Introduction
Renal cell carcinoma (RCC) is the most common type
of kidney cancer with an incidence of 5–10 per 100,000; it accounts
for ~3% of all cancer diagnoses in adults (1,2). In the
United States alone, >65,000 individuals were diagnosed with RCC
in 2013, with >13,600 mortalities recorded (3). At present, surgical resection remains
the most common treatment method for renal cancer because of its
poor response to chemotherapy and radiotherapy; however, nearly 30%
of patients develop metastatic disease following surgery, showing a
median survival time of only 13 months (4,5).
Furthermore, the 5-year survival rate of renal cancer is estimated
to be ~55%, and that of metastatic renal cell carcinoma is ~10%
(6). Despite significant advances in
the diagnosis and treatment of RCC, its mechanism of carcinogenesis
is largely unknown and the prognosis remains poor. Further
understanding of RCC pathogenesis and the identification of novel
molecules involved in its progression may provide new strategies
for treatment.
The enzyme neuregulin receptor degradation protein-1
(Nrdp1), a RING finger domain-containing E3 ubiquitin ligase, is a
member of a family of E3 ubiquitin ligases designated tripartite
motif/RING finger, B-box, and coiled-coil proteins, which are
involved in numerous cellular processes such as apoptosis, cell
cycle regulation and viral response (7,8). Nrdp1
was originally found to be involved in the ubiquitination and
proteasomal degradation of Erb-B2 receptor tyrosine kinase 3
(ErbB3), a member of the epidermal growth factor receptor (EGFR)
family (9). In the past decades,
Nrdp1-mediated degradation of ErbB3 has been shown to be correlated
with a number of diseases, such as human breast and colorectal
cancers (10,11). Furthermore, Nrdp1 is also implicated
in the ubiquitination and degradation of two other E3 ubiquitin
ligases, namely baculovirus inhibitor of apoptosis repeat
(BIR)-containing ubiquitin-conjugating enzyme (BRUCE) (an apoptosis
inhibitor) and parkin, which is involved in the pathogenesis of
Parkinson's disease (12,13). Nrdp1 has been reported to increase
Toll-like receptor-mediated interferon-β production by suppressing
myeloid differentiation primary response gene 88 and activating
TANK-binding kinase 1 (14).
However, the role of Nrdp1 in RCC development and progression
remains largely unknown.
BRUCE, also known as apollon, is a giant (528 kDa)
membrane-associated protein that contains a BIR domain at its
N-terminal region. It is a member of the inhibitor of apoptosis
(IAP) family of proteins, and inhibits caspase activity and
apoptosis via its BIR domain (15,16). Qiu
and Goldberg demonstrated that decreasing BRUCE levels by
proteasomal degradation or RNA interference promotes cell apoptosis
(17). In addition, Hao et al
indicated that BRUCE is essential in preventing second
mitochondria-derived activator of caspase (SMAC)-induced apoptosis,
promoting ubiquitination and proteasomal degradation of SMAC
(18). Consistent with this, Chen
et al (15) reported that
BRUCE is upregulated in certain brain cancers (gliomas), suggesting
that this protein plays a role in tumorigenesis of gliomas by
protecting cells from apoptosis. Previous studies have revealed
that Nrdp1 overexpression results in increased cell apoptosis
through the induction of BRUCE ubiquitination and proteasomal
degradation; consistent with this, decreasing Nrdp1 levels by RNA
interference causes decreased cell apoptosis, suggesting that
Nrdp1-mediated degradation of BRUCE represents a novel mechanism of
apoptosis (12,19).
The present study aimed to investigate the roles of
Nrdp1 and one of its targets, BRUCE, in RCC. First, the protein
expression levels of Nrdp1 and BRUCE were assessed in RCC and
adjacent normal tissues by western blot analysis. Secondly, Nrdp1
was overexpressed or silenced in 786-O RCC cells to determine the
biological role of Nrdp1. Finally, BRUCE-specific small interfering
RNA (si-BRUCE) was transfected into cells to assess whether the
levels of BRUCE are associated with the effects of Nrdp1 observed
in RCC.
Materials and methods
Tissue specimens
A total of 24 paired tumor specimens and adjacent
normal tissues were obtained from patients with primary RCC who had
undergone radical nephrectomy at the Department of Urology,
Shanghai Tenth People's Hospital, Tongji University (Shanghai,
China), between 2009 and 2013. None of the patients received
preoperative treatment. Following surgical resection, samples were
placed immediately into liquid nitrogen for cryopreservation until
use; tumor specimens were all confirmed by postoperative
pathological analysis. This study was approved by the Ethics
Committee of the Shanghai Tenth People's Hospital, and written
informed consent was obtained from all patients.
Cell culture and transfection
The human RCC cell line 786-O was purchased from
American Type Culture Collection (ATCC; Manassas, VA, USA). Cells
were cultured at 37°C in a humid environment containing 5%
CO2 in Dulbecco's modified Eagle's medium (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Thermo Fisher Scientific, Inc.), 50 U/ml
penicillin and 50 µg/ml streptomycin.
Transfection was performed using the Lipofectamine
2000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) following the manufacturer's protocol. An Nrdp1 construct was
generated by subcloning the human Nrdp1 cDNA into the expression
vector pcDNA3.1-EGFR (both Shanghai GenePharma, Co., Ltd.,
Shanghai, China). For Nrdp1 silencing, the corresponding human
small interference short hairpin RNA (shRNA; Shanghai GenePharma,
Co., Ltd.) sequences were cloned into pcDNA3.1-EGFR to generate a
shRNA construct (shNrdp1). Cells transfected with null plasmid were
used as controls. For functional analysis of BRUCE, shNrdp1 and
si-BRUCE (Shanghai GenePharma, Co., Ltd.) were simultaneously
transfected into pretreated cells. The following sequence was used
for si-BRUCE: 5′-CCUGACAAUGCAGAAGGAAUCCAU-3′.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from pretreated cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The RNA samples were
reverse-transcribed into cDNA using the PrimeScript™ RT-PCR kit
(Takara Biotechnology Co., Ltd., Dalian, China). The relative gene
expression levels of Nrdp1 or BRUCE were evaluated by RT-qPCR using
an Applied Biosystems 7900HT Fast Real-Time PCR System (Thermo
Fisher Scientific, Inc.) with 1.0 µl cDNA and SYBR Green Real-time
PCR Master mix (Takara Biotechnology Co., Ltd.). The PCR
amplification was carried out for 40 cycles of 94°C for 30 sec,
60°C for 30 sec, and 72°C for 30 sec. The following primers were
used: Nrdp1, forward 5′-TGAACCGACGCTACTATGAGAACT-3′ and reverse
5′-CTGGTTCTCACAGGCCATCAC-3′; BRUCE, forward
5′-CTCAGTCAGTCCTGCCTCATC-3′ and reverse
5′-CTCAGCAGTCCTCTGGATGTC-3′; glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), forward 5′-GTAAGACCCCTGGACCACCA-3′ and
reverse 5′-CAAGGGGTCTACATGGCAACT-3′. The relative expression levels
were normalized to GAPDH and analyzed using the 2−ΔΔCq
method (20). All experiments were
carried out in triplicate.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay
Cell viability was measured by the MTT assay
(Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer's
protocol. Briefly, following effective transfection, 786-O cells
were seeded into 96-well plates at a density of 5×103
cells per well. The plates were incubated at 37°C in a humidified
atmosphere containing 5% CO2 for 24, 48, 72 and 96 h,
respectively. Afterwards, 20 µl MTT solution (5 mg/ml) was added to
each well, followed by 4 h incubation at 37°C. After careful
removal of the medium, 150 µl dimethyl sulfoxide (Sigma-Aldrich)
was used to solubilize the crystals for 15 min. Finally, cell
viability was assessed spectrophotometrically at 490 nm. Each
experiment was repeated at least three times.
Apoptosis assay
Cell apoptosis was assessed using a fluorescein
isothiocyanate (FITC)-Annexin V Apoptosis Detection kit (BD
Biosciences, San Jose, CA, USA) following the manufacturer's
guidelines. Transfected cells were cultured in serum-free medium.
After trypsinization, cells were washed twice with
phosphate-buffered saline (TBS), and stained with FITC-Annexin V
and propidium iodide (PI; BD Biosciences) in the dark for 15 min at
room temperature. Finally, apoptotic cells were quantified by flow
cytometry using a BD FACSCalibur flow cytometer (BD Biosciences).
The experiments were repeated at least three times.
Western blot analysis
Total protein was extracted from tissues (RCC tumors
and adjacent normal tissues) and cultured cells with
radioimmunoprecipitation assay (RIPA) lysis buffer [50 mM Tris/HCl,
pH 7.4, 150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS)]
containing a protease inhibitor cocktail (Sigma-Aldrich) on ice.
The supernatants were collected following centrifugation for 20 min
at 25,155 × g and 4°C. Protein lysates were subjected to 10%
SDS-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). For immunoblotting, the membranes were blocked
with 5% non-fat milk in TBS and Tween 20 at room temperature for 1
h and incubated overnight at 4°C with mouse anti-Nrdp1 (1:500; cat.
no. sc-374120; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
mouse anti-BRUCE (1:1,000; cat. no. 611192; BD Biosciences) and
mouse anti-β-actin (1:5,000; cat. no. ab8226; Abcam, Cambridge, UK)
monoclonal antibodies. After three washes, blots were incubated
with horseradish peroxidase-conjugated goat anti-mouse secondary
antibody (1:10,000; cat. no. ab97040; Abcam) for 2 h at room
temperature. Finally, protein bands were detected using an enhanced
chemiluminescence ECL kit (EMD Millipore, Billerica, MA, USA) and
quantified using ImageJ 1.x software (National Institutes of
Health, Bethesda, MD, USA). β-actin was used as an internal
control, and three independent experiments were performed.
Statistical analysis
Data presented are the mean ± standard deviation
(SD) from three independent experiments. Statistical analyses were
performed with GraphPad Prism software, version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). Groups were compared using a
Student's t-test (two groups) or one-way analysis of variance
(ANOVA; more than two groups). P<0.05 was considered to indicate
a statistically significant difference.
Results
Decreased Nrdp1 and increased BRUCE
levels in RCC
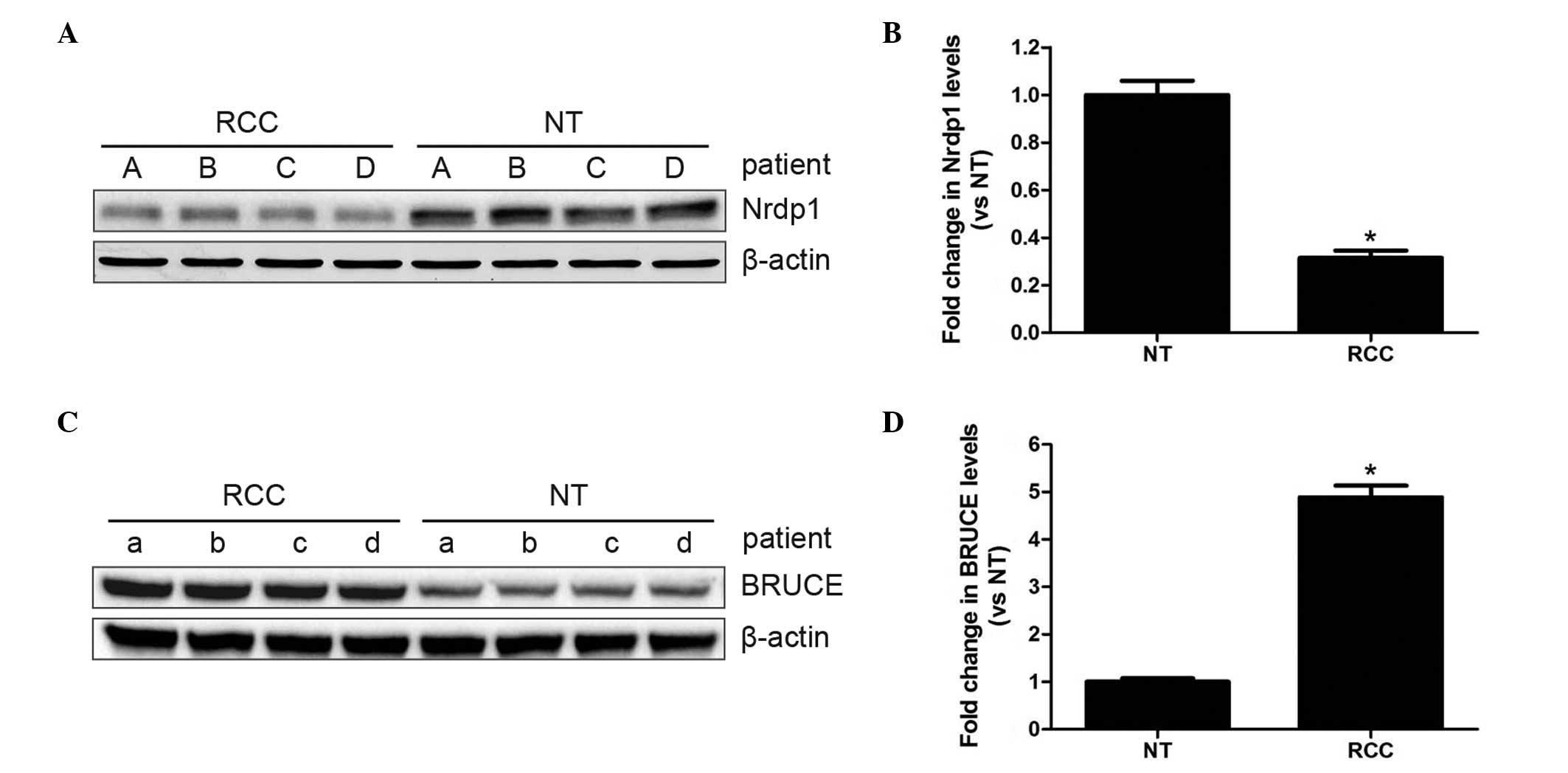
To determine whether Nrdp1 and BRUCE expression
levels were altered in RCC specimens, western blot analysis was
performed on paired RCC tumor and adjacent normal tissues from the
same patients. As shown in Fig. 1,
Nrdp1 levels were significantly lower in RCC tumors compared with
adjacent normal tissues, while the levels of BRUCE were
significantly higher (P<0.05). This suggests that both Nrdp1 and
BRUCE may be involved in RCC progression.
Nrdp1 levels affect RCC cell viability
and apoptosis
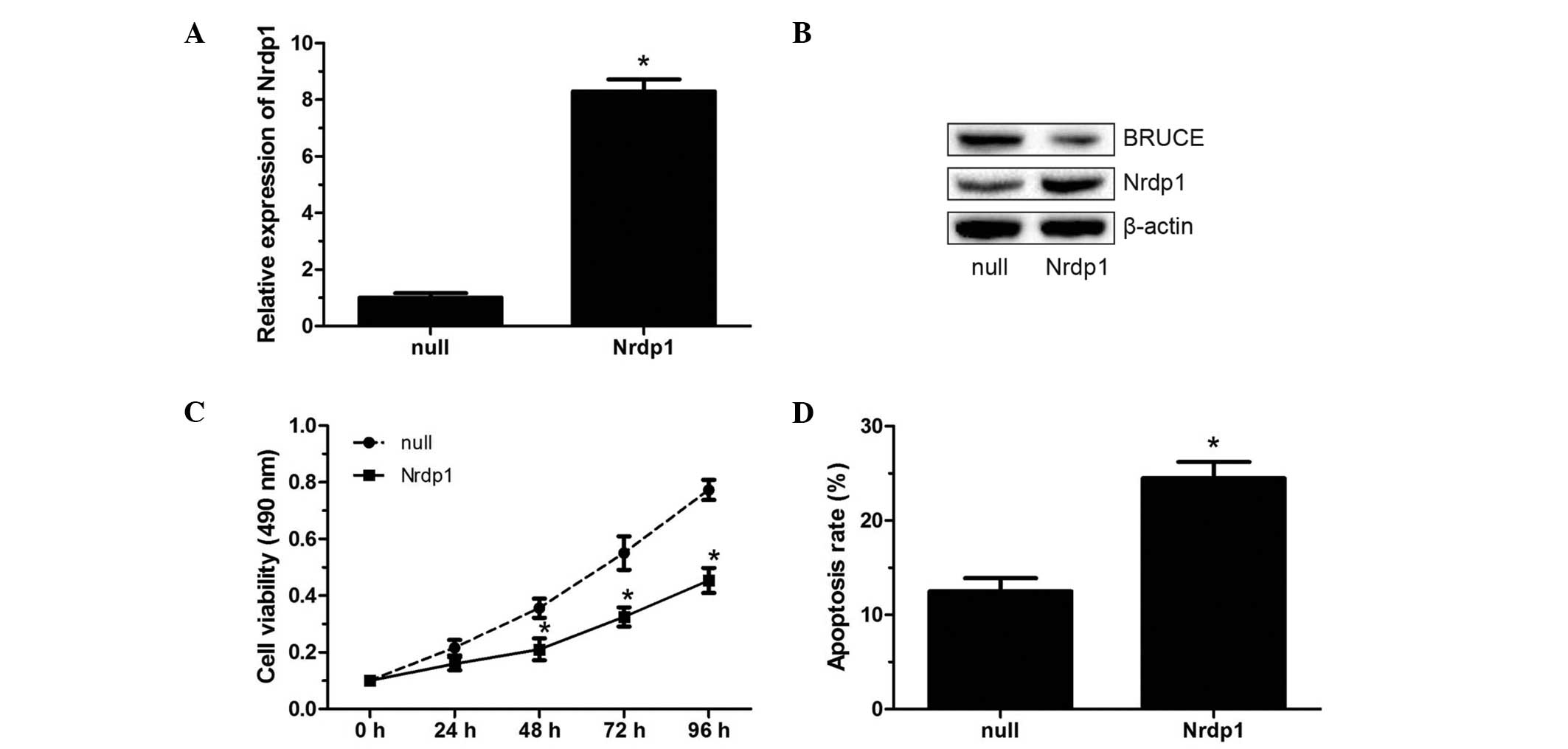
In order to investigate the biological role of Nrdp1
in RCC cells, in vitro assays were carried out using the
human RCC 786-O cell line. Nrdp1 was overexpressed or silenced in
786-O cells. The overexpression of Nrdp1, which was confirmed by
RT-qPCR and western blotting (Fig. 2A
and B), resulted in significantly decreased cell viability in
the MTT assay, and significantly increased apoptosis in 786-O cells
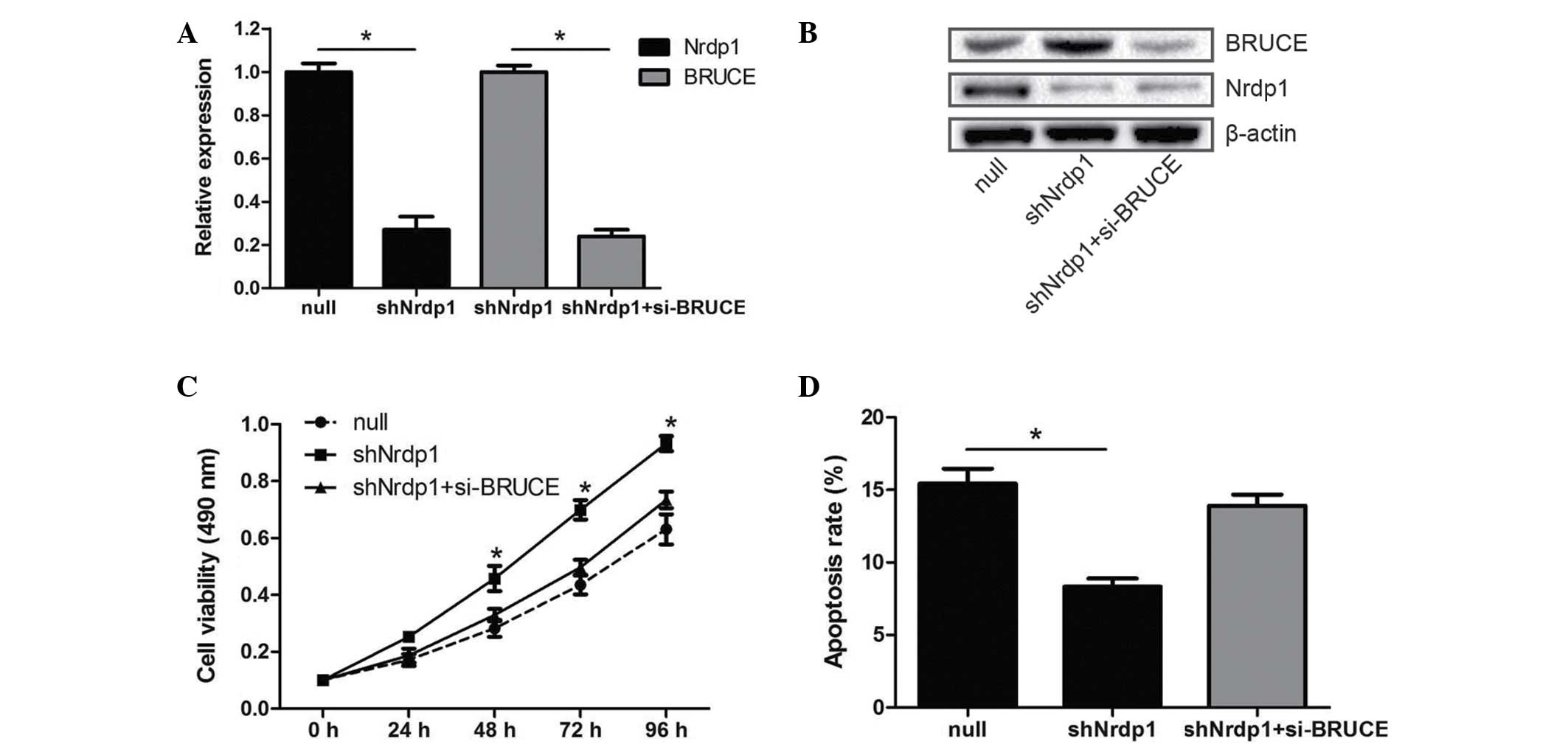
as evaluated by flow cytometry (P<0.05; Fig. 2C and D). Conversely, Nrdp1 knockdown
using shNrdp1, also confirmed by RT-qPCR and western blotting
(Fig. 3A and B), caused the cell
viability to increase and reduced apoptosis in the 786-O cells
(Fig. 3C and D). These results
indicate that Nrdp1 may act as a tumor suppressor in RCC.
Nrdp1 affects RCC cell viability and
apoptosis, likely by degrading BRUCE
Considerable evidence suggests that BRUCE is a
target of Nrdp1, and that Nrdp1-mediated degradation of BRUCE is
correlated with cell apoptosis in human cancers (12,19). To
verify these findings in RCC, the small interfering RNA si-BRUCE
was transfected into 786-O cells with Nrdp1 knockdown. Reduced
levels of BRUCE expression following transfection were confirmed by
RT-qPCR and western blot analysis (Fig.
3A and B). Notably, the effects of Nrdp1 knockdown on the
viability and apoptosis of 786-O cells were partially attenuated by
BRUCE silencing (Fig. 3C and D).
Moreover, the levels of BRUCE protein exhibited an inverse
association with Nrdp1 protein levels as shown by western blot
analysis (Figs. 2B and 3B). These findings suggest that Nrdp1 may
affect the cell viability and apoptosis of RCC via the degradation
of BRUCE.
Discussion
RCC is the third most common urological cancer after
prostate and bladder cancers, but has the highest mortality rate
(21,22). More seriously, its incidence and
mortality rates are on the rise around the globe. As this cancer
has a rather poor prognosis, the identification of novel molecules
involved in RCC progression that may provide new strategies for
therapeutic intervention would be valuable. Previous studies have
shown that the Nrdp1-mediated degradation of BRUCE induces cell
apoptosis, and is closely associated with the progression of human
cancers (12,19). It is acknowledged that multiple
genetic or epigenetic regulations in RCC carcinogenesis and
progression are correlated with cell viability and apoptosis
(23,24). Hence, Nrdp1-mediated degradation of
BRUCE may also affect cell viability and apoptosis in RCC.
In the present study, the biological significance of
Nrdp1 and one of its targets, BRUCE, in RCC were investigated.
Significantly lower Nrdp1 expression levels were found in RCC
specimens compared with the corresponding normal tissues obtained
from patients. In addition, a significant upregulation of BRUCE was
observed in RCC specimens. Functional analyses revealed that the
forced overexpression of Nrdp1 markedly decreased cell viability
and induced apoptosis in the human RCC 786-O cell line; conversely,
Nrdp1 knockdown markedly increased cell viability and inhibited
apoptosis in 786-O cells. These data suggest that Nrdp1 is
downregulated in RCC and that the low Nrdp1 levels may be
associated with RCC development and progression.
Apoptosis is an ordered and orchestrated cellular
process that plays a critical role in normal tissue development and
homeostasis. Nevertheless, apoptosis inhibition is considered to
contribute to carcinogenesis and progression of human cancers
(25,26). BRUCE functions as an inhibitor of
apoptosis, and is regulated by Nrdp1-mediated degradation (12,27).
Therefore, we hypothesized that BRUCE may be associated with the
effects of Nrdp1 on RCC cell viability and apoptosis. In
vitro assays showed that the downregulation of BRUCE partially
attenuates the effects of Nrdp1 knockdown on RCC cell viability and
apoptosis. Moreover, the levels of BRUCE exhibited an inverse
association with Nrdp1 levels as demonstrated by western blot
analysis. These findings suggest that Nrdp1 may affect RCC cell
viability and apoptosis through the proteasomal degradation of
BRUCE.
As a limitation of the present study, the downstream
mechanism through which BRUCE inhibits cell apoptosis in RCC was
not explored. It has previously been reported that BRUCE binds to,
ubiquitinates and facilitates the proteasomal degradation of SMAC
and caspase-9, which play key roles in the initiation of apoptosis,
thereby inhibiting cell apoptosis in mammals (18). Furthermore, the tumor suppressor p53
has been identified as a downstream effector of BRUCE, and the
induction of apoptosis following BRUCE knockdown has been shown to
be associated with upregulation and nuclear localization of p53
(28). Therefore, further study is
warranted to investigate how the Nrdp1-mediated degradation of
BRUCE modulates cell viability and apoptosis in RCC.
The present study demonstrates for the first time,
to the best of our knowledge, that Nrdp1 is significantly decreased
in RCC specimens compared with adjacent normal tissues, and
decreases viability while inducing apoptosis in RCC cells. In
addition, the biological significance of Nrdp1 in RCC cells is
associated with its proteasomal degradation of BRUCE. These
findings suggest that the Nrdp1-mediated degradation of BRUCE
decreases cell viability and induces apoptosis in RCC cells,
highlighting Nrdp1 as a potential target for RCC treatment.
Acknowledgements
This study was partially supported by grants from
the National Natural Science Foundation of China (no. 81000311 and
no. 81270831).
References
|
1
|
Yang J, Yang J, Gao Y, Zhao L, Liu L, Qin
Y, Wang X, Song T and Huang C: Identification of potential serum
proteomic biomarkers for clear cell renal cell carcinoma. PLoS One.
9:e1113642014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lasseigne BN, Burwell TC, Patil MA, Absher
DM, Brooks JD and Myers RM: DNA methylation profiling reveals novel
diagnostic biomarkers in renal cell carcinoma. BMC Med. 12:2352014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Janowitz T, Welsh SJ, Zaki K, Mulders P
and Eisen T: Adjuvant therapy in renal cell carcinoma - past,
present, and future. Semin Oncol. 40:482–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li W, Liu M, Xu YF, Feng Y, Che JP, Wang
GC and Zheng JH: Combination of quercetin and hyperoside has
anticancer effects on renal cancer cells through inhibition of
oncogenic microRNA-27a. Oncol Rep. 31:117–124. 2014.PubMed/NCBI
|
|
6
|
Zhang HM, Yang FQ, Yan Y, Che JP and Zheng
JH: High expression of long non-coding RNA SPRY4-IT1 predicts poor
prognosis of clear cell renal cell carcinoma. Int J Clin Exp
Pathol. 7:5801–5809. 2014.PubMed/NCBI
|
|
7
|
Bouyain S and Leahy DJ: Structure-based
mutagenesis of the substrate-recognition domain of Nrdp1/FLRF
identifies the binding site for the receptor tyrosine kinase ErbB3.
Protein Sci. 16:654–661. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meroni G and Diez-Roux G: TRIM/RBCC, a
novel class of ‘single protein RING finger’ E3 ubiquitin ligases.
Bioessays. 27:1147–1157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu XB and Goldberg AL: Nrdp1/FLRF is a
ubiquitin ligase promoting ubiquitination and degradation of the
epidermal growth factor receptor family member, ErbB3. Proc Natl
Acad Sci USA. 99:14843–14848. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yen L, Cao Z, Wu X, Ingalla ER, Baron C,
Young LJ, Gregg JP, Cardiff RD, Borowsky AD, Sweeney C and Carraway
KL III: Loss of Nrdp1 enhances ErbB2/ErbB3-dependent breast tumor
cell growth. Cancer Res. 66:11279–11286. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu H, Li H, Mao D, Zhu Z and Sun H: Nrdp1
inhibits growth of colorectal cancer cells by nuclear retention of
p27. Tumour Biol. 35:8639–8643. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiu XB, Markant SL, Yuan J and Goldberg
AL: Nrdp1-mediated degradation of the gigantic IAP, BRUCE, is a
novel pathway for triggering apoptosis. EMBO J. 23:800–810. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu F and Zhou J: Parkin is ubiquitinated
by Nrdp1 and abrogates Nrdp1-induced oxidative stress. Neurosci
Lett. 440:4–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang C, Chen T, Zhang J, Yang M, Li N, Xu
X and Cao X: The E3 ubiquitin ligase Nrdp1 ‘preferentially’
promotes TLR-mediated production of type I interferon. Nat Immunol.
10:744–752. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Z, Naito M, Hori S, Mashima T, Yamori
T and Tsuruo T: A human IAP-family gene, apollon, expressed in
human brain cancer cells. Biochem Biophys Res Commun. 264:847–854.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartke T, Pohl C, Pyrowolakis G and
Jentsch S: Dual role of BRUCE as an antiapoptotic IAP and a
chimeric E2/E3 ubiquitin ligase. Mol Cell. 14:801–811. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu XB and Goldberg AL: The
membrane-associated inhibitor of apoptosis protein, BRUCE/Apollon,
antagonizes both the precursor and mature forms of Smac and
caspase-9. J Biol Chem. 280:174–182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hao Y, Sekine K, Kawabata A, Nakamura H,
Ishioka T, Ohata H, Katayama R, Hashimoto C, Zhang X, Noda T, et
al: Apollon ubiquitinates SMAC and caspase-9, and has an essential
cytoprotection function. Nat Cell Biol. 6:849–860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi H, Du J, Wang L, Zheng B, Gong H, Wu
Y, Tang Y, Gao Y and Yu R: Lower expression of Nrdp1 in human
glioma contributes tumor progression by reducing apoptosis. IUBMB
Life. 66:704–710. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Valsechi MC, Oliveira AB, Conceição AL,
Stuqui B, Candido NM, Provazzi PJ, de Araújo LF, Silva WA Jr,
Calmon Mde F and Rahal P: GPC3 reduces cell proliferation in renal
carcinoma cell lines. BMC Cancer. 14:6312014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiao HP, Gao WS, Huo JX and Yang ZS: Long
non-coding RNA GAS5 functions as a tumor suppressor in renal cell
carcinoma. Asian Pac J Cancer Prev. 14:1077–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang T, Zheng J, Jiang N, Wang G, Shi Q,
Liu C and Lu Y: Overexpression of DLC-1 induces cell apoptosis and
proliferation inhibition in the renal cell carcinoma. Cancer Lett.
283:59–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu G, Lai P, Liu M, Xu L, Guo Z, Liu H, Li
W, Wang G, Yao X, Zheng J and Xu Y: MiR-203a regulates
proliferation, migration, and apoptosis by targeting glycogen
synthase kinase-3β in human renal cell carcinoma. Tumour Biol.
35:11443–11453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bartke T, Pohl C, Pyrowolakis G and
Jentsch S: Dual role of BRUCE as an antiapoptotic IAP and a
chimeric E2/E3 ubiquitin ligase. Mol Cell. 14:801–811. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren J, Shi M, Liu R, Yang QH, Johnson T,
Skarnes WC and Du C: The Birc6 (Bruce) gene regulates p53 and the
mitochondrial pathway of apoptosis and is essential for mouse
embryonic development. Proc Natl Acad Sci USA. 102:565–570. 2005.
View Article : Google Scholar : PubMed/NCBI
|