Introduction
Despite recent advances in oncologic therapies,
cancer remains a significant cause of morbidity and mortality
worldwide (1). Lung cancer is the
most common cancer worldwide, and non-small cell lung carcinoma
accounts for ~80% of all lung cancers (2). Surgical excision is the foundation of
treatment for this type of cancer; however, metastatic disease is
the most common cause of cancer-associated mortality (3–5).
It has previously been demonstrated that anesthetics
and anesthesia techniques have an impact on the invasive and
migratory ability of cancer cells, and may possibly affect the
long-term prognosis of patients who have undergone cancer surgery
(3). Pain management is a mandatory
procedure in patients with cancer, since it improves the patient's
quality of life and compliance to therapy (6). Opioids, particularly morphine,
represent a mainstay of treatment for postoperative pain and for
many types of chronic pain, including pain associated with cancer
(6–8). However, previous studies have suggested
that morphine analgesia may lead to a reduction in the activity and
number of natural killer cells, which may weaken the immunologic
barrier function and promote the differentiation of T helper (Th)1
lymphocytes into Th2 lymphocytes (9–11).
Furthermore, previous preclinical data has suggested that morphine
is proangiogenic and thus promotes cancer cell growth (12). One recent epidemiological study
demonstrated that the replacement of postoperative opioids with
epidural analgesia successfully reduced the risk of biochemical
cancer recurrence following prostatectomy surgery, suggesting that
opioids may favor cancer recurrence (13). Therefore, the selection of
appropriate analgesics is vital in order to decrease the risk of
metastasis and improve the quality of life of patients with cancer
(14).
Oxycodone hydrochloride is a semi-synthetic opioid
agent extracted from the alkaloid thebaine (15). The affinity of oxycodone
hydrochloride for the µ-opioid receptor is one-fifth to
one-fortieth that of morphine, however, it is able to fully
activate the κ-opioid receptor (15,16).
Although oxycodone possesses similar analgesic effects to morphine,
its effects on the growth, apoptosis and migration of cancer are
yet to be elucidated. The present in vitro study compared
the effects of morphine and oxycodone on the proliferation,
apoptosis and migration of the A549 human lung cancer cell
line.
Materials and methods
Methods
The A549 human lung cancer cell line was provided by
the Department of Oncology, Jinling Hospital, Nanjing University
(Nanjing, China). RPMI 1640 medium was purchased from HyClone
Laboratories (GE Healthcare Life Sciences, Logan, UT, USA). Fetal
calf serum (FCS), trypsin, penicillin and streptomycin were
obtained from Gibco (Thermo Fisher Scientific Inc., Waltham, MA,
USA). Oxycodone hydrochloride was purchased from Mundipharma
Pharmaceutical Co., Ltd. (Cambridge, UK) and morphine hydrochloride
was purchased from Shenyang First Pharmaceutical Factory of
Northeast Pharmaceutical Group Co., Ltd. (Shenyang, China). The
kits for apoptosis detection, human vascular endothelial growth
factor (VEGF) and urokinase-type plasminogen activator (uPA) were
purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China).
The PCR primers were synthesized by Invitrogen (Thermo Fisher
Scientific Inc.) and the polymerase chain reaction (PCR) Master mix
reagents were purchased from Promega Corporation (Madison, WI,
USA). Intercellular cell adhesion molecule (ICAM)-1 antibody was
purchased from Nanjing Dizhao Biological Technology Co., Ltd.
(Nanjing, China).
Drug preparation
The oxycodone hydrochloride and morphine
hydrochloride preparations were diluted to the desired
concentrations with culture media under sterile conditions.
Cell culture
A549 cells were cultured in RPMI 1640 medium
supplemented with 10% FCS, 100 U/µl penicillin and 100 g/µl
streptomycin, and incubated at 37°C in an atmosphere containing 5%
CO2. Once the cells were confluent, they were digested
with 0.25% trypsin and passaged in order to maintain cells in a
logarithmic phase of growth. Cell proliferation was observed under
an inverted microscope (Olympus Corporation, Tokyo, Japan) every 24
h and cells were routinely passaged every 2–3 days.
Methyl thiazolyl tetrazolium
assay
The cells were digested and seeded in 96-well plates
at a concentration of 1×105 cells/µl, with the exception
of the outermost wells, which were filled with sterile saline
solution. The cells were then washed twice with phosphate-buffered
saline (PBS), and the study agents were diluted to the desired
concentrations using culture solution and added to each well to a
total volume of 100 µl. The control and blank control wells were
set up and 15 µl methyl thiazolyl tetrazolium solution (5 mg/µl;
Biocam GmbH, Berlin, Germany) was added to each well 48 h after
adding the study drugs. The control well indicates the cells
without adding the drug, while the blank control was exposed to
culture solution only. After 4 h, dimethyl sulfoxide (150 µl) was
added to each well, and the plate was agitated for 10 min in a
shaker, in order to dissolve the crystals. Optical density (OD) was
measured at 490 nm using the enzyme-linked immunosorbent assay
method, and the cell inhibition rate was subsequently calculated
for each treatment group.
Flow cytometry
Cell apoptosis was analyzed using an Annexin
V-Fluorescein Isothiocyanate (FITC) Apoptosis Detection kit (BD
Pharmingen, San Diego, CA, USA), according to the manufacturer's
protocol.
In the logarithmic growth phase, the A549 cells were
seeded into 6-well plates (2 µl/well; 1×105 cells/µl),
cultured in an incubator for 24 h and the supernatant was discarded
prior to the addition of the drug-containing media to the
appropriate wells. Subsequently, the 6-well plate was incubated for
48 h, and the cells were collected by digestion with trypsin. The
cells were then washed twice with PBS, centrifuged at 375 × g for 5
min and resuspended in 500 µl binding buffer. A total of 5 µl
Annexin V-FITC was added and mixed into the cell suspension,
followed by the addition of 5 µl propidium iodide and subsequent
mixing. The reaction was incubated for 5–15 min in a dark room. The
early apoptotic cells were detected by flow cytometry within 1
h.
Scratch assay
Lines were drawn on the back of a 6-well plate with
a marker pen; the transverse lines were drawn uniformly at
distances of 0.5–1 cm between the lines using a ruler, and the
lines passed through the wells. Each well was subsequently
inoculated with ~5×105 cells, and vertical scratches
perpendicular to the transverse lines on the underside of the plate
were made the following day using a pen tip and a ruler. The cells
were washed three times with PBS in order to remove the excess cell
debris, in order to retain a clean wounding line. The plate was
incubated at 37°C for 24 h in an atmosphere containing 5%
CO2, prior to sampling, and images were captured using a
DSC-HX1 digital camera (Sony Corporation, Tokyo, Japan).
Reverse transcription (RT)-PCR
A549 cells were treated with various concentrations
of oxycodone and morphine, and subsequently cultured for 48 h. cDNA
was synthesized using a Takara Reverse Transcription kit (Clontech
Laboratories, Inc., Mountain View, CA, USA) according to the
manufacturer's protocol. Briefly, total RNA samples were isolated
from human lung cancer cells (A549) using a Simply P Total RNA
isolation Kit (BioFlux Corporation, Tokyo, Japan) according to the
manufacturers instructions. The quantity of total RNA was
determined using an ND-1000 spectrophotometer (NanoDrop; Thermo
Fisher Scientific, Wilmington, DE, USA) by measuring the optical
density at A260 and A280 nm. Subsequently, the concentration of
mRNA was adjusted to 1.0 g/ml. According to the manufacturer's
protocol for the PCR Master Mix kit (Promega Corporation), 25 mg
reaction mixture was prepared in order to perform the PCR reaction.
The cycling program was performed as follows: One cycle at 95°C for
5 min; 32 cycles of 95°C for 30 sec, 55°C for 30 sec, 72°C for 45
sec; followed by a final elongation step at 72°C for 10 min, using
a MultiGene Gradient PCR thermal cycler (Labnet International,
Inc., Edison, NJ, USA). For p53, the forward primer was
5-GAAACTACTTCCTGAAAACAACGT-3 and the reverse was
5-GCCTCACAACCTCCGTCAT-3, the amplicon size is 455 bp. For Bax, the
forward primer was 5-TTCTGACGGCAACTTCAACTG-3, and the reverse was
5-TGAGGAGTCTCACCCAACCA-3, the amplicon size is 188 bp. For Bcl-2,
the forward primer was 5-GACGCTTTGCCACGGTGGTG-3, and the reverse
was 5-GGGGCAGGCATGTTGACTTCAC-3, the amplicon size is 356 bp. For
β-actin, the forward primer was 5-AACAAGATGAGATTGGCA-3, and the
reverse was 5-AGTGGGGTGGCTTTTAGGAT-3, the amplicon size is 251 bp.
The 10 µl PCR products were separated by electrophoresis on a 1.5%
agarose gel, and images were captured using a Gel Doc XR
gel-imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The experiment was repeated three times. The mean grayscale values
of the electrophoretic bands were analyzed using Quantity One 4.4.0
software (Bio-Rad Laboratories, Inc.).
Enzyme-linked immunosorbent assay
The cells were washed with PBS solution, the lysis
buffer (Beyotime Biotech, Jiangsu, China) was added and the cell
mixture was pipetted up and down several times. Following complete
pyrolysis, the mixture was centrifuged at 14,000 × g for 5 min, and
the supernatant was retained. The samples were prepared as follows:
i) Blank wells, the blank control wells contained chromogenic
reagents A and B and stop solution, and the operation steps were
consistent with the other treatments; ii) testing sample wells, 100
µl cell culture supernatant was added and incubated at 37°C for 90
min prior to the addition of 100 µl biotin-labeled anti-uPA (1:100;
SCS415Hu) and VEGF (1:100; SEA150Hu) antibodies (Dizhao Co., Ltd,
Nanjing, China), which were incubated at 37°C for 60 min. Following
washing three times, 100 µl horseradish peroxidase (HRP)-conjugated
streptavidin secondary antibody was added to each well, and the
plate was covered with a microplate sealer, gently shaken and
incubated at 37°C for 30 min; and iii) standard wells, 100 µl
standard substance was added and incubated at 37°C for 90 min,
following this no washing was performed and 100 µl biotin-labeled
anti-uPA antibodies were subsequently added to the wells, and the
plate was incubated at 37°C for 60 min. The wells were washed three
times, 100 µl HRP-conjugated streptavidin was added, the plate was
covered with a microplate sealer and gently shaken, and incubated
at 37°C for 30 min. Following this, 50 µl chromogenic reagents A
and B was added to each well and the plate was shaken gently and
placed in the dark at 37°C for 15-min color development. To
terminate the reaction, 100 µl stop solution was added to each
well. A blank well was taken as zero, and the OD of each well was
successively measured at 450 nm within 10 min of administering the
stop solution, using a microplate reader (Bio-Rad Laboratories,
Inc.). The standard curve and the concentrations of uPA and VEGF
were calculated according to the concentration and the
corresponding OD value of the standard wells.
Immunofluorescence
Cells were seeded into six-well plates with a 22×22
cm coverslip. The following day, when the cells had adhered to the
walls, the cells were fixed with 4% paraformaldehyde and washed
three times with PBS. Following this, Triton X-100 (0.2%-0.5% in
PBS) was used to permeabilize the cells for 10 min, prior to
washing three times with PBS. Subsequently, the cells were blocked
with 2% bovine serum albumin for 30 min, washed twice with PBS and
incubated with primary antibodies at room temperature for 1 h.
Following washing three times with PBS, the cells were incubated
with secondary antibodies for 30–40 min at room temperature and
subsequently washed with PBS four times. ICAM-1 (0.5 µg/µl) was
then added to the cells and incubated for 10 min prior to washing
three times with PBS. Finally, the cells were mounted with 20 µl
mounting medium and observed using an Axio Observer A1 microscope
(Carl Zeiss AG, Oberkochen, Germany).
Statistical analysis
Data analysis was performed using SPSS software
(version 16.0; SPSS, Inc., Chicago, IL, USA). Statistical analysis
was performed using analysis of variance followed by Tukey's test
for individual comparisons between group means. Data are presented
as the mean ± standard error of the mean. P<0.05 was considered
to indicate a statistically significant difference.
Results
Effects of oxycodone and morphine on
the cell morphology of the A549 human lung cancer cell line
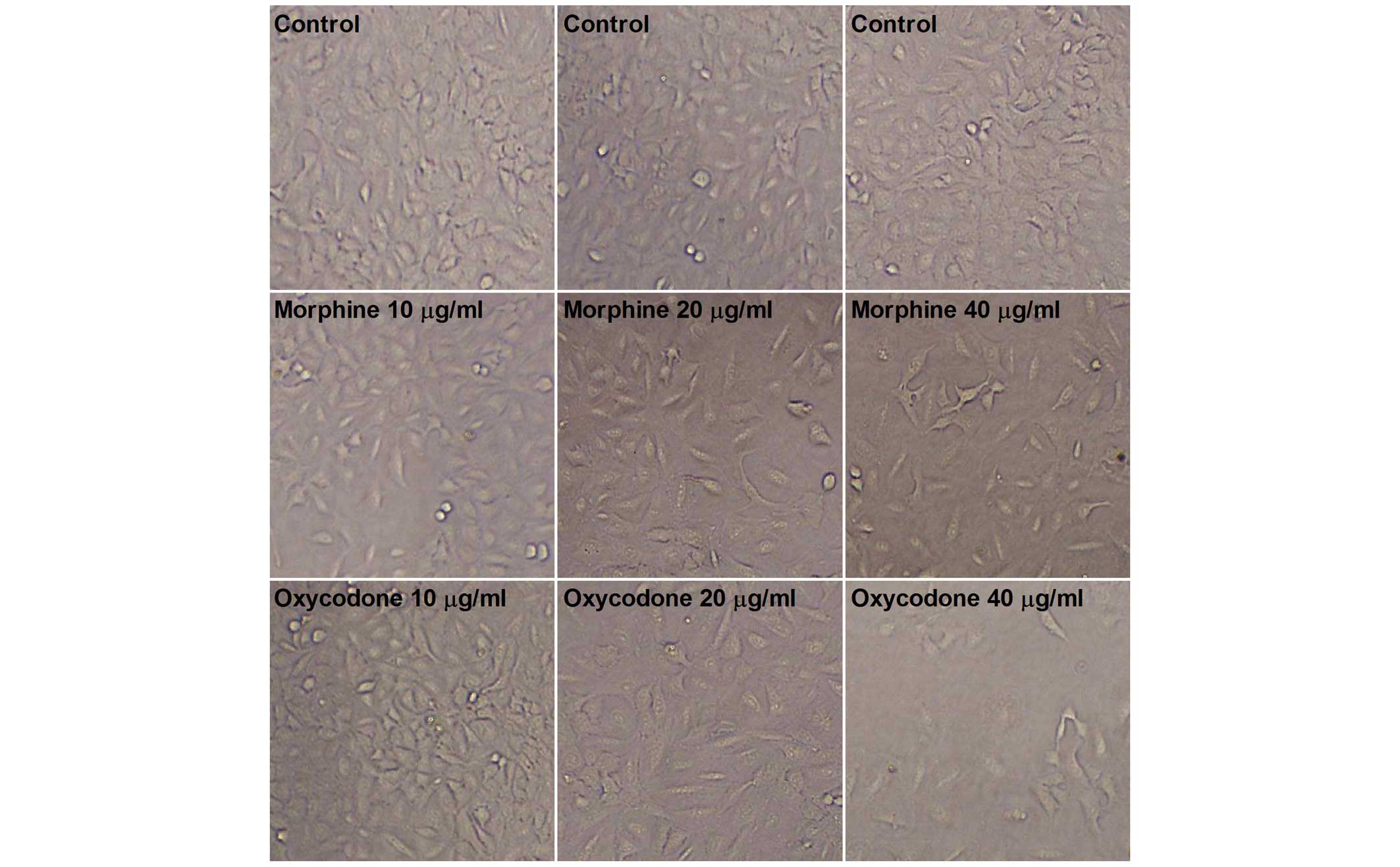
As shown in Fig. 1,
microscopic observation of the A549 cells in the control group
demonstrated that they were uniform in size and grew adherently
with a fusiform shape. Following treatment with oxycodone and
morphine, the morphology of the A549 cells altered; the cells were
no longer uniform size and exhibited irregular contours.
Furthermore, the cells exhibited typical apoptotic characteristics,
including total atrophy and decreased cellular refractivity
following treatment with 40 µg/ml oxycodone or morphine, with a
more obvious inhibitory effect demonstrated following oxycodone
administration.
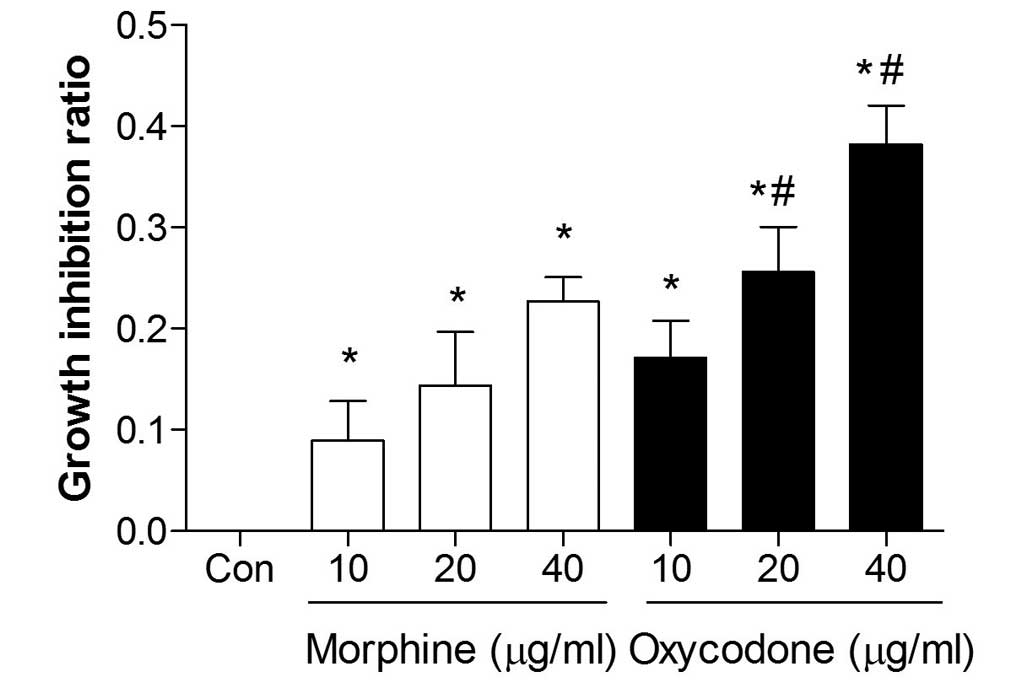
Effects of oxycodone and morphine on
the proliferation of A549 cells
The proliferation of A549 cells was significantly
inhibited following treatment with oxycodone or morphine, and both
agents demonstrated a significant inhibitory effect (P<0.05) in
a dose-dependent manner (Fig. 2).
Furthermore, when the concentrations were >20 µg/ml the
inhibitory effects of oxycodone were increased, as compared with
morphine administration (P<0.05).
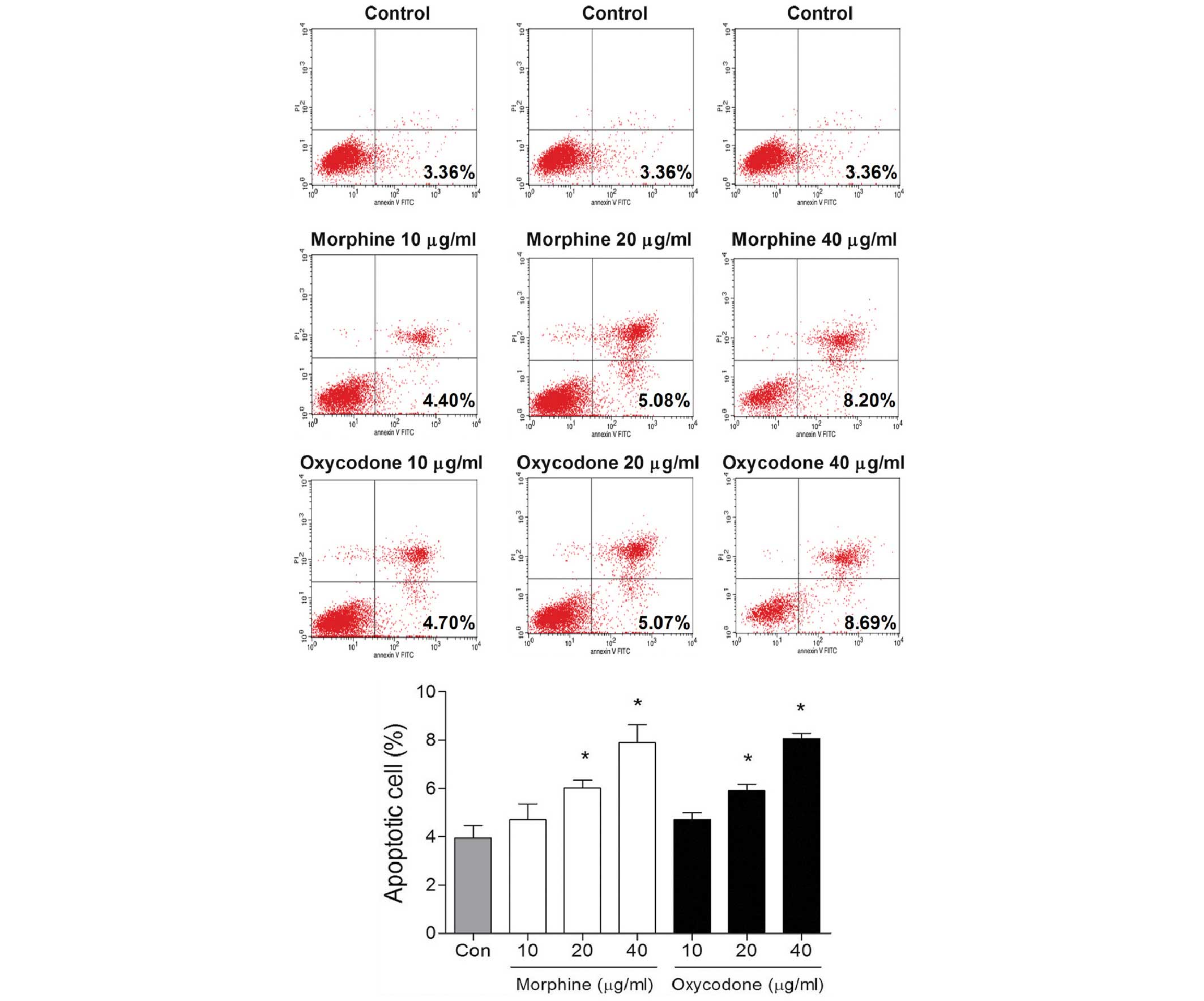
Effects of oxycodone and morphine on
the apoptosis of A549 cells
Following treatment with 10 µg/ml oxycodone or
morphine, no differences in the rates of early apoptosis were
demonstrated after 48 h, as compared with the control group
(P>0.05; Fig. 3). However,
oxycodone or morphine treatment was able to significantly promote
apoptosis when the concentrations were >20 µg/ml
(P<0.05).
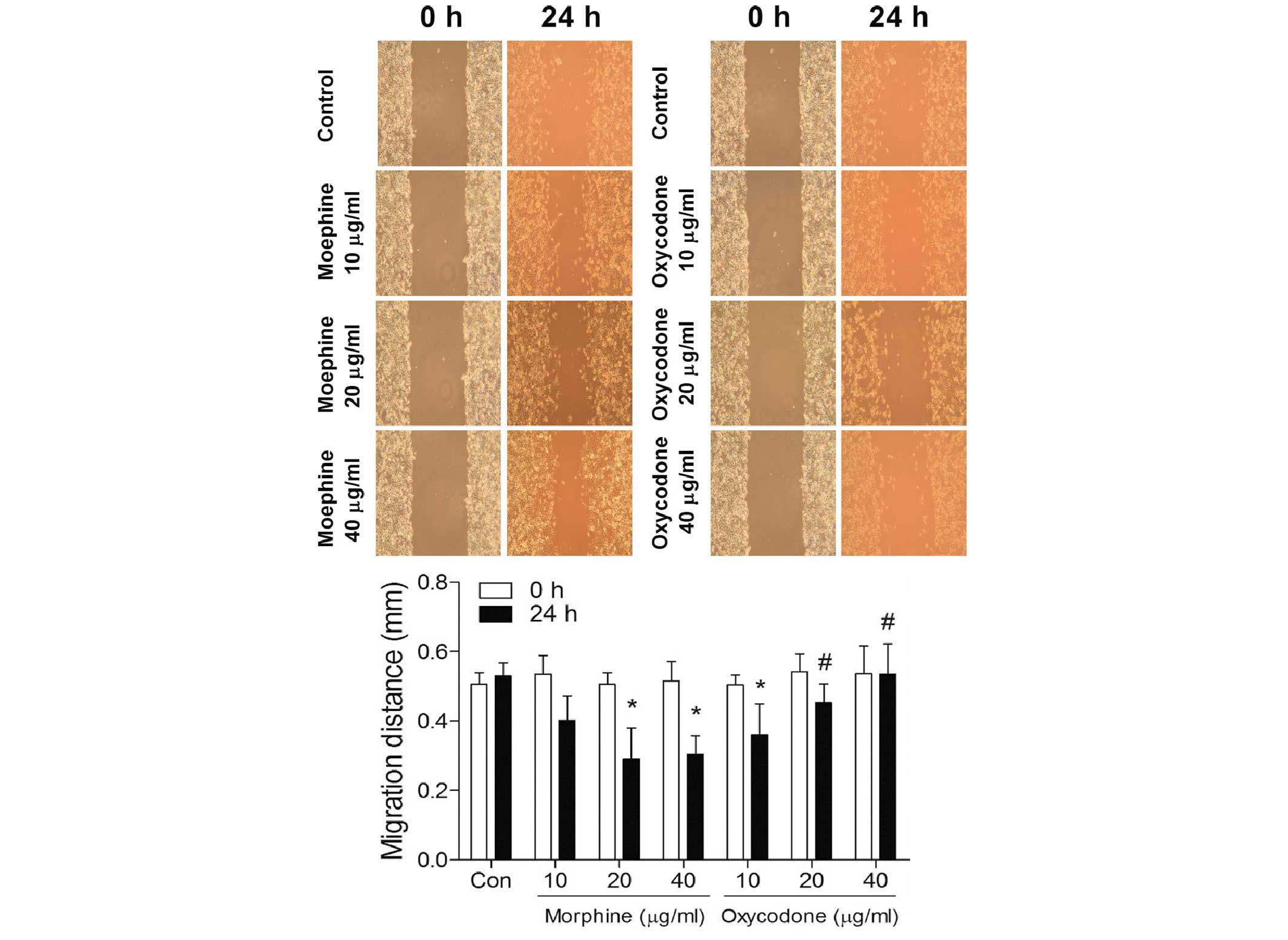
Effects of oxycodone and morphine on
the migratory ability of A549 cells at 24 h
As outlined in Fig.
4, the scratch assay suggested that morphine may significantly
increase (P<0.05) the migratory ability of A549 lung cancer
cells in a dose-dependent manner; whereas the migratory abilities
of the A549 lung cancer cells in the oxycodone group were
significantly decreased (P<0.05) in a dose-dependent manner.
Furthermore, when the concentrations were >20 µg/ml, the
migration distances were significantly greater in the oxycodone
groups, as compared with the morphine groups (P<0.05).
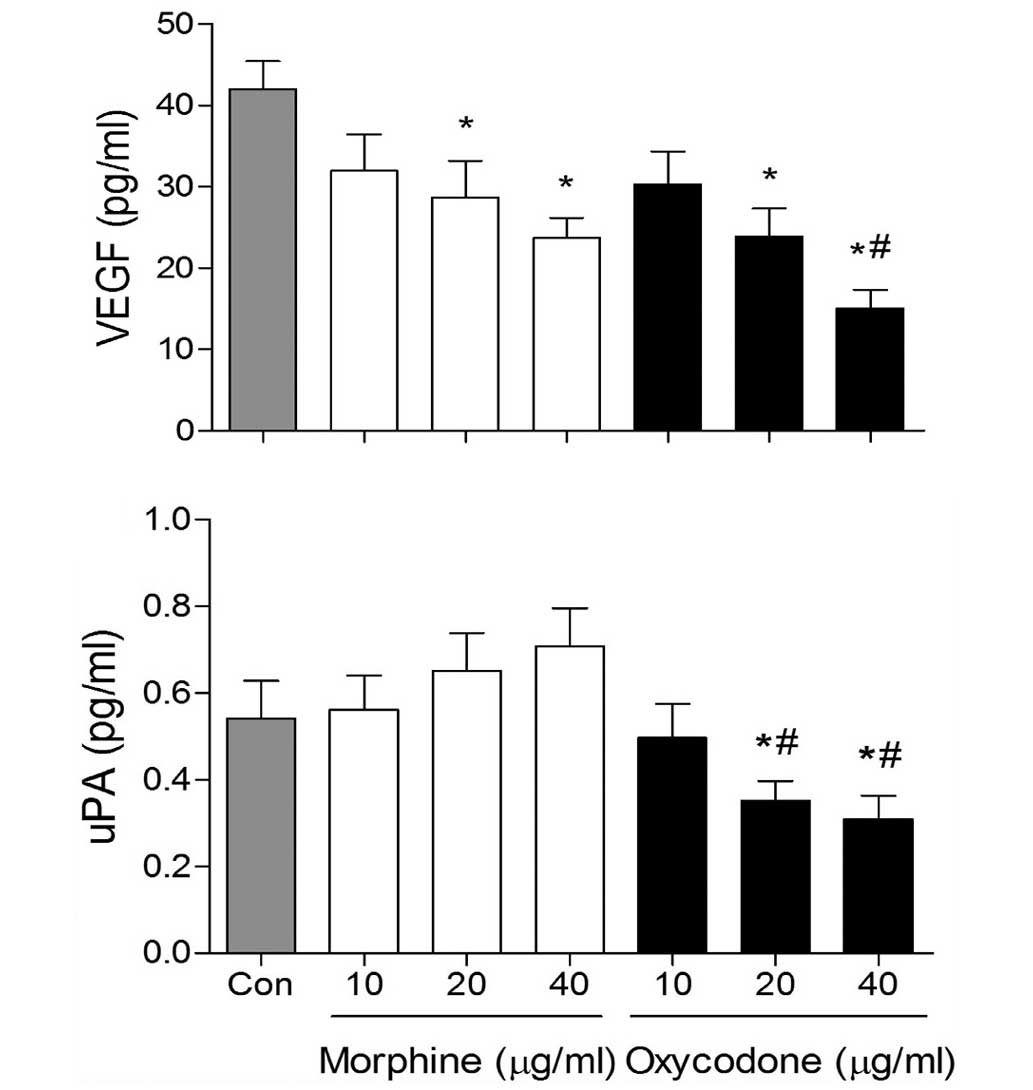
Effects of oxycodone and morphine on
the expression levels of VEGF and uPA in A549 cells
VEGF expression levels in the morphine and oxycodone
groups were significantly decreased (P<0.05), as compared with
the control group (Fig. 5).
Furthermore, at the concentrations of 40 µg/ml, the expression
levels of VEGF were reduced in the oxycodone group, as compared
with the morphine group (P<0.05). In addition, oxycodone
downregulated the expression levels of uPA, whereas morphine
upregulated uPA expression levels in A549 cells.
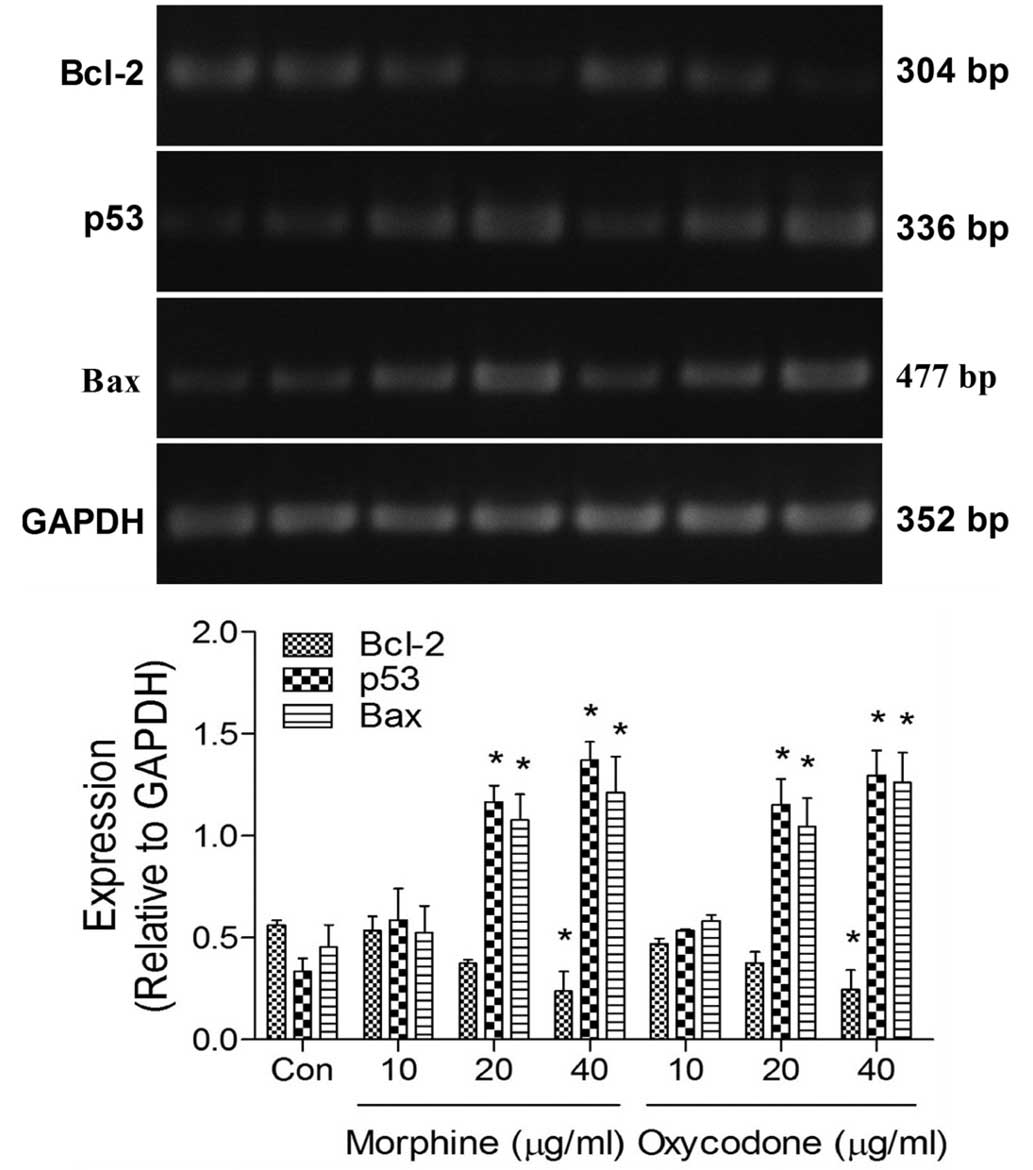
Effects of oxycodone and morphine on
the expression levels of apoptosis-related mRNA in A549 cells
RT-PCR analysis demonstrated that the expression
levels of B-cell lymphoma (Bcl)-2 were significantly decreased
(P<0.05) in the oxycodone and morphine groups, whereas the
expression levels of p53 and Bcl-2-associated X protein (Bax) were
significantly increased (P<0.05), as compared with the control
group (Fig. 6). No differences were
demonstrated between the effects of oxycodone and morphine when
applied at equal concentrations.
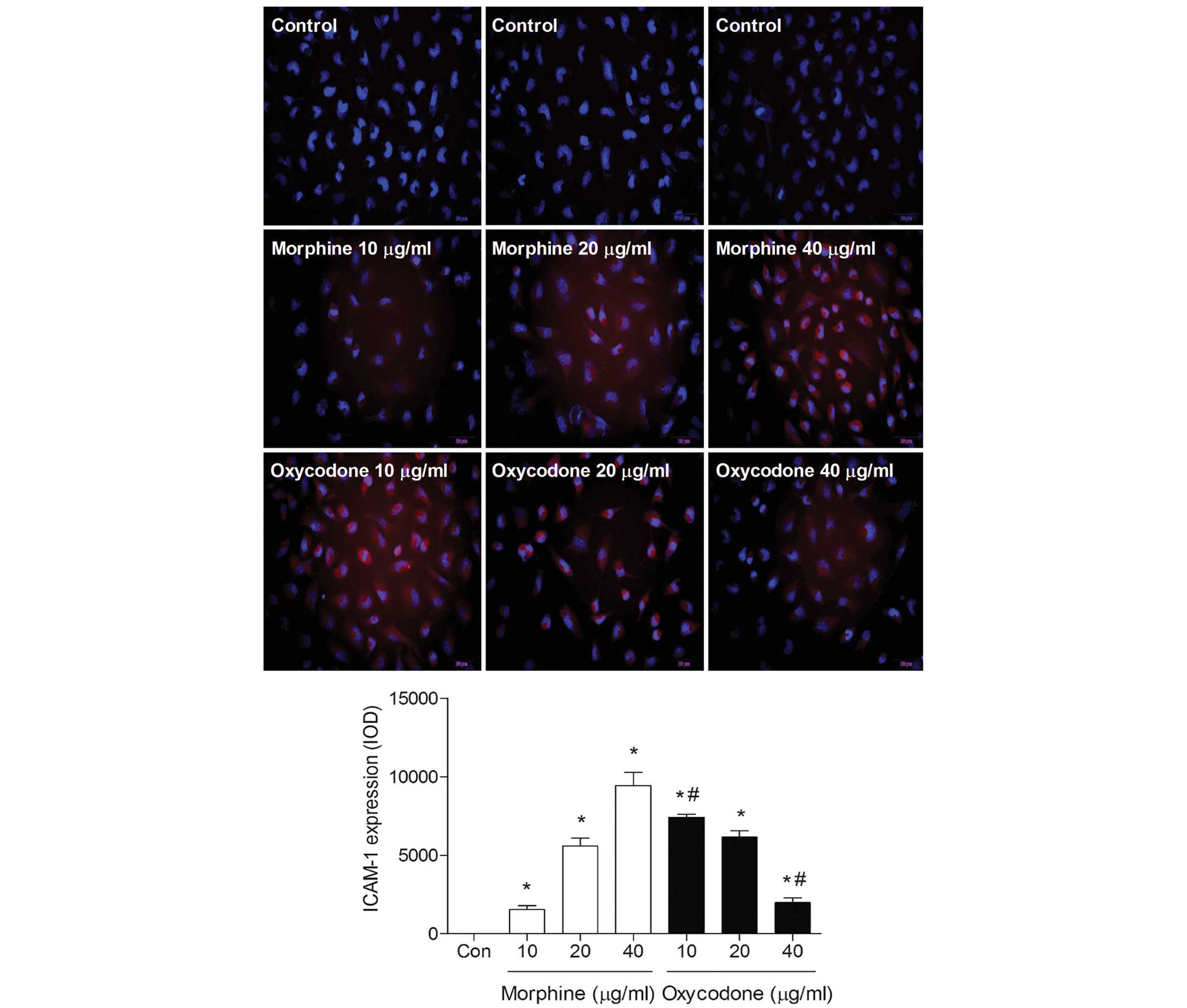
Effects of oxycodone and morphine on
the expression levels of ICAM-1 in A549 cells
Immunofluorescence analysis demonstrated that the
fluorescence intensity of ICAM-1 gradually decreased with
increasing oxycodone concentrations; whereas morphine induced a
dose-dependent increase in the fluorescence intensity of ICAM-1
(P<0.05; Fig. 7).
Discussion
The present study demonstrated that oxycodone is
capable of inhibiting the proliferation of A549 cells, inducing
apoptosis, and weakening the invasive ability of A549 cells. These
findings support the hypothesis that oxycodone may exert these
effects on A549 tumor cells by modulating the expression levels of
p53, Bax, Bcl-2, VEGF, ICAM-1 and uPA.
Previous studies have demonstrated that chemical
mediators are released in response to surgical stress, which may
upregulate malignant pathways and promote the recurrence of cancer
(4,5). Adequate pain management is essential in
patients with cancer, in order to reduce immune deficiency against
cancer recurrence and/or the spread of residual cancer cells
promoted by surgical stress (17).
However, opioids have also been demonstrated to promote cancer
recurrence or progression by affecting the immune system or cancer
cells (18). Opioid receptors are
expressed in cancer cells (19) and
opioids are capable of regulating the growth, proliferation and/or
apoptosis of cancer cells by directly activating the respective
receptors (20). Previous studies
have suggested that anesthetics may have negative effects on the
outcome of postoperative cancer recurrence by: Inducing molecular
changes in cancer cells; modulating proliferation, angiogenesis and
apoptosis; and exacerbating immunosuppression in patients with
cancer undergoing surgery (3–6).
Therefore, inhibition of the invasive and migratory potential of
cancer cells may improve the outcome of cancer treatment.
Mitigation of the metastatic potential of cancer cells during the
perioperative period is a challenging topic for anesthesiologists.
In the present study, oxycodone, which is a semi-synthetic opioid,
significantly suppressed the invasion and migration of A549 cells.
Furthermore, oxycodone and morphine successfully inhibited the
growth of A549 cells. This corroborates the findings of a previous
study, which demonstrated that >10 µM morphine inhibited the
growth of SH-SY5Y cancer cells (21). Opioids are capable of stimulating the
production and release of nitric oxide and reactive oxygen species,
which may explain the anti-cancer effects of oxycodone and morphine
(22,23). However, a previous study has
demonstrated that, at clinically relevant doses, morphine is
capable of promoting neovascularization in a human breast tumor
xenograft model in mice (12). These
findings suggested that the effects of morphine on cancer cells may
be dependent on concentration, cell-type specificity and the
administration methods.
The proliferation of cancer cells depends on
numerous factors, with angiogenesis of particular importance. It is
well established that VEGF regulates angiogenesis (12). The results of the present study
demonstrated that, at higher concentrations (40 µg/ml), oxycodone
decreased the expression levels of VEGF in A549 cells more
profoundly than morphine was able to. Notably, Gupta et al
observed that morphine at concentrations observed in a patients
blood may cause the opposite effect, resulting in the stimulation
of angiogenesis, and that only increased doses of morphine may
inhibit it (12,24). Furthermore, the present study
demonstrated that morphine significantly promoted the migration of
A549 cells, whereas oxycodone inhibited migration. Oxycodone also
downregulated the expression levels of uPA and ICAM-1 in A549
cells, whereas morphine upregulated the expression levels of these
proteins. These findings suggested that oxycodone may be an
improved candidate for pain management, as compared with morphine,
in the treatment of patients with cancer.
In cancer cells, apoptosis is generally impaired
which leads to increased cell proliferation. The p53 gene has a
directly negative regulatory effect on the promoters of some cell
proliferation-related genes, and thus has a vital role in the
regulation of apoptosis. Bcl-2 and Bax belong to the Bcl-2 family
and the mechanism by which Bcl-2 inhibits apoptosis may be related
to its antagonistic effects on the apoptosis-promoting Bax gene
(25–27). The results of the present study
demonstrated that morphine and oxycodone upregulated the mRNA
expression levels of p53 and Bax, and downregulated the Bcl-2 mRNA
expression levels in A549 cells, thus suggesting that morphine and
oxycodone may promote apoptosis in these cells. At concentrations
>20 µg/ml, oxycodone and morphine were able to significantly
increase apoptosis in this cell line. Another major characteristic
of cancer cells is their ability to migrate into surrounding and
distant tissues, which, according to the findings of the present
study, may be more preferably suppressed by oxycodone in A549 lung
cancer cells, as compared with morphine treatment.
In conclusion, the results of the present study
demonstrated that oxycodone and morphine are capable of inducing
apoptosis and inhibiting the proliferation of A549 lung cancer
cells. In addition, oxycodone, but not morphine, exhibited
prominent anti-migration effects in these cells. Taken together,
these findings support favorable anti-cancer properties of
oxycodone over morphine. Future in vivo studies are required
in order to further characterize the anti-malignant potential of
oxycodone.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81271216, 81300946)
and the Natural Science Foundation of Jiangsu Province (grant no.
BK2012778) attributed to the Department of Anesthesiology, Jinling
Hospital, School of Medicine, Nanjing University (Nanjing,
China).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liang H, Gu M, Yang C, Wang H, Wen X and
Zhou Q: Sevoflurane inhibits invasion and migration of lung cancer
cells by inactivating the p38 MAPK signaling pathway. J Anesth.
26:381–392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Snyder GL and Greenberg S: Effect of
anaesthetic technique and other perioperative factors on cancer
recurrence. Br J Anaesth. 105:106–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Afsharimani B, Cabot P and Parat MO:
Morphine and tumor growth and metastasis. Cancer Metastasis Rev.
30:225–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cata JP, Bauer M, Sokari T, Ramirez MF,
Mason D, Plautz G and Kurz A: Effects of surgery, general
anesthesia, and perioperative epidural analgesia on the immune
function of patients with non-small cell lung cancer. J Clin
Anesth. 25:255–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koodie L, Yuan H, Pumper JA, Yu H,
Charboneau R, Ramkrishnan S and Roy S: Morphine inhibits migration
of tumor-infiltrating leukocytes and suppresses angiogenesis
associated with tumor growth in mice. Am J Pathol. 184:1073–1084.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boland JW, Foulds GA, Ahmedzai SH and
Pockley AG: A preliminary evaluation of the effects of opioids on
innate and adaptive human in vitro immune function. BMJ Support
Palliat Care. 4:357–367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki M, Sakurada T, Gotoh K, Watanabe S
and Satoh N: Correlation between the administration of morphine or
oxycodone and the development of infections in patients with cancer
pain. Am J Hosp Palliat Care. 30:712–716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Welden B, Gates G, Mallari R and Garrett
N: Effects of anesthetics and analgesics on natural killer cell
activity. AANA J. 77:287–292. 2009.PubMed/NCBI
|
|
10
|
Nguyen J, Luk K, Vang D, Soto W, Vincent
L, Robiner S, Saavedra R, Li Y, Gupta P and Gupta K: Morphine
stimulates cancer progression and mast cell activation and impairs
survival in transgenic mice with breast cancer. Br J Anaesth.
113(Suppl 1): i4–i13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roy S, Balasubramanian S, Sumandeep S,
Charboneau R, Wang J, Melnyk D, Beilman GJ, Vatassery R and Barke
RA: Morphine directs T cells toward T(H2) differentiation. Surgery.
130:304–309. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta K, Kshirsagar S, Chang L, Schwartz
R, Law PY, Yee D and Hebbel RP: Morphine stimulates angiogenesis by
activating proangiogenic and survival-promoting signaling and
promotes breast tumor growth. Cancer Res. 62:4491–4498.
2002.PubMed/NCBI
|
|
13
|
Biki B, Mascha E, Moriarty DC, Fitzpatrick
JM, Sessler DI and Buggy DJ: Anesthetic technique for radical
prostatectomy surgery affects cancer recurrence: A retrospective
analysis. Anesthesiology. 109:180–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mantyh PW: Cancer pain and its impact on
diagnosis, survival and quality of life. Nat Rev Neurosci.
7:797–809. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ordóñez Gallego A, González Barón M and
Espinosa Arranz E: Oxycodone: A pharmacological and clinical
review. Clin Transl Oncol. 9:298–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamura A, Hasegawa M, Minami K, Kanbara
T, Tomii T, Nishiyori A, Narita M, Suzuki T and Kato A:
Differential activation of the µ-opioid receptor by oxycodone and
morphine in pain-related brain regions in a bone cancer pain model.
Br J Pharmacol. 168:375–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fodale V, D'Arrigo MG, Triolo S, Mondello
S and La Torre D: Anesthetic techniques and cancer recurrence after
surgery. ScientificWorldJournal. 2014:3285132014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mathew B, Lennon FE, Siegler J,
Mirzapoiazova T, Mambetsariev N, Sammani S, Gerhold LM, LaRiviere
PJ, Chen CT, Garcia JG, et al: The novel role of the mu opioid
receptor in lung cancer progression: A laboratory investigation.
Anesth Analg. 112:558–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lennon FE, Mirzapoiazova T, Mambetsariev
B, Salgia R, Moss J and Singleton PA: Overexpression of the
µ-opioid receptor in human non-small cell lung cancer promotes Akt
and mTOR activation, tumor growth, and metastasis. Anesthesiology.
116:857–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kharmate G, Rajput PS, Lin YC and Kumar U:
Inhibition of tumor promoting signals by activation of SSTR2 and
opioid receptors in human breast cancer cells. Cancer Cell Int.
13:932013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin X, Wang YJ, Li Q, Hou YY, Hong MH, Cao
YL, Chi ZQ and Liu JG: Chronic high-dose morphine treatment
promotes SH-SY5Y cell apoptosis via c-Jun N-terminal
kinase-mediated activation of mitochondria-dependent pathway. FEBS
J. 276:2022–2036. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Finley MJ, Happel CM, Kaminsky DE and
Rogers TJ: Opioid and nociception receptors regulate cytokine and
cytokine receptor expression. Cell Immunol. 252:146–154. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsiao PN, Chang MC, Cheng WF, Chen CA, Lin
HW, Hsieh CY and Sun WZ: Morphine induces apoptosis of human
endothelial cells through nitric oxide and reactive oxygen species
pathways. Toxicology. 256:83–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koodie L, Ramakrishnan S and Roy S:
Morphine suppresses tumor angiogenesis through a HIF-1alpha/p38MAPK
pathway. Am J Pathol. 177:984–997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki S, Chuang LF, Doi RH and Chuang RY:
Morphine suppresses lymphocyte apoptosis by blocking p53-mediated
death signaling. Biochem Biophys Res Commun. 308:802–808. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singhal PC, Bhaskaran M, Patel J, Patel K,
Kasinath BS, Duraisamy S, Franki N, Reddy K and Kapasi AA: Role of
p38 mitogen-activated protein kinase phosphorylation and Fas-Fas
ligand interaction in morphine-induced macrophage apoptosis. J
Immunol. 168:4025–4033. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: Roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|