Introduction
Osteoporosis is a disease characterized by low bone
mass and deterioration of bone structure that causes bone fragility
and increases the risk of fracture (1). Osteoporosis has become a major health
problem that is expected to worsen with aging populations
worldwide; the incidence is also rising in Asian countries. The
results of a randomized controlled trial in healthy postmenopausal
women, conducted by the Women's Health Initiative, has reported
that the health risks of hormone replacement therapy exceed the
benefits (2). Isoflavonoids are
increasing considered as a promising preventive treatment for
osteoporosis in a clinical setting (3). Of all the natural alternatives
currently undergoing investigation, Chinese medicines have received
considerable attention. Herba Epimedii (HEP), one of the foremost
Chinese anti-osteoporosis formulas (4), is an important traditional Chinese
herbal medicine used widely as a tonic, aphrodisiac and
antirheumatic in China and Korea. Icariin (ICA;
C33H40O15; molecular weight,
676.67 g/mol) is a flavonoid found in HEP and is considered to be
the major bioactive component (5).
Experiments in rats have indicated that ICA prevents bone loss and
reduction in femoral strength induced by ovariectomy (OVX)
(6,7).
As yet, the molecular mechanism of action of ICA in
the prevention of osteoporosis has not been investigated in
vivo. As a step towards addressing this, the present study
aimed to evaluate the effects of ICA in preventing osteoporosis and
ameliorating bone loss in rats subjected to OVX. The study also
attempted to elucidate the molecular mechanism of action of ICA in
OVX model rats. In particular, the β-catenin pathway, including
low-density lipoprotein receptor-related protein 6 (Lrp6), glycogen
synthase kinase-3β (GSK-3β) and runt-related transcription factor 2
(Runx2) was investigated.
Materials and methods
Animals and diets
A total of 84 specific-pathogen-free female
Sprague-Dawley rats, 3 months of age, were purchased (Animal Center
of Guangdong Province, Guangzhou, China) and acclimated to
laboratory conditions for 2 weeks prior to the study. The animals
were housed in an air-conditioned room at constant temperature
(23±2°C) and humidity (45–50%), with 12 h/12 h light-dark
illumination cycles. The animals were fed in the Laboratory Animal
Management Center of Jinan University Medical College (Guangzhou,
China). At the beginning of the study, the animals were 3 months of
age with an average weight of 250±20 g. The present study was
approved by the Animal Ethics Committee of Jinan University
(Guangzhou, China).
Chemicals
ICA (EPE-120215; purity, 98%) was purchased from
Changsha Nutramax Inc. (Changsha, China). Reagent kits for the
measurement of serum osteoprotegerin (OPG) and bone Gla protein
(BGP) were purchased from Cloud-Clone Corp., (Houston, TX, USA).
Reagent kits for the measurement of serum alanine aminotransferase
(ALT) and aspartate aminotransferase (AST) were purchased from
Sekisui Medical Co., Ltd. (Tokyo, Japan). Reagent kits for the
measurement of blood urea nitrogen (BUN) and creatinine (Cr) were
obtained from Mike Biotechnology Co., Ltd. (Sichuan, China).
Ovariectomy and administration of
ICA
The rats were randomly divided into two groups: The
sham-operated group (n=14) and the OVX group (n=70). Surgery was
performed under intraperitoneal pentobarbital sodium (0.15 ml/100
g) anesthesia. Bilateral ovariectomies were performed from the
midline with an abdominal skin incision ~1.5 cm long. The
sham-operated rats were subjected to surgery exposing the ovaries,
but these were not removed. After 3 months, the bone mineral
density (BMD) of the lumbar vertebrae and femurs of all rats was
determined using dual-energy X-ray absorptiometry (DEXA; Lunar
iDXA; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA)) to evaluate
whether the model had been successfully established.
Once it had been determined that the model was a
success, the OVX model rats were randomly divided into five groups,
each comprising 14 animals: OVX group, treated with 0.2 ml/100 g
double distilled water, daily, administered orally; positive group
treated with 5.04 mg/kg Fosamax (alendronate sodium; Merck Sharp
& Dohme, Kenilworth, NJ, USA) weekly, administered orally; and
three OVX-ICA groups, treated with 125, 250 or 500 mg/kg ICA,
daily, administered orally. The treatments were administered for 12
weeks. The 125, 250 and 500 mg/kg doses of ICA were designated as
low (L-ICA), medium (M-ICA) and high (H-ICA), respectively.
After 12 weeks of treatment, blood samples were
withdrawn from rats in all groups using the abdominal aorta method
to assess biochemical parameters. The animals were then sacrificed
using pentobarbital sodium, and the femurs, tibias and lumbar
vertebrae were dissected and stored in a freezer at −80°C.
Measurement of BMD
BMD (g/cm2) is log-normally distributed
for all individuals at the femoral neck but normally distributed at
the trochanter in men and postmenopausal women (8); therefore, the assessment of BMD can be
used to evaluate osteoporosis in the clinic. Following 12 weeks of
treatment, the BMD (g/cm2) was assessed using DEXA in
the small animal scan mode with the rats under intraperitoneal
pentobarbital sodium (0.15 ml/100 g) anesthesia. The BMD of the
fourth and fifth lumbar vertebrae and the femur was measured in all
rats.
Biomechanical parameters
The freshly isolated right femur and the fifth
lumbar vertebra were assessed using the three-point bending test
and the compression test (9).
Measurements were carried out using a precision biological
materials testing system (MTS MINI 858; MTS Systems Corp., Eden
Prairie, MN, USA) and the readings were recorded in Newtons.
Assessment of bone microstructure
using micro-computed tomography (CT)
In recent years, high resolution micro-CT has become
feasible for in vitro or in vivo evaluation of bone
architecture. In comparison with other imaging techniques, micro-CT
is more effective for detecting early bone changes that allow
fracture prediction and assessment of potential antiosteoporotic
agents (10). The freshly isolated
fourth lumbar vertebrae were assessed using micro-CT (µCT 80;
Scanco Medical AG, Brüttisellen, Switzerland). The fourth lumbar
vertebrae were placed in the micro-CT scanning device and scanned
images were obtained from different sections of the same specimen.
Each plane was 20 µm. Morphometric parameters, including trabecular
thickness (Tb.Th, µm), trabecular number (Tb.N, 1/mm) and
trabecular separation (Tb.Sp, µm) were measured using MedProject
4.1 software and bone tissue was reconstructed through 3D
images.
Serum parameters
ALT, AST, BUN and Cr were measured using an
automatic analyzer (Ciba-Corning 550; Ciba Corning Diagnostics,
East Walpole, MA, USA) with the appropriate diagnostic reagent kit.
Levels of OPG and BGP were determined using an enzyme-linked
immunosorbent assay reagent kit (Cloud-Clone Corp.).
Histopathology of femur bone
Femur samples were dehydrated through a graded
ethanol series, defatted with chloroform and subsequently hydrated
and decalcified for 1–2 weeks in formic acid. Following this, the
decalcified tissues were dehydrated using increasing concentrations
of ethanol, embedded in paraffin and cut into 4-mm-thick sections
along the femoral axis in the sagittal plane. Serial sections were
mounted on slides and dried overnight prior to staining with
hematoxylin and eosin (H&E) to observe pathological changes in
rats from each group. Images of the distracted zones were captured
under ×40 magnification using a light microscope (CFX41; Olympus
Corp., Tokyo, Japan).
RNA isolation, cDNA synthesis and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analysis
Femur samples were ground in liquid nitrogen using a
mortar and pestle to avoid RNA degradation. Extraction of total RNA
was performed using TRIzol according to the manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
purified RNA sample was incubated with QuantiNova gDNA Removal Mix
(Qiagen GmbH, Hilden, Germany) at 45°C for 2 min. Following genomic
DNA removal, 1 µg RNA was reverse transcribed to cDNA using a
QuantiNova reverse transcription kit (Qiagen GmbH). The total
reaction volume of 20 µl contained 15 µl RNA, 1 µl QuantiNova
Reverse Transcription Enzyme and 4 µl QuantiNova Reverse
Transcription Mix. Following a 3 min primer annealing step at 25°C,
the reaction was performed at 45°C for 10 min and subsequently
inactivated at 85°C. qPCR analysis was performed using a
LightCycler 480 instrument (Roche Diagnostics GmbH, Mannheim,
Germany) with a total reaction volume of 50 µl comprising 25 µl
SYBR green PCR master mix (Takara Bio, Inc., Otsu, Japan), 5 µl
cDNA, 2 µl each primer and 16 µl RNAase-free water. The qPCR
analysis was performed with an initial incubation temperature of
95.0°C for 5 min, followed by a two-step cycling protocol, with a
denaturation step at 95°C for 5 sec and a combined
annealing/extension step at 60°C for 30 sec, carried out for 40
cycles. Melting curve analysis was performed to determine the
product specificity. The respective primer sequences are shown in
Table I. As a control gene,
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) exhibits stable
and rich expression, therefore it was chosen to assess the
performance of the machine via the melting and amplification
curves. Expression levels were normalized against GAPDH according
to the 2−∆∆Cq method (11).
 | Table I.Primer sequences used in the reverse
transcription-quantitative polymerase chain reaction assay. |
Table I.
Primer sequences used in the reverse
transcription-quantitative polymerase chain reaction assay.
| Gene | Forward sequence
(5′ to 3′) | Reverse sequence
(5′ to 3′) |
|---|
| β-catenin |
ACTCTAGTGCAGCTTCTGGGTTCTG |
CTCGGTAATGTCCTCCCTGTCA |
| GSK-3β |
ACACCTGCCCTCTTCAACTTTACC |
ATTGGTCTGTCCACGGTCTCCA |
| Lrp6 |
GGAAGCCTAAGATTGACAGAGCG |
GTTGAGTCCGAGCATATTTGAAG |
| Runx2 |
AGCGGACGAGGCAACAGTTT |
CCTAAATCACTGAGGCGGTCAG |
| GAPDH |
CAACGGGAAACCCATCACCA |
ACGCCAGTAGACTCCACGACAT |
Western blot analysis
A cell lysis method was used to extract total
protein from the rat femoral heads and the bicinchoninic acid
method was used to measure the total protein concentration in each
group. Samples were run on a 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis gel and transfered onto
nitrocellulose membranes. Then, immunoblotting was assayed using
rabbit anti-β-catenin (1:2,000; 9562) and GAPDH (1:1,000; 2118;
both Cell Signaling Technology, Inc., Danvers, MA, USA) polyclonal
antibodies at 4°C for 12 h. Blots were visualized using horseradish
peroxidase (HRP)-conjugated anti-rabbit secondary antibody
(1:2,000; 7074S; Cell Signaling Technology, Inc.) at room
temperature for 1 h, with ECL Western Blotting Substrate (EMD
Millipore, Billerica, MA, USA) as the substrate of HRP. Bands were
detected and analyzed using ImageJ software (version 1.48; National
Institutes of Health, Bethesda, MA, USA)
Statistical analysis
Statistical analyses were performed using SPSS
software (version 19.0: IBM SPSS, Armonk, NY, USA). Data are
presented as mean value ± standard deviation (SD). The effects of
ICA in OVX rats were analyzed by one-way analysis of variance
followed by Dunnett's test (post hoc analysis). Differences between
two groups were analyzed using the independent t-test or
Mann-Whitney test (two-tailed), as appropriate. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of OVX and ICA on BMD
The BMD of OVX rats was significantly lower than
that of sham-operated rats (P<0.05). Following 12 weeks of
treatment, the BMD of the OVX model rats treated with ICA was
significantly higher than that of OVX rats (Table II). Compared with the OVX group, the
BMD of the lumbar vertebrae, right femur and left femur
significantly increased (P<0.01) in the positive group; whereas
the BMD of the right and left femurs significantly increased
(P<0.01 and P<0.05, respectively) in the L-ICA group. In the
M-ICA group, the lumbar vertebrae, right femur and left femur BMD
significantly increased (P<0.01); and, in the H-ICA group, the
BMD of the right and left femurs significantly increased (P<0.01
and P<0.05, respectively). The BMD of the OVX model rats treated
with ICA were significantly increased, as compared with the OVX
rats. The M-ICA was most effective in comparison with the positive
group (Table II).
 | Table II.Effects of OVX and ICA on BMD 12
weeks after surgery (g/cm2). |
Table II.
Effects of OVX and ICA on BMD 12
weeks after surgery (g/cm2).
| A, Effect of OVX on
BMD |
|---|
|
|---|
| Group | Lumbar
vertebrae |
| Right femur | Left femur |
|---|
| Sham | 0.285±0.009 |
| 0.340±0.020 | 0.310±0.013 |
|---|
| OVX |
0.212±0.006a |
|
0.262±0.006a |
0.247±005a |
|---|
|
| B, Effect of ICA on
BMD in OVX rats |
|
| Group | Lumbar
vertebrae | Right femur | Left femur | Whole body |
|
| Sham | 0.266±0.026 | 0.274±0.034 | 0.255±0.030 | 0.184±0.003 |
| OVX |
0.196±0.009b |
0.185±0.010b |
0.194±0.010b |
0.174±0.007a |
| Positive |
0.261±0.024c |
0.263±0.038c |
0.260±0.045c |
0.176±0.005a |
| L-ICA | 0.226±0.037 |
0.257±0.020c |
0.249±0.030d |
0.173±0.006a |
| M-ICA |
0.240±0.029c |
0.254±0.017c |
0.285±0.029c |
0.174±0.006a |
| H-ICA |
0.218±0.027a |
0.243±0.016c |
0.251±0.044d |
0.171±0.007a |
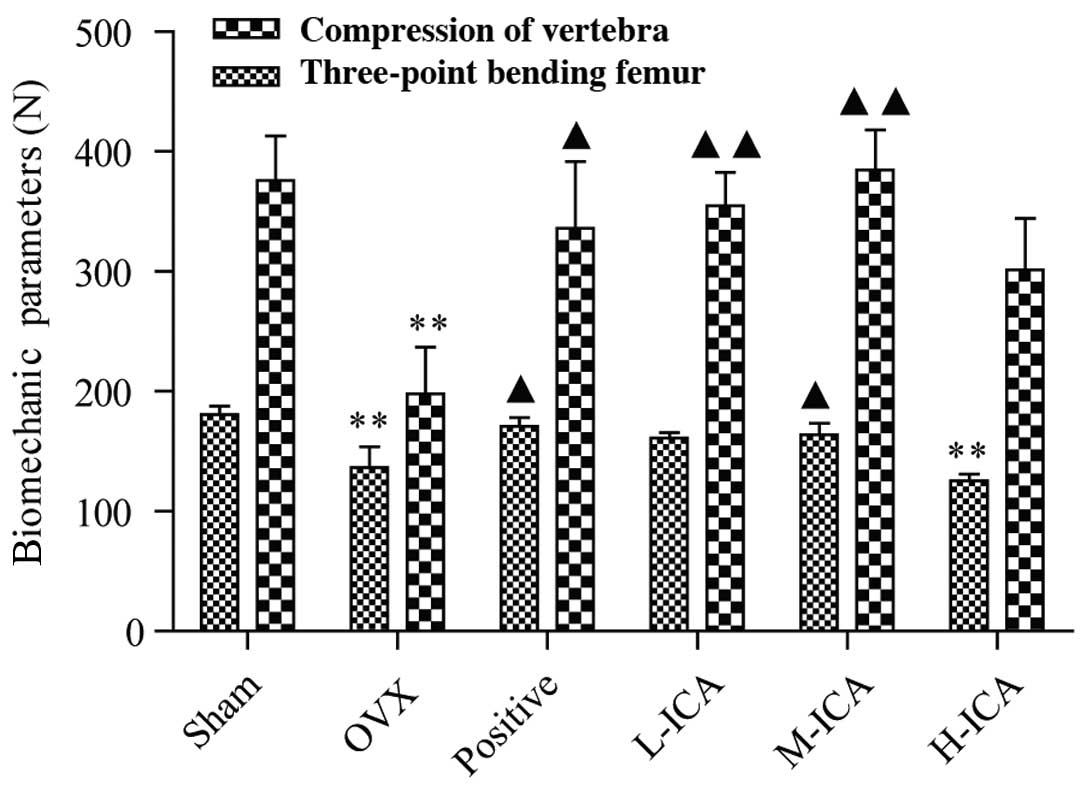
Biomechanical tests
The bone strengths of the femurs and vertebrae,
which were assessed by the three-point bending test and the
compression test, respectively, decreased significantly in the OVX
group compared with the sham-operated group (P<0.01). Following
the administration of ICA to the OVX model rats, the biomechanical
strength increased significantly and was comparable to that of the
sham-operated group (Fig. 1).
Following 12 weeks of treatment, as compared with the OVX group,
vertebral and femoral strength significantly increased (P<0.05)
in the positive group, vertebral strength increased significantly
(P<0.01) in the L-ICA group, and femoral and vertebral strength
increased significantly (P<0.05 and P<0.01, respectively) in
the M-ICA group. M-ICA was most effective in comparison with the
positive group.
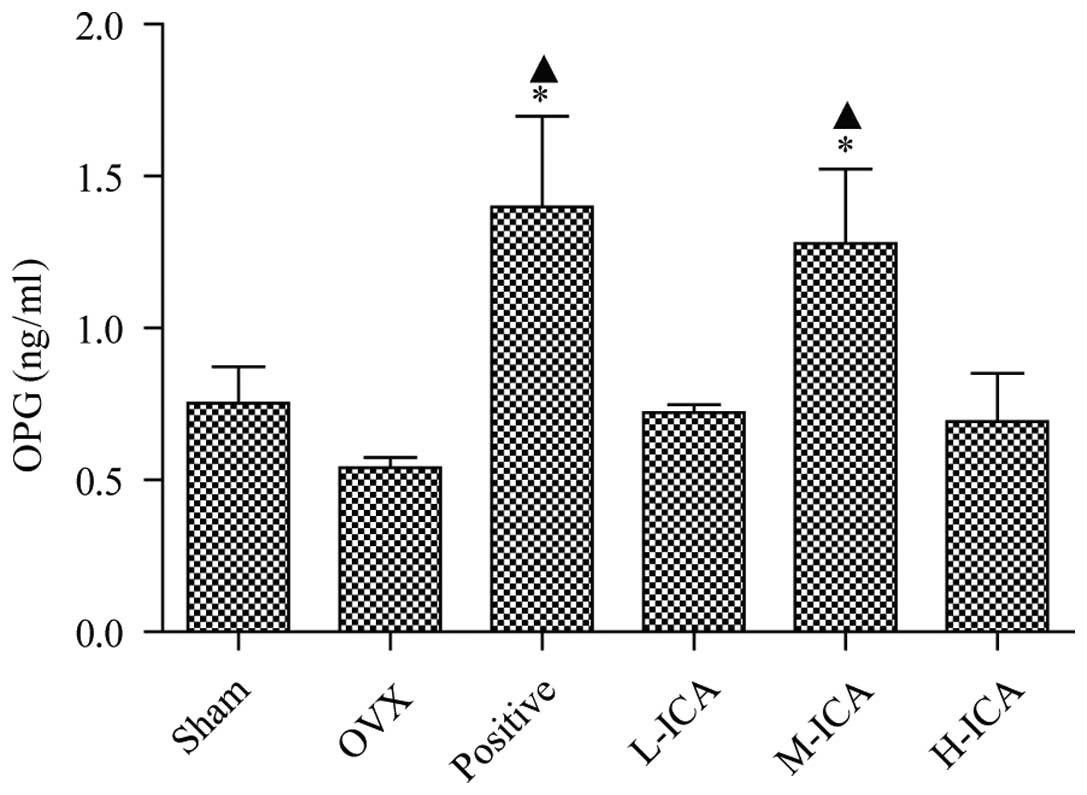
Effect of OVX and ICA on OPG and BGP
serum biochemical indicators
OPG levels in sham-operated and OVX rats were
0.752±0.121 and 0.541±0.034 ng/ml, respectively. OPG levels were
significantly increased (P<0.05) to 1.28±0.244 ng/ml following
treatment with M-ICA (Fig. 2). BGP
levels in the sham-operated group were 4,328±0.476 ng/ml. As
compared with the sham-operated group, BGP levels were
significantly increased (P<0.05) to 5,983±0.645 ng/ml in the OVX
group; however, they were significantly decreased (P<0.05) to
1.713±0.202 ng/ml in the positive group. As compared with the OVX
group, BGP levels in the positive, L-ICA, M-ICA and H-ICA groups
were significantly decreased (P<0.05) to 1.713±0.202,
4.436±0.477, 4.055±0.273 and 3.939±0.269 ng/ml, respectively
(Fig. 3).
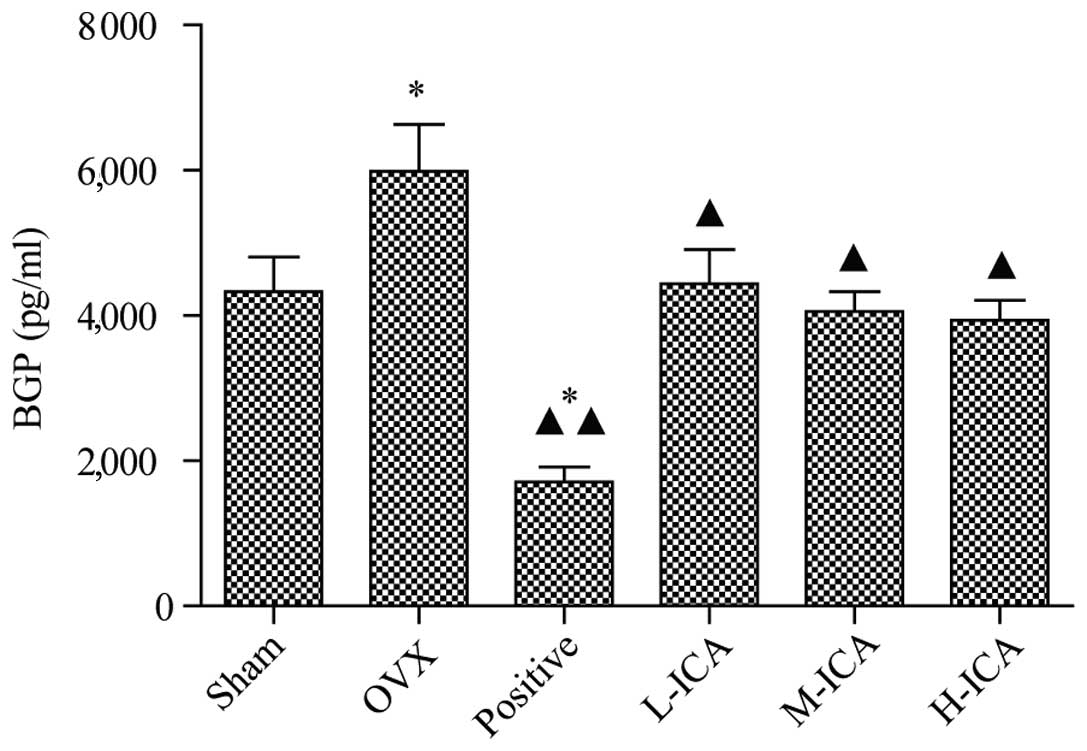 | Figure 3.BGP levels in the six groups. Compared
with the sham-operated group, BGP levels in the OVX group increased
significantly (P<0.05). Compared with OVX group, levels of BGP
in the positive group (P<0.01), and the L-ICA, M-ICA and H-ICA
groups were significantly reduced (P<0.05). *P<0.05 vs.
sham-operated; ▲P<0.05 and ▲▲P<0.01 vs.
OVX. BGP, bone Gla protein; OVX, ovariectomy; ICA, icariin; Sham,
sham-operated; Positive, treated with alendronate sodium; L-ICA,
low dose ICA (125 mg/kg/day); M-ICA, medium dose ICA (250
mg/kg/day); H-ICA, high dose ICA (500 mg/kg/day). |
Effects of OVX and ICA on liver and
kidney function
Following 12 weeks of treatment, BUN and ALT were
significantly increased (P<0.05), as compared with the
sham-operated group. As compared with the OVX group, M-ICA
successfully restored BUN and ALT to normal levels (P<0.05),
whereas H-ICA significantly increased Cr (P<0.05). Liver and
kidney function index tests demonstrated the M-ICA group exhibited
reduced liver and kidney damage, as compared with the OVX group
(Table III).
 | Table III.Effect of ICA on liver and kidney
function in OVX rats after 12 weeks. |
Table III.
Effect of ICA on liver and kidney
function in OVX rats after 12 weeks.
| Group | BUN | Cr | ALT | AST |
|---|
| Sham |
4.84±0.69 |
35.20±8.64 |
51.00±12.98a |
81.75±18.25 |
| OVX |
6.64±1.85b |
32.00±13.74 |
87.80±11.90b |
121.40±71.90 |
| Positive |
6.84±1.19 |
35.78±6.53 |
53.00±9.68a |
101.±7.49 |
| L-ICA |
5.40±1.04 |
36.00±4.76 |
68.80±31.59 |
104.40±41.4 |
| M-ICA |
5.19±0.73a |
37.17±3.37 |
41.86±13.20a |
75.71±8.28 |
| H-ICA |
6.05±0.68b |
41.14±5.70a |
59.59±29.97 |
63.66±29.08a |
Morphometric measurements
Morphological observations were quantitated by the
histomorphometric analysis of longitudinal cross-sections collected
2 cm away from the proximal vertebra using micro-CT. Marked bone
loss was observed in the OVX rats compared with the sham-operated
group (Fig. 4A). This bone loss was
accompanied by a significant reduction in Tb.Th and Tb.N together
with a significant increase in Tb.Sp (P<0.01). Treatment with
M-ICA normalized Tb.Th, Tb.N and Tb.Sp compared with the OVX group.
Compared with the sham-operated group, in the positive group and
the H ICA group, Tb.Th was significantly reduced (P<0.01) but
Tb.Sp was significantly increased (P<0.05). In the L-ICA group,
Tb.N decreased (P<0.05). In the L-ICA group, Tb.N decreased
(P<0.05). Compared with the sham-operated group, the bone
trabecula was looser in the OVX group. Following 12 weeks of
treatment, as compared with the OVX group, the bone trabecula was
more dense in the positive group and ICA groups, suggesting that
ICA may recover bone trabecula to varying levels. M-ICA was most
effective in comparison with the positive group (Fig. 4B).
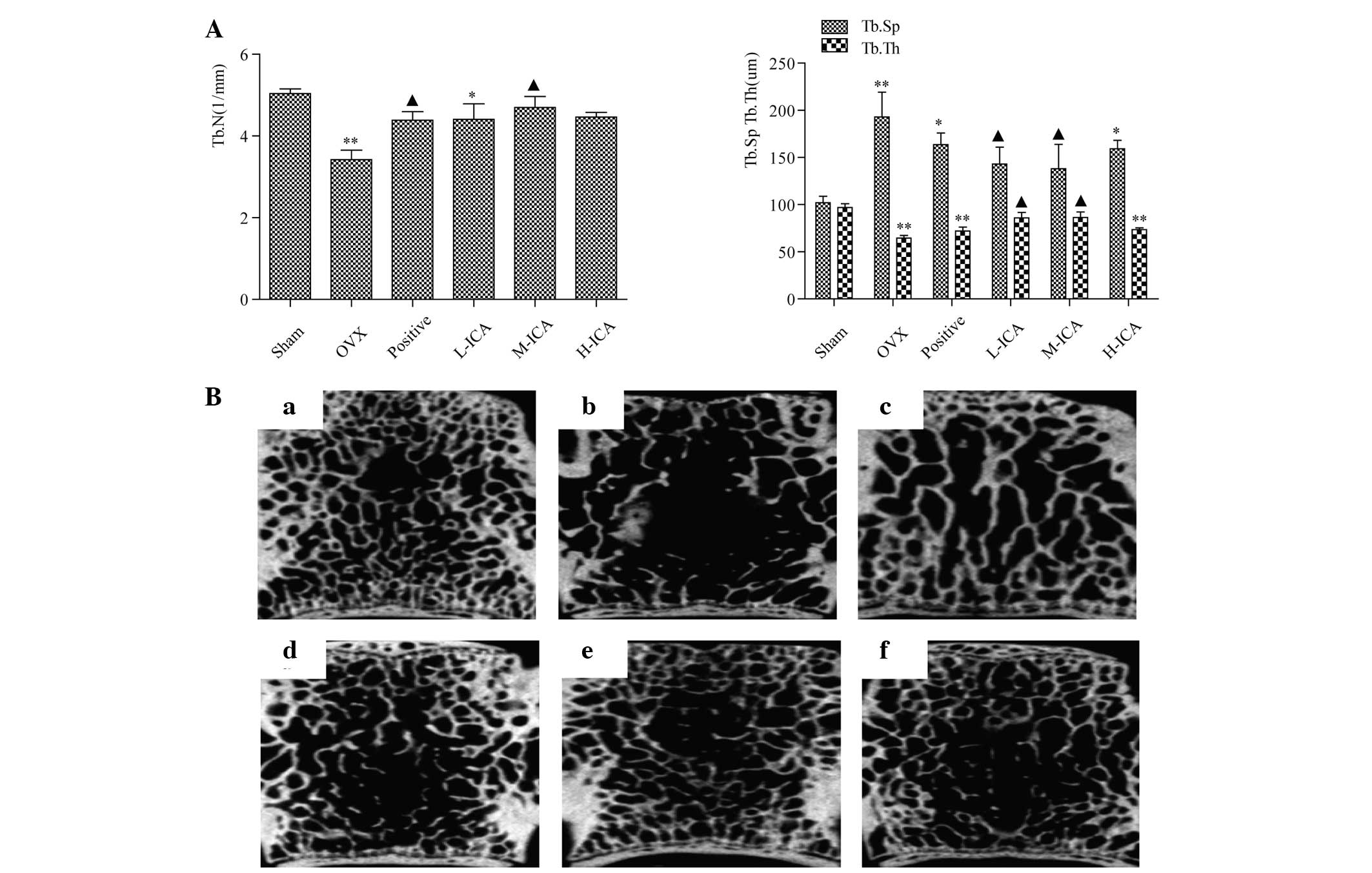 | Figure 4.Morphometric analysis. (A) Tb.N and
Tb.Th decreased significantly in the OVX group compared with the
sham group (P<0.01). Compared with the OVX group, the M-ICA
group had significantly increased Tb.N and Tb.Th (P<0.05), while
Tb.Sp decreased significantly (P<0.05), whereas in the L-ICA
group, Tb.Th increased significantly and Tb.Sp decreased
significantly (P<0.05). *P<0.05, **P<0.01 vs.
sham-operated; ▲P<0.05 vs. OVX. (B) Micro-computed
tomography analysis for the (a) sham, (b) OVX, (c) positive, (d)
L-ICA, (e) M-ICA and (f) H-ICA groups. In comparison with the sham
group, the bone trabecula is loose, fractured and exhibits loss in
the OVX group. Following oral administration of ICA, the number of
bone trabecula was clearly increased and the bone trabecula was
more densely concatenated than that in the OVX group. Tb.N,
trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular
separation; OVX, ovariectomy; ICA, icariin; Sham, sham-operated;
Positive, treated with alendronate sodium; L-ICA, low dose ICA (125
mg/kg/day); M-ICA, medium dose ICA (250 mg/kg/day); H-ICA, high
dose ICA (500 mg/kg/day). |
Histopathology of femural bone
Histopathological changes in the femur were examined
at the end of the 12-week study period. Representative histological
sections of femur tissue were stained with H&E. Tissue from the
sham-operated group revealed normal compactness of the diaphysis
and a competent trabecular meshwork (Fig. 5A). Tissue from the OVX group showed
sparse, uniform thinning of the trabeculae, resulting in widened
inter-trabecular spaces (Fig. 5B).
In the positive group, the trabecular was significantly recovered
(Fig. 5C). Tissues from the OVX-ICA
groups exhibited significant restoration of structure, with
mineralization. Uniform trabeculae with dense matrices and shaft
sizes were also observed (Fig.
5D–F). Histomorphological parameters were within normal limits
and complete restoration of the trabecular meshwork was observed.
Bone from the M-ICA group (Fig. 5E)
was essentially the same as normal bone.
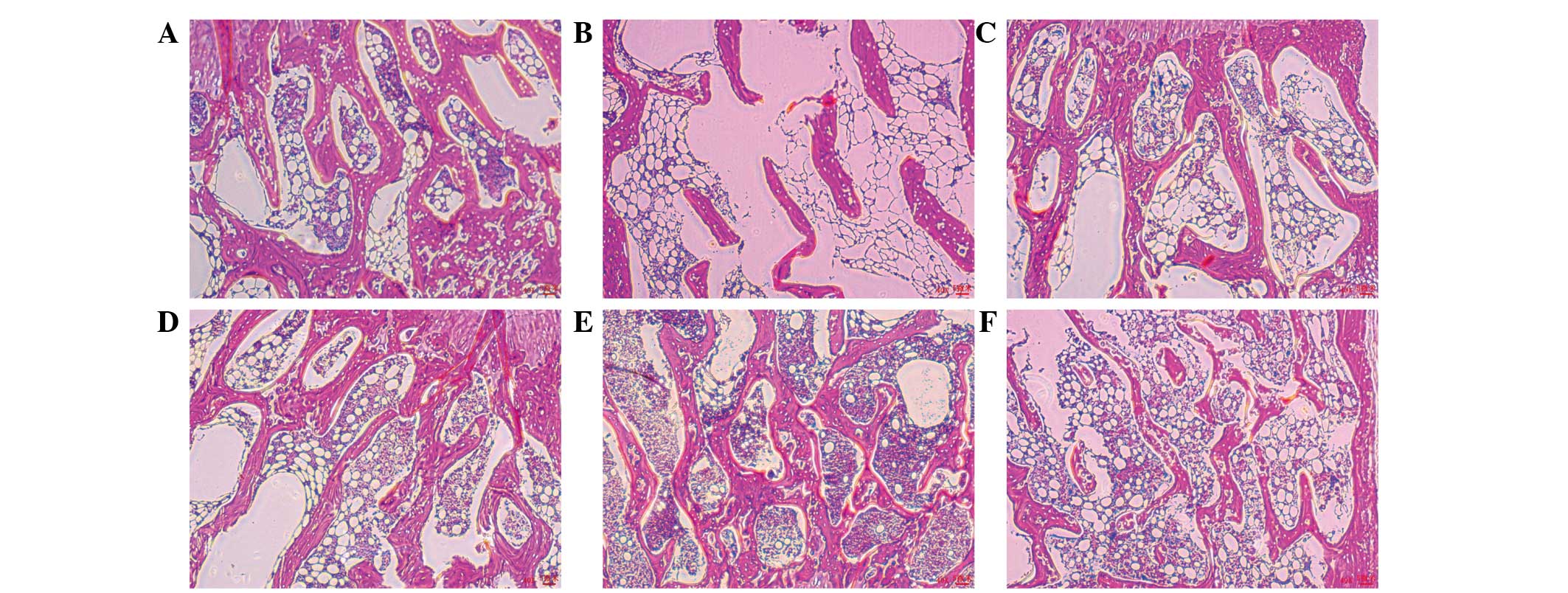 | Figure 5.Histopathology of femurs from the six
groups. (A) Sham, (B) OVX (C) positive, (D) L-ICA, (E) M-ICA and
(F) H-ICA groups. Hematoxylin and eosin staining (magnification,
×40). OVX, ovariectomy; ICA, icariin; Sham, sham-operated;
Positive, treated with alendronate sodium; L-ICA, low dose ICA (125
mg/kg/day); M-ICA, medium dose ICA (250 mg/kg/day); H-ICA, high
dose ICA (500 mg/kg/day)..ask for letters to be removed. |
Effect of ICA on expression of
β-catenin, GSK-3β and Runx2 and Lrp6 mRNA
The expression levels of β-catenin, Runx2 and Lrp6
mRNA were reduced in the OVX animals compared with their respective
levels in the sham-operated group (P<0.05; Fig. 6). Levels of β-catenin, Runx2 and Lrp6
mRNA in the M-ICA group were significantly increased in comparison
with those in the OVX group (P<0.05). The levels of GSK-3β were
significantly increased in the OVX group compared with those in the
sham-operated group (P<0.05). Furthermore, treatment with M-ICA
significantly reduced the expression of GSK-3β compared with that
in the OVX group (P<0.05).
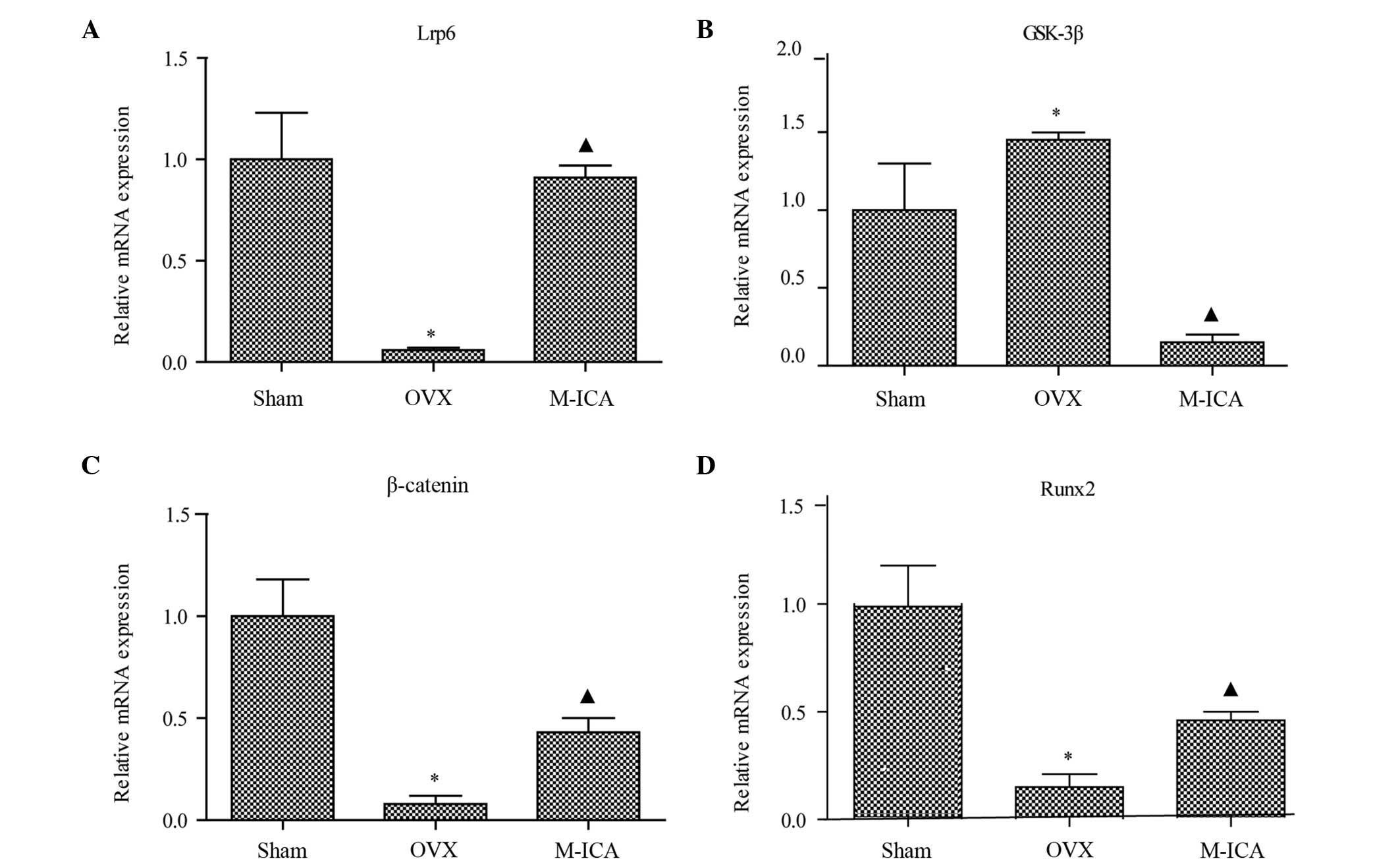 | Figure 6.mRNA expression levels determined by
reverse transcription-quantitative polymerase chain reaction.
Expression levels of (A) Lrp6, (B) GSK-3β, (C) β-catenin and (D)
Runx2 mRNA. *P<0.05 vs. sham; ▲P<0.05 vs. OVX.
Lrp6, low-density lipoprotein receptor-related protein 6; GSK-3β,
glycogen synthase kinase-3β; Runx2, runt-related transcription
factor 2; OVX, ovariectomy; ICA, icariin; Sham, sham-operated;
Positive, treated with alendronate sodium; L-ICA, low dose ICA (125
mg/kg/day); M-ICA, medium dose ICA (250 mg/kg/day); H-ICA, high
dose ICA (500 mg/kg/day). |
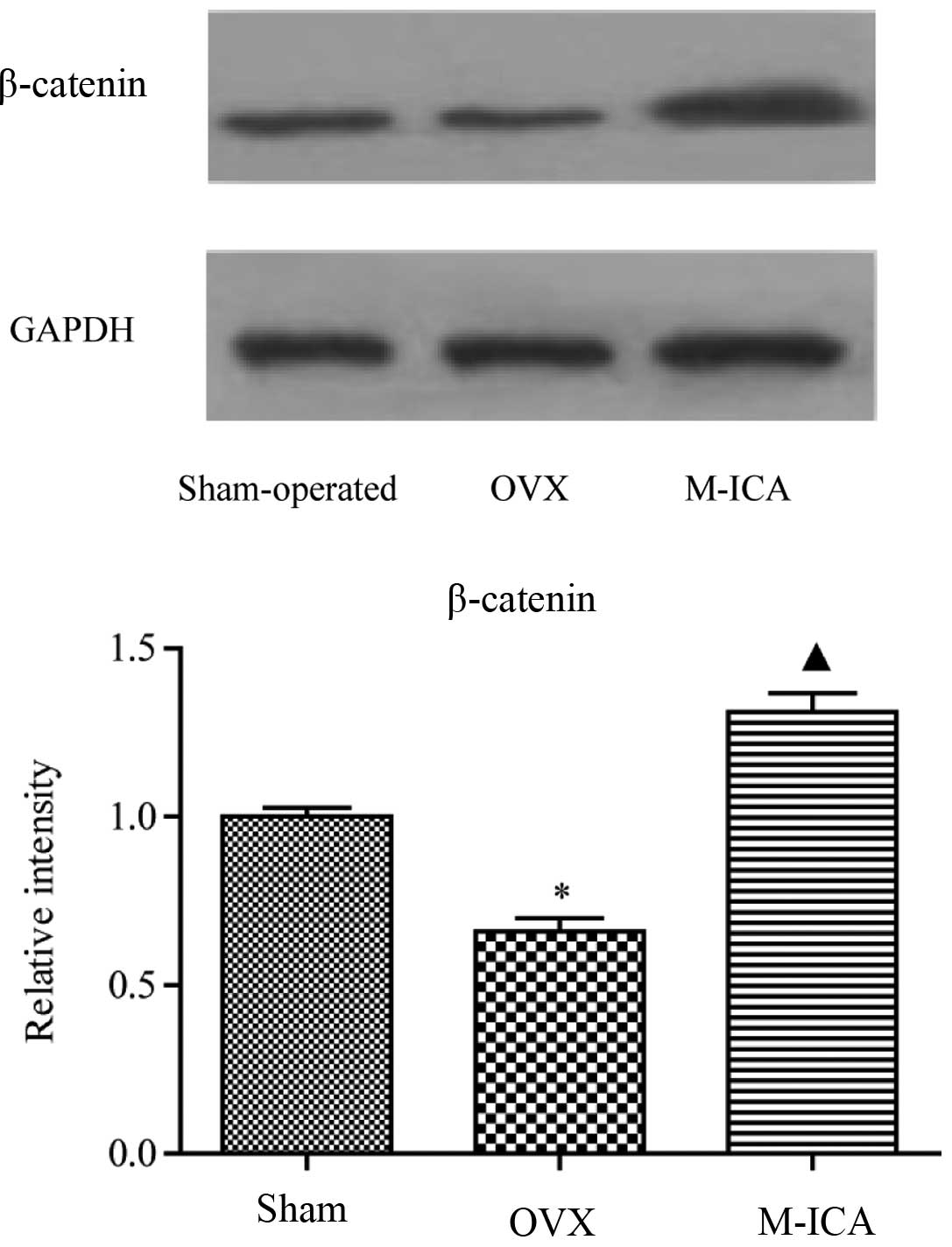
Effect of ICA on expression levels of
β-catenin protein in OVX rats
As shown in Fig. 7,
following bilateral removal of the ovaries, expression of β-catenin
was significantly decreased (P<0.05). Treatment with M-ICA led
to significantly increased levels of β-catenin compared with those
in OVX rats (P<0.05).
Discussion
There has been much interest in the potential of
phytoestrogen flavonoids as an alternative therapy for improving
bone formation and for preventing or treating osteoporosis. ICA, a
prenylated flavonol glycoside isolated from HEP, has been shown to
prevent osteoporosis in late postmenopausal women (12) and has also been shown to have a
strong estrogen-like activity (13).
The main index for evaluating the effect of
treatments on postmenopausal osteoporosis (POP) is the increase in
BMD. Estrogen deficiency, in both OVX rats and in postmenopausal
women, can lead to a reduction in BMD (14,15). In
the present study, treatment with ICA significantly improved BMD
and effectively prevented the loss of bone mass caused by the
OVX-induced lack of estrogen.
The bone changes observed in the OVX rats, most
notably a decline in BMD, are similar to those in postmenopausal
osteoporotic women. ICA treatment significantly increased BMD in
the OVX rats and can effectively prevent bone loss and be effective
in the treatment of osteoporosis in the present study.
Biomechanical testing showed that less force was
needed to fracture the femur and vertebrae in OVX rats compared
with sham-operated animals. Administration of ICA greatly improved
the biomechanical properties, as demonstrated by the increased
fracture force required to break the femur and vertebrae in OVX
rats. Bone strength is associated with BMD, architecture,
connectivity and mineralization (16). The results of the present study are
consistent with those of this earlier study, in which ovariectomy
resulted in reduced BMD as well as decreased biomechanical
strength.
In the OVX rats, the bone tissue pathology and the
morphology of bone microstructure changed markedly. Bone
microstructure is an important factor affecting the quality of
bone. Both bone microstructure and bone mass together determined
bone strength. In OVX rats, the trabecular bone was clearly reduced
in thickess and length, with a fine rod- or button-shaped
morphology. Parts of the trabecular bone were destroyed, or even
disappeared, and there were significant lesions in the secondary
spongy bone area. The number of trabecular surface absorption
pouches increased noticeably and there was a visible osteoclast
(OC) reaction. Treatment with ICA reduced trabecular bone lesions,
particularly in the secondary spongy areas, and reduced the number
of bone resorption pouches and OCs. ICA can effectively improve
BMD, increase trabecular bone numbers and thickness, and reduce
their degree of separation.
The present study shows that an imbalance in the
physiological processes of bone turnover, with loss of equilibrium
between the activity of osteoblasts (OBs) and OC, contributes to
the development of osteoporosis. Therapy at a molecular level
should mainly aim to correct the imbalance between bone resorption
and bone formation and thus protect skeletal integrity and reduce
the risk of fractures. A previous study found that ICA strongly
enhanced the differentiation and mineralization of OB in
vitro (17), but the
antiosteoporotic mechanism of ICA in vivo was not
investigated. The present study indicates that ICA has the ability
to correct the imbalance between bone resorption and bone formation
in vivo.
Changes in serum biochemical indicators reflect the
balance of bone metabolism in the body. These changes can be used
to evaluate bone conversion, and to objectively measure bone
resorption and bone formation. OPG and BGP are widely accepted as
phenotypic markers of bone formation (18). OPG is a key cytokine secreted by OB
that plays an important role in bone remodeling (19,20). A
negative correlation has been observed between OPG and BMD
(21). BGP is regarded as a marker
of bone turnover and serum levels of BGP have been shown to
increase with increasing age (22).
OPG produced by OB plays an essential role in the formation,
differentiation and bone-resorbing activity of OC (23). It has been shown in the present study
that OVX rats have reduced OPG activity compared with sham-operated
animals. Administration of ICA to OVX rats upregulated OPG activity
and downregulated BGP activity. In addition, the M-ICA dose did not
cause liver or kidney damage.
ICA has been reported to enhance fracture healing
(24), and treatment of OVX rats
with ICA has been shown to ameliorate POP (6,25,26). The
main cause of osteoporosis is an imbalance between bone resorption
and bone formation; healthy bones are required to maintain a
dynamic balance between OB and OC for appropriate bone remodeling
(27).
The Wnt receptors Lrp5/6 and Frizzled, when
activated, promote phosphorylation of GSK-3β and block
phosphorylation of β-catenin. Thus, the degradation of β-catenin is
reduced and an increase in cytoplasmic and nuclear levels of
β-catenin promotes pairing with the TCF/LEF family of transcription
factors and activates the Wnt/β-catenin signaling pathway (28,29).
Using RT-qPCR, the present study found that expression levels of
Lrp6, β-catenin and Runx2 mRNA decreased significantly in OVX rats,
while the expression of GSK-3β mRNA increased significantly.
Western blot analysis of the tibia showed that β-catenin expression
also decreased significantly in OVX rats at the protein level.
Treatment with ICA significantly increased the expression of Lrp6,
β-catenin and Runx2 mRNA and decreased the expression of GSK-3β
mRNA. Upregulation of Lrp6 receptor should promote GSK-3β mRNA
phosphorylation and increase the stability of intracellular
β-catenin. Aggregation of β-catenin and transfer to the nucleus
will activate the Wnt/β-catenin signaling pathway. This then
activates the target gene, Runx2, which promotes OB proliferation
and differentiation and inhibits OC differentiation (30–32).
An ideal antiostoporotic agent should restore the
balance between bone resorption and bone reconstruction, enhance
bone strength and BMD, increase Th.N and reduce bone loss. The
present in vivo study demonstrated that ICA effectively
increased the BMD of the fourth and fifth vertebrae of the lumbar
spine and femur in OVX rats. In addition, lumbar spinal Tb.N and
Tb.Th were increased and Tb.Sp was reduced by ICA treatment. Bone
strength was enhanced and bone loss was reduced. ICA was thus
indicated to be an effective treatment for POP, that did not cause
liver and kidney damage when used at an effective dosage. The
action of ICA as an antiosteoporotic agent in OVX rats has been
shown to involve upregulation of the expression of Lrp6 receptor
mRNA and downregulation of GSK-3β mRNA. This promotes GSK-3β mRNA
phosphorylation and increases the stability of intracellular
β-catenin. The β-catenin aggregates and is transferred to the
nucleus, where it activates the Wnt/β-catenin signaling pathway.
The downstream target gene, Runx2, is then activated, promoting OB
proliferation and differentiation and inhibiting OC differentiation
and bone resorption. This restores the balance between bone
resorption and bone reconstruction, enhances bone strength and BMD,
increases Th.N and reduces bone loss, all of which contribute to
the treatment of osteoporosis.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81473509
and 81503384), the Cultivation for Scientific Research of First
Affiliated Hospital of Jinan University (grant no. 2015103),
Guangdong Provincial Natural Science Foundation (grant no.
S2012040007531), the Cultivation and Innovation Fund for Scientific
Research of Jinan University Youth Fund Project (grant no.
21612341), the Fundamental Research Funds for the Central
Universities (grant no. 21614309), the Medical Scientific Research
Foundation of Guangdong Province (grant no. B2014227) and the
administration of Traditional Chinese Medicine of Guangdong
Province (grant no. 20151178).
References
|
1
|
Kelly PJ: Is osteoporosis a genetically
determined disease? Br J Obstet Gynaecol. 103(Suppl 13): 20–27.
1996.PubMed/NCBI
|
|
2
|
Rossouw JE, Anderson GL, Prentice RL,
LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA,
Howard BV, Johnson KC, et al: Writing Group for the Women's Health
Initiative Investigators: Risks and benefits of estrogen plus
progestin in healthy postmenopausal women: Principal results from
the Woman's Health Initiative randomized controlled trial. JAMA.
288:321–333. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma DF, Qin LQ, Wang PY and Katoh R: Soy
isoflavone intake inhibits bone resorption and stimulates bone
formation in menopausal women: Meta-analysis of randomized
controlled trials. Eur J Clin Nutr. 62:155–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao SQ, Fu DX and Zhang HM: Advances in
the study on the treatment of osteoporosis with Herba Epimedii and
its compound prescriptions. China J Chin Mater Med. 24:249–251.
1999.
|
|
5
|
Li C, Li Q, Mei Q and Lu T:
Pharmacological effects and pharmacokinetic properties of icariin,
the major bioactive component in Herba Epimedii. Life Sci.
126:57–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nian H, Ma MH, Nian SS and Xu LL:
Antiosteoporotic activity of icariin in ovariectomized rats.
Phytomedicine. 16:320–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mok SK, Chen WF, Lai WP, Leung PC, Wang
XL, Yao XS and Wong MS: Icariin protects against bone loss induced
by oestrogen deficiency and activates oestrogen receptor-dependent
osteoblastic functions in UMR 106 cells. Br J Pharmacol.
159:939–949. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pearson J, Dequeker J, Reeve J, Felsenberg
D, Henley M, Bright J, Lunt M, Adams J, Diaz Curiel M and Galan F:
Dual X-ray absorptiometry of the proximal femur: normal European
values standardized with the European Spine Phantom. J Bone Miner
Res. 10:315–324. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fradinho MJ, Vale AC, Bernardes N,
Caldeira RM, Vaz MF and Ferreira-Dias G: Biomechanical properties
of the equine third metacarpal bone: In vivo quantitative
ultrasonography versus ex vivo compression and bending techniques.
J Equine Vet Sci. 35:198–205. 2015. View Article : Google Scholar
|
|
10
|
Effendy NM, Khamis MF and Shuid AN:
Micro-CT assessments of potential anti-osteoporotic agents. Curr
Drug Targets. 14:1542–1551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noguchi A, Nakamura K, Sakata K,
Sato-Fukuda N, Ishigaki T, Mano J, Takabatake R, Kitta K, Teshima
R, Kondo K and Nishimaki-Mogami T: Development and interlaboratory
validation of a simple screening method for genetically modified
maize using a DeltaDeltaCq-based multiplex real-time PCR assay.
Analytical Chem. 88:4285–4293. 2016. View Article : Google Scholar
|
|
12
|
Zhang G, Qin L and Shi Y:
Epimedium-derived phytoestrogen flavonoids exert beneficial effect
on preventing bone loss in late postmenopausal women: A 24-month
randomized, double-blind and placebo-controlled trial. J Bone Miner
Res. 22:1072–1079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye HY and Lou YJ: Estrogenic effects of
two derivatives of icariin on human breast cancer MCF-7 cells.
Phytomedicine. 12:735–741. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gambacciani M and Levancini M: Hormone
replacement therapy and the prevention of postmenopausal
osteoporosis. Przeglad Menopauzalny. 13:213–220. 2014.PubMed/NCBI
|
|
15
|
Agata U, Park JH, Hattori S, Aikawa Y,
Kakutani Y, Ezawa I, Akimoto T and Omi N: The impact of different
amounts of calcium intake on bone mass and arterial calcification
in ovariectomized rats. J Nutr Sci Vitaminol (Tokyo). 61:391–399.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Einhorn TA: Bone strength: The bottom
line. Calcif Tissue Int. 51:333–339. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma HP, Ming LG, Ge BF, Zhai YK, Song P,
Xian CJ and Chen KM: Icariin is more potent than genistein in
promoting osteoblast differentiation and mineralization in vitro. J
Cell Biochem. 112:916–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Evans DB, Bunning RA and Russell RG: The
effects of recombinant human interleukin-1 beta on cellular
proliferation and the production of prostaglandin E2, plasminogen
activator, osteocalcin and alkaline phosphatase by osteoblast-like
cells derived from human bone. Biochem Biophys Res Commun.
166:208–216. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hofbauer LC, Khosla S, Dunstan CR, Lacey
DL, Boyle WJ and Riggs BL: The roles of osteoprotegerin and
osteoprotegerin ligand in the paracrine regulation of bone
resorption. J Bone Miner Res. 15:2–12. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie GQ, Lei DD, He HB, Gong JJ, Chen C,
Chen P, Zhang H, Luo XH, Liao EY and Wu XP: Relationship between
serum TGF-β1, OPG levels and osteoporotic risk in native Chinese
women. Clinica Chim Acta. 423:116–121. 2013. View Article : Google Scholar
|
|
22
|
Plantalech L, Guillaumont M, Vergnaud P,
Leclercq M and Delmas PD: Impairment of gamma carboxylation of
circulating osteocalcin (bone gla protein) in elderly women. J Bone
Miner Res. 6:1211–1216. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwan Tat S, Padrines M, Théoleyre S,
Heymann D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: Interrelations
in bone resorption pathophysiology. Cytokine Growth Factor Rev.
15:49–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin L, Zhang G, Hung WY, Shi Y, Leung K,
Yeung HY and Leung P: Phytoestrogen-rich herb formula ‘XLGB’
prevents OVX-induced deterioration of musculoskeletal tissues at
the hip in old rats. J Bone Miner Metab. 23(Suppl): S55–S61. 2005.
View Article : Google Scholar
|
|
25
|
Qian G, Zhang X, Lu L, Wu X, Li S and Meng
J: Regulation and of Cbfa1 expression by total flavonoids of Herba
epimedii. Endocr J. 53:87–94. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mok SK, Chen WF, Lai WP, Leung PC, Wang
XL, Yao XS and Wong MS: Icariin protects against bone loss induced
by oestrogen deficiency and activates oestrogen receptor-dependent
osteoblastic functions in UMR 106 cells. Br J Pharmacol.
159:939–949. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahmed Y, Nouri A and Wieschaus E:
Drosophila Apc1 and Apc2 regulate Wingless transduction throughout
development. Development. 129:1751–1762. 2002.PubMed/NCBI
|
|
29
|
Liu C, Li Y, Semenov M, Han C, Baeg GH,
Tan Y, Zhang Z, Lin X and He X: Control of β-Catenin
phosphorylation and degradation by a dual-kinase mechanism. Cell.
108:837–847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cui Y, Niziolek PJ, MacDonald BT, Zylstra
CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon
RA, et al: Lrp5 functions in bone to regulate bone mass. Nat Med.
17:684–691. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan Y, Tang D, Chen M, Huang J, Xie R,
Jonason JH, Tan X, Hou W, Reynolds D, Hsu W, et al: Axin2 controls
bone remodeling through the beta-Catenin-BMP signaling pathway in
adult mice. J Cell Sci. 122:3566–3578. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gambardella A, Nagaraju CK, O'Shea PJ,
Mohanty ST, Kottam L, Pilling J, Sullivan M, Djerbi M, Koopmann W,
Croucher PI and Bellantuono I: Glycogen synthase kinase-3α/β
inhibition promotes in vivo amplification of endogenous mesenchymal
progenitors with osteogenic and adipogenic potential and their
differentiation to the osteogenic lineage. J Bone Miner Res.
26:811–821. 2011. View
Article : Google Scholar : PubMed/NCBI
|