq.M | 0.103±0.010 | 0.095±0.008 | 0.025±0.007 |
0.948±0.154b |
19.15±0.96b |
| ASMq.L | 0.098±0.009 | 0.081±0.014 | 0.017±0.006 | 0.673±0.085 | 18.57±1.17 |
 | Table II.Effect of ASMq on A + 5-FU-induced
changes in organ/body weight ratios (x̅±S). |
Table II.
Effect of ASMq on A + 5-FU-induced
changes in organ/body weight ratios (x̅±S).
| Group | Kidney/body weight
(mg/10 g) | Heart/body weight
(mg/10 g) | Spleen/body weight
(mg/10 g) | Liver/body weight
(mg/10 g) |
|---|
| Normal |
63±13 | 55±7 | 33±6 | 497±50 |
| A + 5-FU | 61±8 |
54±10 |
10±3a |
383±64a |
| ASMq.H | 56±6 | 50±8 | 13±5 |
443±37b |
| ASMq.M |
54±3b | 50±5 |
13±3b |
493±62b |
| ASMq.L |
53±3b |
44±8b |
9±3 | 363±43 |
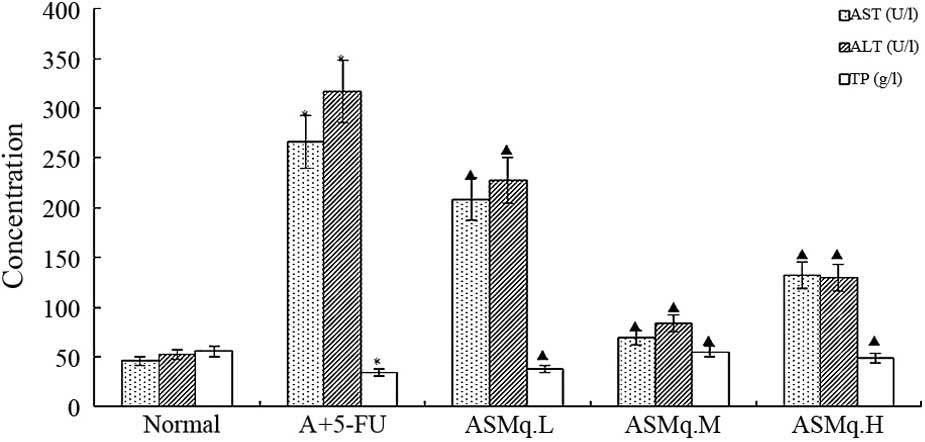
ALT, AST and TP concentrations
The concentrations of ALT and AST were significantly
increased and the TP level was significantly decreased in the blood
of doxorubicin + 5-FU mice compared with normal control mice,
indicating the failure of liver function due to doxorubicin +
5-FU-induced hepatotoxicity (Fig. 1)
(P<0.05). Treatment with ASMq significantly reduced the levels
of ALT and AST (P<0.05). The ASMq.M group was most similar to
the normal group. By contrast, a significant increase in TP content
(P<0.05) was produced by ASMq treatment as compared to the
doxorubicin + 5-FU group (Fig.
1).
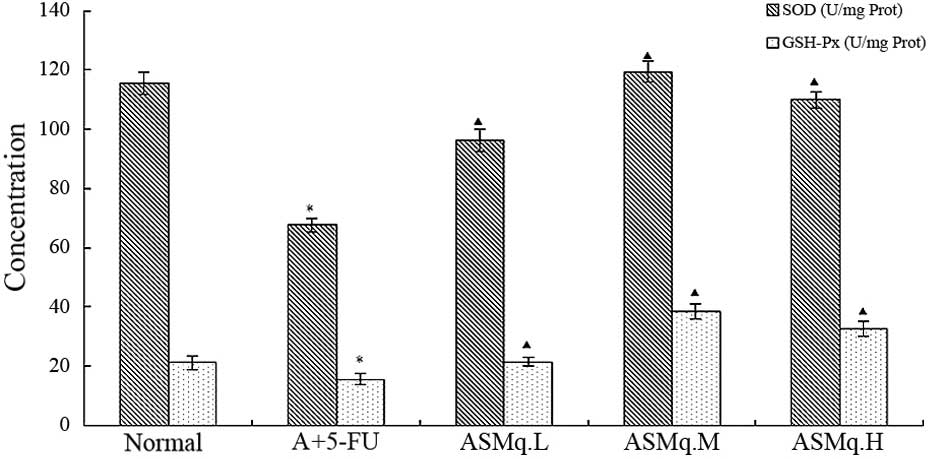
Effects of ASMq on heart homogenate
SOD activity, GSH-Px and MDA content in doxorubicin + 5-FU
mice
As oxidative stress contributes to the development
of doxorubicin + 5-FU-induced liver injury, the levels of the
antioxidative enzymes SOD and GSH-Px were measured. Levels of SOD
and GSH-Px were significantly decreased in the doxorubicin + 5-FU
group compared with the normal group (P<0.05) (Fig. 2). Pre-treatment with ASMq
significantly increased the SOD and GSH-Px levels as compared with
the mice that received doxorubicin + 5-FU treatment. Results showed
that the activities of SOD and GSH-Px were significantly increased
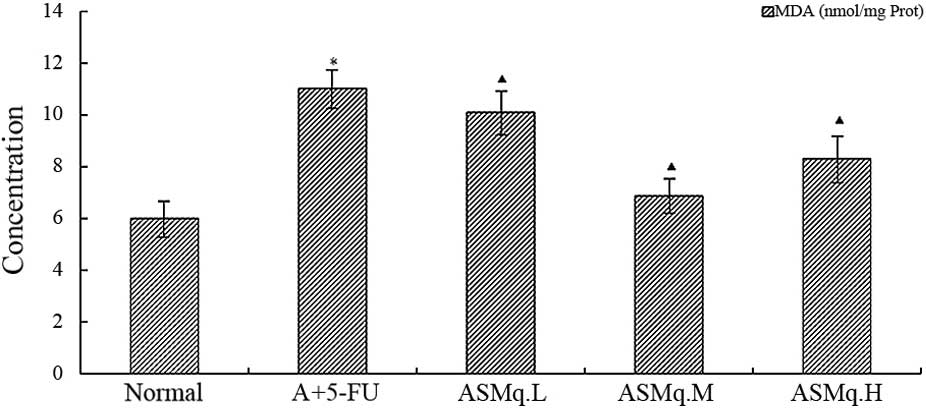
(P<0.05) by ASMq at the doses of 2, 4 and 8 g/kg (Fig. 2). MDA is an end-product of the
breakdown of polyunsaturated fatty acids and related esters, and
its formation is an index of lipid peroxidation in numerous organ
homogenates (26). Administration
with doxorubicin + 5-FU resulted in a significant increase in MDA
concentration when compared with the normal group (P<0.05).
However, ASMq significantly reduced MDA in the heart homogenate as
compared with doxorubicin + 5-FU-intoxicated group (P<0.05)
(Fig. 3).
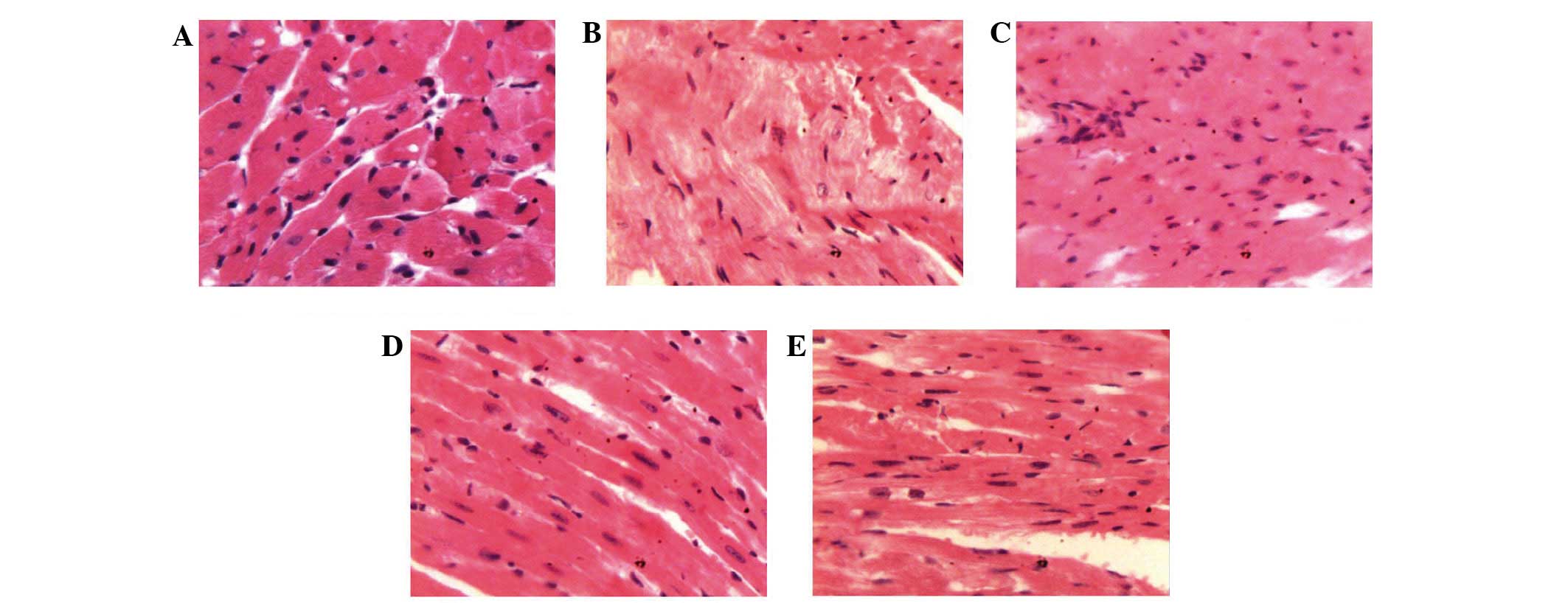
Histopathological analysis of mouse
hearts
As shown in Fig. 4,
histological analysis of the heart sections of normal group animals
showed normal cells with well-preserved cytoplasm, prominent
nucleus and nucleolus. Compared to normal group mice, extensive
necrosis and mineralization of cardiomyocytes combined with
cardiomyocyte vacuolation and myofibril breakage was observed in
the doxorubicin + 5-FU treated group. ASMq.M and ASMq.H mice showed
evidence of cardiomyocyte pathological changes, although in the
absence of cardiac edema, spotty necrosis, heart vacuolar
degeneration and abnormal myocardial interstitial changes (Fig. 4).
Histopathological analysis of mouse
livers
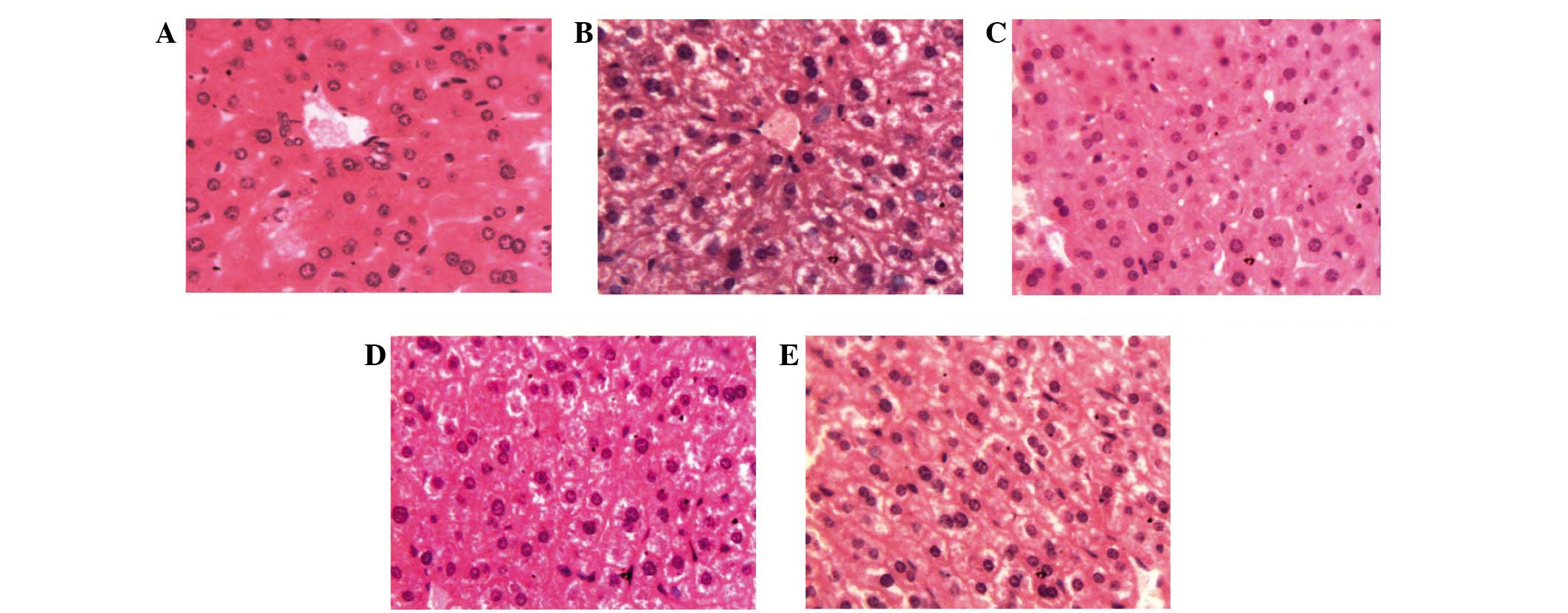
Liver histopathology was observed to determine the
protective effects of ASMq on doxorubicin + 5-FU mice at the
cellular level. Normal mice livers showed limpid central vein and
hepatic cells with prominent nuclei and uniform cytoplasm.
doxorubicin + 5-FU-treated mice liver sections showed vacuolization
of hepatocytes, sinusoidal dilation and congestion, infiltration of
cells, loss of cell boundaries and ballooning degeneration, loss of
architecture and cell necrosis. The histopathological changes were
prominent compared to those of mice in normal group. As
demonstrated by the liver histopathological observations, ASMq at
all doses showed reduced liver structure damage compared with the
doxorubicin + 5-FU group (Fig. 5).
Treatment with ASMq reduced the level of hepatic lesions induced by
doxorubicin + 5-FU. Photomicrographs indicated reduced damage of
liver tissue, on account of the absence of focal or bridging
necrosis. Doxorubicin + 5-FU-induced hepatic lesions were most
notably reversed by ASMq.M and ASMq.H, which appeared to be
comparable to the normal group. These histopathological
observations further suggest the hepatoprotective potential of ASMq
as an anti-cancer agent in vitro (Fig. 5).
Discussion
At present, chemotherapy is an crucial modality in
the treatment of cancer and in numerous instances may be the single
best agent for treatment (27).
However, toxic side effects of chemotherapy drugs may be a major
limitation to their effective use, in addition to affecting quality
of life (11). A key problem
associated with cancer chemotherapy are the severe side effects
resulting from normal tissue damage, including the heart, liver and
spleen (3). Increasing studies have
investigated approaches to improve the sensitivity of tumors to
chemotherapeutic drugs and to reduce the associated side effects
(27). Herbal drugs may offer an
alternative to chemotherapeutic compounds, and have been considered
to be non-toxic or less toxic, which has led to studies screen
herbal drugs for their protective ability of chemotherapy drug
toxicity (28,29).
The development of combination chemotherapy produced
new evidence of hepatotoxicity, and more instances can be
anticipated in the future (30).
Combination chemotherapy uses a number of chemotherapeutic agents,
each with a different mechanism of action and toxicity profile.
Along with the potential for greater tumor kill, however, the
possibility for enhanced toxicity occurs (31). Among combination therapies, 5-FU and
doxorubicin (sold under the brand name Adriamycin) is the most
prevalent combination (32). 5-FU, a
pyrimidine analogue, is a cytotoxic agent which is extensively used
in different solid tumors such as breast, lung and gastrointestinal
tract cancers (33,34). Doxorubicin, an anthracycline
glycoside, is commonly used as chemotherapeutic agent in various
malignant disorders; however, the possibility of the adverse effect
of irreversible cardiomyopathy limits its use (35). The hepatotoxic effects of anticancer
drugs may manifest as symptoms other than liver injury, such as
necrosis, steatosis, fibrosis, cholestasis and vascular injury
(36). 5-FU has been shown to
exhibit hepatotoxic effects, such as increased activity of
aminotransferases, lactate dehydrogenase and alkaline phosphatase,
which indicates hepatic damage (37).
The present results demonstrated that, following
administration of doxorubicin, the levels of AST and ALT were
elevated and were associated with idiosyncratic drug reactions,
including focal infiltration by inflammatory cells and steatosis on
liver biopsies. This was considered an idiosyncratic reaction.
Chemotherapeutic agents are extensively metabolized in the liver
and decrease the antioxidant capacity of the liver, including
decreasing glutathione production, which protects against free
radical injury (38). Doxorubicin
acts via DNA intercalation, alteration of membrane function and
free radical formation (39).
In the present study, injection of doxorubicin and
5-FU in mice resulted in decreased liver index and deterioration of
hepatic function, as indicated by elevations in ALT and AST and by
a significant reduction in total protein. In addition, the
examination of liver function correlated with the histopathological
changes observed by photomicroscopy. These results are consistent
with the previous reports on chemotherapy induced hepatotoxicity
(40,41). In the present study it was shown that
ASMq protected the liver tissue against damage induced by
doxorubicin and 5-FU combination. This protective effect was
indicated by a reduction in serum levels of ALT and AST and by a
significant elevation in total protein. However, ASMq showed
controversial effects to heart and kidney indices, and these
require further investigation. The results of the present study
indicated that ASMq is able to exert a protective against
doxorubicin and 5-FU induced hepatotoxicity. The histological
observations supported the results obtained from biochemical
analyses.
The clinical application of doxorubicin is
complicated by its potential toxicity in heart tissue (15,42,43). The
mechanism by which doxorubicin or its metabolites cause chronic
cardiomyopathy is not fully understood. Hypotheses regarding the
mechanism underlying this cardiac toxicity include perturbation of
calcium homeostasis, formation of iron complexes, generation of
radical oxygen species, mitochondrial dysfunction and damage to
cell membranes (44). The heart is
particularly vulnerable to injury from free radicals as it has
reduced levels of protective enzymes, such as superoxide dismutase,
compared with other tissues (45–47).
The pathophysiology of 5-FU-induced cardiotoxicity
is controversial, and conclusions are based on clinical studies and
case reports to a greater extent than on experimental evidence
(48). Furthermore, 5-FU
cardiotoxicity is suspected to be mediated by coronary vasospasm
and free radical damage to the myocardium (13,49).
Prior studies showed that the administration of doxorubicin caused
an increase in MDA levels, as well as reductions in GSH, SOD and
glutathione-S-transferase expression in treated rats compared with
a control group. Single injection of doxorubicin increased SOD,
MDA, NO and xanthine oxidase and myeloperoxidase expression in
kidney tissues in rats at 10 days after administration (50–52).
Previous approaches to reduce doxorubicin or 5-FU related
toxicities have centred on the use of antioxidants to minimize the
generation of reactive oxygen species.
Lipid peroxidation is an crucial mechanism in the
pathogenesis of cell damage (53).
MDA is the final product of cell membrane lipid peroxidation, and
thus its concentration may reflect the intensity of lipid
peroxidation (26). SOD and GSH-Px
are indicators of resistance to oxidative processes (54). SOD is able to specifically eliminate
free oxygen radicals by transforming surplus oxygen free radicals
into hydrogen peroxide, which is transformed by catalase and GSH-Px
into water, thus reducing free radical-mediated cell damage
(54). Furthermore, GSH-Px is an
important antioxidase (19–21), and may cause lipid peroxide to change
into a alcohol type fatty acid, to complement SOD.
Chemicals such as doxorubicin + 5-FU may induce
massive production of free radicals, which accumulate causing cell
toxicity and leading to lipid peroxidation of the cell membrane,
resulting in cell damage or death (55). The present study found that the
administration of doxorubicin and 5-FU was accompanied by signs of
oxidative damage, including reduced SOD and GSH-Px activity and
increased MDA. These results were consistent with previous studies
(56,57), and may be primary to the organ
toxicity, or a result of organ toxicity and cell death. ASMq
reduced the indicators of oxidative damage, increased SOD and
GSH-Px levels to values similar to normal, while partially
normalizing the increased levels of MDA. Furthermore, ASMq was
found to reverse or oppose the negative effects of doxorubicin +
5-FU on liver function and liver and heart morphology, which was
consistent with previous studies (20,21,58–60). In
the present study, significant differences were detected in the
heart homogenate levels of MDA, GSH-Px and SOD between normal
groups and doxorubicin + 5-FU treatment groups. ASMq exhibited a
significant effect (P<0.05) by increasing levels of SOD, GSH-Px
and by reducing MDA levels. However, the histological findings show
that of doxorubicin + 5-FU induced a degree of cardiotoxicity. The
severity of the histological change was notably reduced in sections
from animals treated with ASMq. The most marked effects were
observed in the ASMq.M (4 mg/kg) group, with moderate efficacy
shown by ASMq.H (8 mg/kg).
Future studies may be warranted involving whole
organisms treated with toxic chemotherapy, to assess the capacity
of ASMq to reduce the adverse reactions of chemotherapeutics that
may be dose- and treatment-limiting. As ASMq also has anti-tumoral
effects, it may be useful to monitor the beneficial and toxic
effects of this treatment. The presently observed reversal of a
number of the signs of toxicity associated with doxorubicin + 5-FU
by ASMq are consistent with its known anti-oxidative properties
in vitro, and warrant further exploratory in vivo
studies.
Acknowledgements
This study was supported by the Ministry of
Education Changjiang Scholar and Innovative Team Development
Program of China (grant no. IRT0977) and the National Natural
Science Foundation of China (grant no. 30260128).
References
|
1
|
Powathil GG, Adamson DJ and Chaplain MA:
Towards predicting the response of a solid tumour to chemotherapy
and radiotherapy treatments: Clinical insights from a computational
model. PLoS Comput Biol. 9:e10031202013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirose A, Sato E, Fujii H, Sun B, Nishioka
H and Aruoma OI: The influence of active hexose correlated compound
(AHCC) on cisplatin-evoked chemotherapeutic and side effects in
tumor-bearing mice. Toxicol Appl Pharmacol. 222:152–158. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dantzer R, Meagher MW and Cleeland CS:
Translational approaches to treatment-induced symptoms in cancer
patients. Nat Rev Clin Oncol. 9:414–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cleeland CS, Allen JD, Roberts SA, Brell
JM, Giralt SA, Khakoo AY, Kirch RA, Kwitkowski VE, Liao Z and
Skillings J: Reducing the toxicity of cancer therapy: Recognizing
needs, taking action. Nat Rev Clin Oncol. 9:471–478. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dong J, Su S, Wang M and Zhan Z: Shenqi
fuzheng, an injection concocted from Chinese medicinal herbs,
combined with platinum-based chemotherapy for advanced non-small
cell lung cancer: A systematic review. J Exp Clin Cancer Res.
29:1372010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arcamone F, Penco S and Vigevani A:
Adriamycin (NSC-123127): New chemical developments and analogs.
Cancer Chemother. 6:123–129. 1975.
|
|
7
|
Huang CH and Chiang CP: Bladder
instillation of adriamycin in the treatment of bladder cancer.
Cancer Chemother Pharmacol. 11(Suppl): S91–S93. 1983.PubMed/NCBI
|
|
8
|
Matsumura Y, Tsushima T, Ozaki Y,
Yoshimoto J, Akagi T, Obama T, Nash Y and Ohmori H: Intravesical
chemotherapy with 4′-epi-Adriamycin in patients with superficial
bladder tumors. Cancer Chemother Pharmacol. 16:176–177. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maksimenko A, Dosio F, Mougin J, Ferrero
A, Wack S, Reddy LH, Weyn AA, Lepeltier E, Bourgaux C, Stella B, et
al: A unique squalenoylated and nonpegylated doxorubicin
nanomedicine with systemic long-circulating properties and
anticancer activity. Proc Natl Acad Sci USA. 111:E217–E226. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nair KL, Jagadeeshan S, Nair SA and Kumar
GS: Biological evaluation of 5-fluorouracil nanoparticles for
cancer chemotherapy and its dependence on the carrier, PLGA. Int J
Nanomedicine. 6:1685–1697. 2011.PubMed/NCBI
|
|
12
|
Mitchell MS and Deconti RC:
Immunosuppression by 5-fluorouracil. Cancer. 26:884–889. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Upur H, Yusup A, Umar A and Moore N:
Uighur traditional medicine syndrome of Abnormal Savda in men is
associated with oxidative stress, which can be improved by Munziq
and Mushil of Abnormal Savda. Therapie. 59:483–484. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yusup A, Upur H, Umar A, Berke B, Yimit D,
Lapham JC, Moore N and Cassand P: Abnormal Savda Munziq, an Herbal
Preparation of Traditional Uighur Medicine, May Prevent
1,2-dimethylhydrazine-Induced Rat Colon Carcinogenesis. Evid Based
Complement Alternat Med. 2011:1520152011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yusup A, Upur H, Baudrimont I, Umar A,
Kader T, Begaud B, Creppy EE and Moore N: Cytotoxicity of abnormal
Savda Munziq aqueous extract in human hepatoma (HepG2) cells.
Fundam Clin Pharmacol. 19:465–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abliz G, Mijit F, Hua L, Abdixkur G,
Ablimit T, Amat N and Upur H: Anti-carcinogenic effects of the
phenolic-rich extract from abnormal Savda Munziq in association
with its cytotoxicity, apoptosis-inducing properties and telomerase
activity in human cervical cancer cells (SiHa). BMC Complement
Altern Med. 15:232015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yusup A, Upur H, Umar A and Moore N:
Protective effects of Munziq and Mushil of abnormal Savda to
mitochondrial oxidative damage. Fundam Clin Pharmacol. 18:471–476.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Parekh H, Advani S and Chitnis M: Bepridil
enhances adriamycin-induced DNA biosynthesis inhibition in human
myeloid leukemia cells. Sel Cancer Ther. 6:183–191. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gupta V, Lahiri S, Sultana S, Tulsawani R
and Kumar R: Anti-oxidative effect of Rhodiola imbricata root
extract in rats during cold, hypoxia and restraint (C-H-R) exposure
and post-stress recovery. Food Chem Toxicol. 48:1019–1025. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ku SK, Seo BI, Park JH, Park GY, Seo YB,
Kim JS, Lee HS and Roh SS: Effect of Lonicerae Flos extracts on
reflux esophagitis with antioxidant activity. World J
Gastroenterol. 15:4799–4805. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salo DC, Lin SW, Pacifici RE and Davies
KJ: Superoxide dismutase is preferentially degraded by a
proteolytic system from red blood cells following oxidative
modification by hydrogen peroxide. Free Radic Biol Med. 5:335–339.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Upur H, Yusup A, Baudrimont I, Umar A,
Berke B, Yimit D, Lapham JC, Creppy EE and Moore N: Inhibition of
cell growth and cellular protein, DNA and RNA synthesis in human
hepatoma (HepG2) cells by ethanol extract of abnormal Savda Munziq
of traditional Uighur medicine. Evid Based Complement Alternat Med.
2011:2514242011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yusup A, Upur H, Tursun X, Berke B,
Baudrimont I and Moore N: Study on mechanism of abnormal savda
munziq flavonoids in induction of apoptosis of Hep2 cells. Zhongguo
Zhong Yao Za Zhi. 32:1068–1071. 2007.(In Chinese). PubMed/NCBI
|
|
24
|
Yusup A, Upur H, Umar A, Berke B and Moore
N: Ethanol extract of abnormal Savda Munziq, a herbal preparation
of traditional Uighur medicine, inhibits Caco-2 cells proliferation
via cell cycle arrest and apoptosis. Evid Based Complement Alternat
Med. 2012:9263292012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yusup A, Upur H, Umar A, Berke B, Yimit D,
Lapham JC, Moore N and Cassand P: Abnormal Savda Munziq, an herbal
preparation of traditional Uighur medicine, may prevent
1,2-dimethylhydrazine-induced rat colon carcinogenesis. Evid Based
Complement Alternat Med. 2011:1520152011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sim AS, Salonikas C, Naidoo D and Wilcken
DE: Improved method for plasma malondialdehyde measurement by high
performance liquid chromatography using methyl malondialdehyde as
an internal standard. J Chromatogr B Analyt Technol Biomed Life
Sci. 758:337–344. 2003. View Article : Google Scholar
|
|
27
|
Gerber DE and Schiller JH: Maintenance
chemotherapy for advanced non-small-cell lung cancer: New life for
an old idea. J Clin Oncol. 31:1009–1020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hedigan K: Cancer: Herbal medicine reduces
chemotherapy toxicity. Nat Rev Drug Discov. 9:7652010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lam W, Bussom S, Guan F, Jiang Z, Zhang W,
Gullen EA, Liu SH and Cheng YC: The four-herb Chinese medicine
PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci
Transl Med. 2:45ra592010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Joensuu H, Holli K, Heikkinen M, Suonio E,
Aro AR, Hietanen P and Huovinen R: Combination chemotherapy versus
single-agent therapy as first- and second-line treatment in
metastatic breast cancer: A prospective randomized trial. J Clin
Oncol. 16:3720–3730. 1998.PubMed/NCBI
|
|
31
|
Floyd J, Mirza I, Sachs B and Perry MC:
Hepatotoxicity of chemotherapy. Semin Oncol. 33:50–67. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jassem J, Pieńkowski T, Płuzańska A, Jelic
S, Gorbunova V, Mrsic-Krmpotic Z, Berzins J, Nagykalnai T, Wigler
N, Renard J, et al: Doxorubicin and paclitaxel versus fluorouracil,
doxorubicin, and cyclophosphamide as first-line therapy for women
with metastatic breast cancer: Final results of a randomized phase
III multicenter trial. J Clin Oncol. 19:1707–1715. 2001.PubMed/NCBI
|
|
33
|
Ciccolini J, Mercier C, Blachon MF, Favre
R, Durand A and Lacarelle B: A simple and rapid high-performance
liquid chromatographic (HPLC) method for 5-fluorouracil (5-FU)
assay in plasma and possible detection of patients with impaired
dihydropyrimidine dehydrogenase (DPD) activity. J Clin Pharm Ther.
29:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Compagnon P, Thiberville L, Moore N,
Thuillez C and Lacroix C: Simple high-performance liquid
chromatographic method for the quantitation of 5-fluorouracil in
human plasma. J Chromatogr B Biomed Appl. 677:380–383. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou Q and Chowbay B: Determination of
doxorubicin and its metabolites in rat serum and bile by LC:
Application to preclinical pharmacokinetic studies. J Pharm Biomed
Anal. 30:1063–1074. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ishak KG and Zimmerman HJ: Morphologic
spectrum of drug-induced hepatic disease. Gastroenterol Clin North
Am. 24:759–786. 1995.PubMed/NCBI
|
|
37
|
Ray S, Roy K and Sengupta C: In vitro
evaluation of protective effects of ascorbic acid and water extract
of Spirulina plantesis (blue green algae) on 5-fluorouracil-induced
lipid peroxidation. Acta Pol Pharm. 64:335–344. 2007.PubMed/NCBI
|
|
38
|
Meredith MJ and Reed DJ: Depletion in
vitro of mitochondrial glutathione in rat hepatocytes and
enhancement of lipid peroxidation by adriamycin and
1,3-bib(2-chloroethyl)1-nitrosurea (BCNU). Biochem Pharmacol.
32:1383–1388. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Farrell GC: Drug-Induced liver disease.
Edinburgh: Churchill Livingstone. 389–412. 1994.
|
|
40
|
Mikalauskas S, Mikalauskiene L, Bruns H,
Nickkholgh A, Hoffmann K, Longerich T, Strupas K, Büchler MW and
Schemmer P: Dietary glycine protects from chemotherapy induced
hepatotoxicity. Amino Acids. 40:1139–1150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sréter I, Kiss A, Cornides A, Vereckei A,
Toncsev H and Fehér J: Inhibition of doxorubicin-induced liver
toxicity by a new dihydroquinoline type antioxidant. Acta Physiol
Hung. 64:431–435. 1984.PubMed/NCBI
|
|
42
|
Singal PK and Iliskovic N:
Doxorubicin-induced cardiomyopathy. N Engl J Med. 339:900–905.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Giri SN, Al-Bayati MA, Du X, Schelegle E,
Mohr FC and Margolin SB: Amelioration of doxorubicin-induced
cardiac and renal toxicity by pirfenidone in rats. Cancer Chemother
Pharmacol. 53:141–150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mordente A, Meucci E, Martorana GE,
Giardina B and Minotti G: Human heart cytosolic reductases and
anthracycline cardiotoxicity. IUBMB Life. 52:83–88. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Doroshow JH, Locker GY and Myers CE:
Enzymatic defenses of the mouse heart against reactive oxygen
metabolites: Alterations produced by doxorubicin. J Clin Invest.
65:128–135. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Keizer HG, Pinedo HM, Schuurhuis GJ and
Joenje H: Doxorubicin (adriamycin): A critical review of free
radical-dependent mechanisms of cytotoxicity. Pharmacol Ther.
47:219–231. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Myers C: The role of iron in
doxorubicin-induced cardiomyopathy. Semin Oncol. 25(4 Suppl 10):
10–40. 1998.PubMed/NCBI
|
|
48
|
Polk A, Vistisen K, Vaage-Nilsen M and
Nielsen DL: A systematic review of the pathophysiology of
5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol.
15:472014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ensley JF, Patel B, Kloner R, Kish JA,
Wynne J and al-Sarraf M: The clinical syndrome of 5-fluorouracil
cardiotoxicity. Invest New Drugs. 7:101–109. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu LL, Li QX, Xia L, Li J and Shao L:
Differential effects of dihydropyridine calcium antagonists on
doxorubicin-induced nephrotoxicity in rats. Toxicology. 231:81–90.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yagmurca M, Erdogan H, Iraz M, Songur A,
Ucar M and Fadillioglu E: Caffeic acid phenethyl ester as a
protective agent against doxorubicin nephrotoxicity in rats. Clin
Chim Acta. 348:27–34. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yilmaz S, Atessahin A, Sahna E, Karahan I
and Ozer S: Protective effect of lycopene on adriamycin-induced
cardiotoxicity and nephrotoxicity. Toxicology. 218:164–171. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yin H, Xu L and Porter NA: Free radical
lipid peroxidation: Mechanisms and analysis. Chem Rev.
111:5944–5972. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Y, Zhang L and Liang J: Activation of
the Nrf2 defense pathway contributes to neuroprotective effects of
phloretin on oxidative stress injury after cerebral
ischemia/reperfusion in rats. J Neurol Sci. 351:88–92. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
El-Sayyad HI, Ismail MF, Shalaby FM,
Abou-El-Magd RF, Gaur RL, Fernando A, Raj MHG and Ouhtit A:
Histopathological effects of cisplatin, doxorubicin and
5-flurouracil (5-FU) on the liver of male albino rats. Int J Biol
Sci. 5:466–473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Andersen HR, Nielsen JB, Nielsen F and
Grandjean P: Antioxidative enzyme activities in human erythrocytes.
Clin Chem. 43:562–568. 1997.PubMed/NCBI
|
|
57
|
Inal ME, Kanbak G and Sunal E: Antioxidant
enzyme activities and malondialdehyde levels related to aging. Clin
Chim Acta. 305:75–80. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gupta V, Lahiri SS, Sultana S, Tulsawani R
and Kumar R: Anti-oxidative effect of Rhodiola imbricata root
extract in rats during cold, hypoxia and restraint (C-H-R) exposure
and post-stress recovery. Food Chem Toxicol. 48:1019–1025. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Andersen HR, Nielsen JB, Nielsen F and
Grandjean P: Antioxidative enzyme activities in human erythrocytes.
Clinical Chem. 43:562–568. 1997.
|
|
60
|
Inal ME, Kanbak G and Sunal E: Antioxidant
enzyme activities and malondialdehyde levels related to aging. Clin
Chim Acta. 305:75–80. 2001. View Article : Google Scholar : PubMed/NCBI
|