Introduction
Graves' disease (GD) affects 1–2% of the adult
population (1). The patient's
quality of life decreases (2) due to
the adverse metabolic effects of elevated thyroid hormone and the
thyrotropin-receptor antibodies; this can affect emotional lability
and sleep, and cosmetic effects such as goiter (3). Graves' ophthalmopathy (GO), which is
the most common extrathyroidal manifestation of GD, is an
autoimmune disorder of the eyes characterized by inflammation of
the orbital connective tissue, inflammation and fibrosis of the
extraocular muscles and adipogenesis in the eyes (4). Between 20–25% of patients with GD have
clinically apparent TAO at the time of diagnosis (5). Rituximab (RTX) is a human/murine
chimeric anti-CD20 monoclonal antibody whose variable
(antigen-binding) region is derived from a mouse antibody (6). The binding of RTX to CD20 blocks the
activation and differentiation of B-cells, since CD20 is expressed
on the surface of pre-B cells and mature B lymphocytes (6). Conversely, CD20 is absent on the
surface of stem cells, pro-B lymphocytes and plasma cells (7–9), such
that treatment with RTX promotes the specific elimination of
B-cells without affecting the regeneration of B-cells from stem
cells and the production of immunoglobulins by plasma cells
(6). The present study reports the
case of a female Chinese patient with severe GO, who eventually
recovered following RTX treatment.
Case report
A 58-year-old woman (non-smoker) was diagnosed with
GD and GO in July 2013 at the First Hospital of Yulin (Yulin,
China), where she received 50 mg propylthiouracil (PTU) and 10 mg
prednisolone (Beijing Shunxin Xiangyun Pharmaceutical Co., Ltd.,
Beijing, China) three times daily (t.i.d.). Prednisolone was
administered in decreasing doses: 30 mg for the initial 2 weeks, 25
mg for 1 week, 20 mg for 7 weeks. PTU was reduced to 100 mg daily
(25 mg in the morning and afternoon, and 50 mg in the evening)
following a reevaluation of the patient's thyroid function in
August 2013. However, a the lack of improvement in the symptoms of
GO meant that the patient was transferred to the First Affiliated
Hospital of Xi'an Jiaotong University (Xi'an, China) in September
2013 for further treatment.
Upon admission to the First Affiliated Hospital of
Xi'an Jiaotong University, the thyroid function of the patient was
normal and so PTU was replaced with 5 mg/day methimazole (Merck
KGaA, Darmstadt, Germany). An eye examination revealed extremely
severe GO, including restriction of the muscles in all directions,
loss of eyesight in the right eye, reduced visual acuity in the
counting fingers test in the left eye, reduced light perception,
proptosis (right eye, 19 mm; left eye, 20 mm), a clinical activity
score (CAS) (10) of 7/7 and a
NOSPECS score (11) of 6c. An
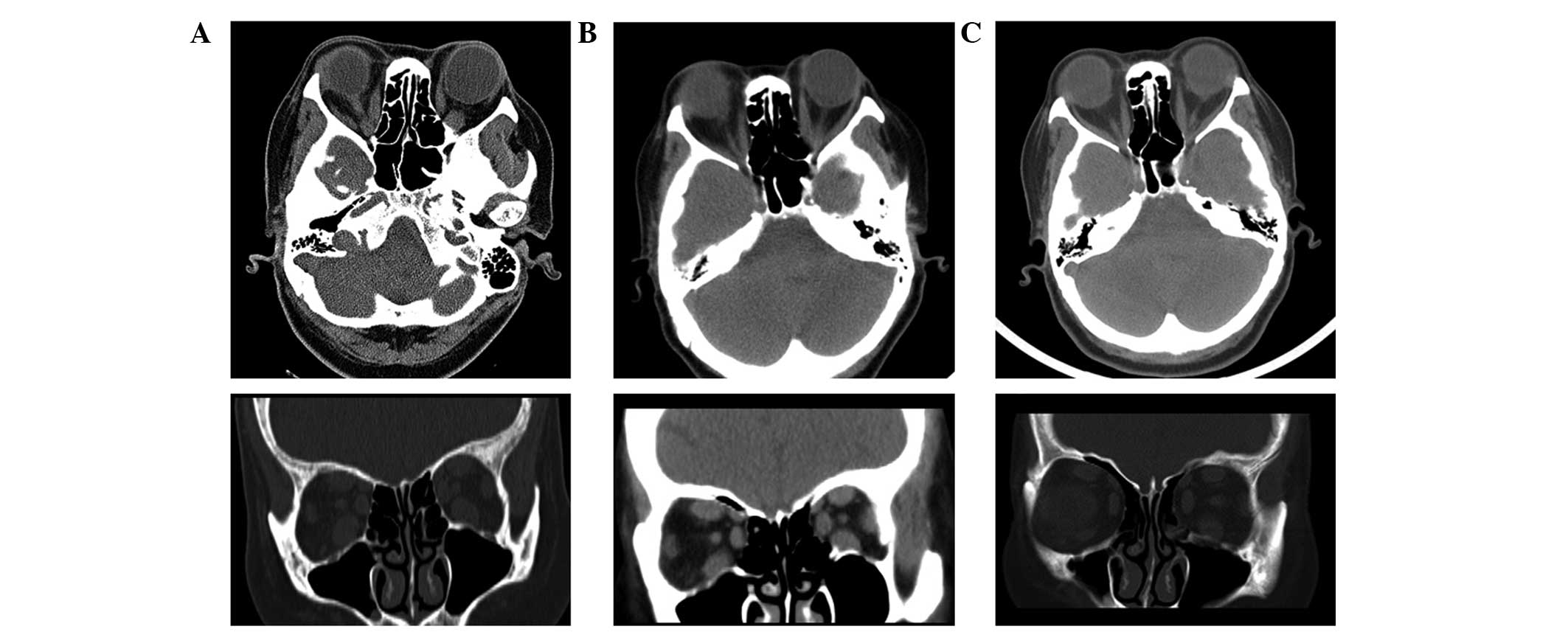
orbital computed tomography (CT) scan on 2nd September 2013 showed
thickening of the extraocular muscles, in particular of the
inferior, superior and medial recti, although with normal optic
nerves (Fig. 1). The subsequent
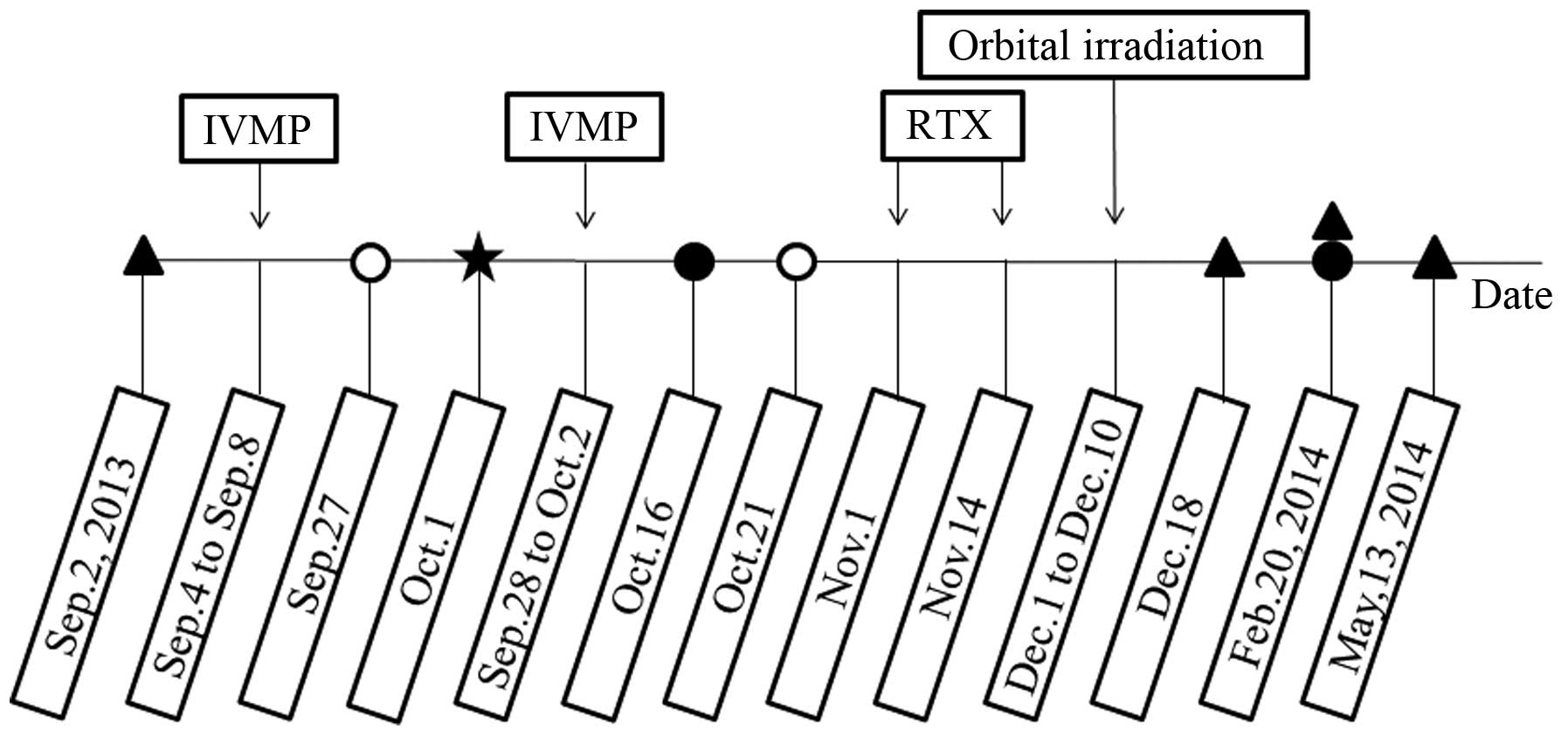
courses of tests and treatments are shown in Fig. 2. No contraindications to high-dose
intravenous methylprednisolone pulse (IVMP; Pfizer, Inc., New York,
NY, USA) therapy were observed in these examinations and so the
patient was administered 1 g intravenous (i.v.) methylprednisolone
every other day (3 times in total), which was repeated 20 days
later. The potential side-effects of IVMP were explained to the
patient and informed consent was obtained. On 1st October 2013, the
patient underwent a temporary tarsorrhaphy on the right eye for the
treatment of keratohelcosis and hypophasis, and the stitches were
removed when the swelling of the conjunctiva was improved. At 2
weeks following the second IVMP regime, without remarkable effect,
the patient was readmitted to the First Affiliated Hospital of
Xi'an Jiaotong University, whereupon RTX therapy was proposed and
accepted by the patient. RTX therapy was administered on 1st and
14th November 2013. The therapeutic protocol was that used for
patients with rheumatoid arthritis. Briefly, it consisted of twice
i.v. infusion of 1 g RTX (Roche Holding AG, Basel, Switzerland)
over ~255 min with a 2-week interval (6,12),
following pretreatment with intramuscular injection of 25 mg
Phenergan (Wellhope Pharmaceutical Co., Ltd., Shanghai, China). In
addition, the patient received a 10 mg i.v. infusion of
dexamethasone during the RTX treatment. The patient did not report
any side effects on the first nor second infusions. One day after
the initial infusion of RTX, the methimazole was reduced to 5 and
2.5 mg/daily, on alternate days, following reevaluation of the
patient's thyroid function. Simultaneous to the RTX therapy, a
cumulative dose of 20 Gy orbital irradiation was administered
between 1st and 10th December 2013, with radiation fractions
comprising of 2 Gy daily (Fig. 2).
The results of an analysis of the serum levels of anti-thyrotropin
(TSH) receptor antibodies (TRAb; competitive radioimmunoassay;
Human TRAb RIA kit; Medipan GmbH, Blankenfelde-Mahlow, Germany) are
also presented in Fig. 2. Secondary
diabetes associated with glucocorticoid use was diagnosed using an
oral glucose tolerance test (OGTT) on 27th September 2013, and 50
mg acarbose (Bayer HealthCare AG, Leverkusen, Germany) treatment
t.i.d. was initiated. The blood glucose and OGTT results were
returned to normal ~3 weeks later and acarbose treatment was
discontinued.
As compared with the orbital CT scan taken prior to
treatment (Fig. 1), the scans taken
following treatment showed signs of gradual improvements (Fig. 1). In addition, the CAS and NOSPECS
scores were decreased to 1/7 and 4b, respectively. Prior to the
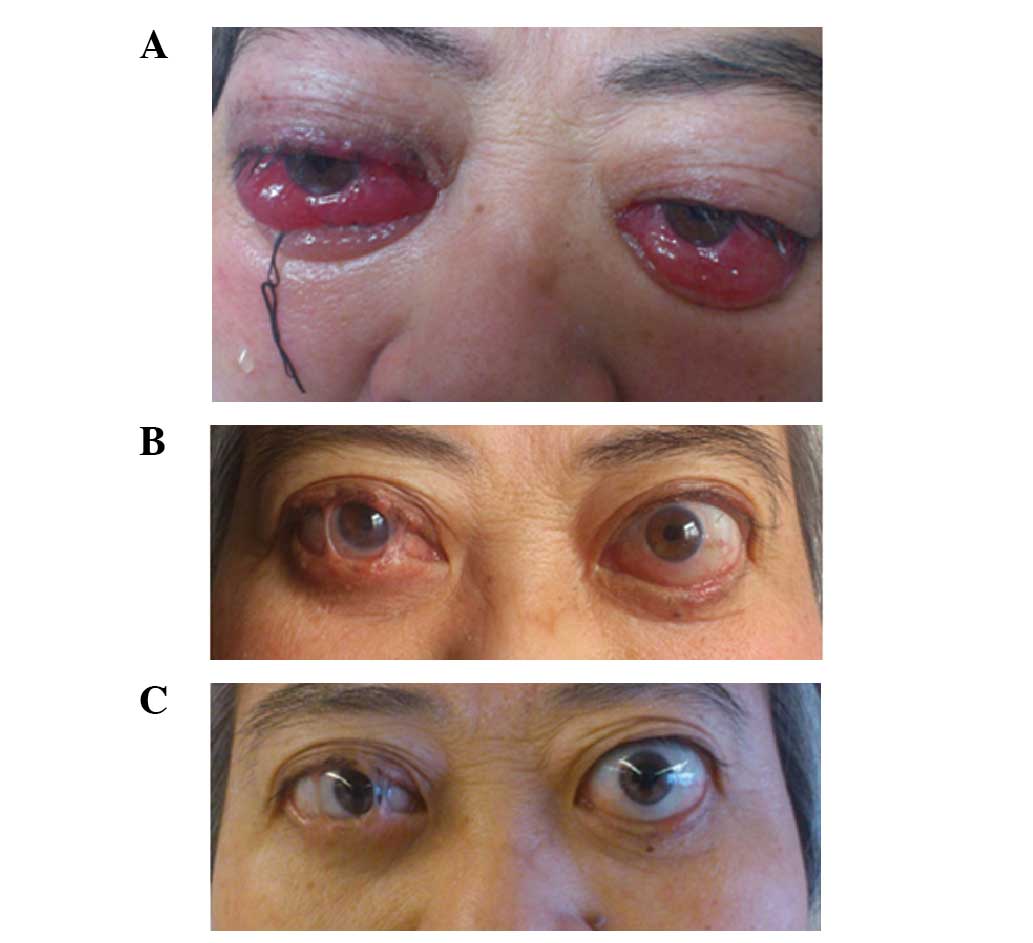
first infusion of RTX, the clinical features of GO were extremely
severe (Fig. 3A; 30th October 2013);
however, at 2 and 5 months following RTX withdrawal, the clinical
features were markedly improved (Fig. 3B
and C). The patient was maintained in a euthyroid state
following RTX therapy by alternate treatment with 5 and 2.5
mg/daily methimazole, which was reduced to 2.5 mg/daily in May
2014. Furthermore, the subsequent blood glucose levels were normal.
The patient was satisfied with the treatment.
Discussion
The clinical symptoms of GO may include periorbital
edema, lid lag and retraction, chemosis, exophthalmos and altered
ocular motility (6). Severe GO may
lead to exposure keratopathy, diplopia and compressive optic
neuropathy, which may cause visual loss (6). The pathophysiology of GO has been shown
to involve the humoral and cell-mediated immune responses (13). Existing treatment options for GO
include glucocorticoids (oral or intravenous) (14), orbital radiation (15), surgical decompression (16) or a combination of these. The present
study reports a novel option of immunosuppressive therapy involving
RTX for the treatment of patients with severe GO.
RTX is an anti-CD20 monoclonal antibody and
functions by depleting B-cells, which are precursors of the
autoantibody-producing plasma cells (6). Treatment with RTX leads to specific
elimination of B-cells without affecting the regeneration of
B-cells from stem cells and the production of immunoglobulins by
plasma cells, since CD20 is absent from stem cells, pro-B
lymphocytes and plasma cells (7–9). It has
been suggested that RTX-induced transient B-cell depletion, which
typically lasts for 4–6 months, may effectively modify the active
inflammatory phase of GO and its subsequent clinical course
(17). However, the exact mechanism
underlying the effects of RTX on GO is unclear, although various
hypotheses have been proposed, including the participation of
B-cells, TRAb and cytokines (6).
Previous studies have reported the involvement of
TRAbs in the pathogenesis of GO (18–20). A
previous clinical review suggested that GO occurs as a result of
immunological cross-reactivity between the thyroid and orbital
tissue antigens, of which the TSH receptor is included (18). Furthermore, it was observed that
hyperstimulation of the TSH receptor in the eyes leads to
glycosaminoglycan secretion by pre-adipocytic fibroblasts, which
subsequently increases the volume of the intraorbital tissues
(19,20). Previous studies have demonstrated
that TRAb levels are decreased following B-cell depletion to a
similar extent to that observed following treatment with
methimazole or prednisolone (17,21,22). The
TRAb level in the present case was decreased following RTX
treatment, which is consistent with the above theory and provides
further rationale for the use of RTX therapy for the treatment of
GO (17,21,22). In
addition, GD has been shown to be caused by B-cell-induced
production of TSH receptor-directed immunoglobulins (23), and the mechanism underlying the
treatment of GO with RTX involves the depletion of B-cells
(6). Therefore, there may be a
potential benefit for the use of RTX therapy in the treatment of
GD, which is consistent with previous studies (24,25).
The potential use of RTX in GO has previously been
reported in the literature (17,26–29).
However, the difference and importance of the present study is that
the GO of this patient was the most severe of the published cases.
Furthermore, to the best of our knowledge, the present study is the
first report of GO in a Chinese patient. Various conventional
therapies were implemented during the treatment of our patient;
however, the combined RTX treatment achieved satisfying efficacy
and allowed the surgical procedure of orbital decompression to be
avoided.
Acknowledgements
The present study was supported by the Major Project
of Technological Development of Nanjing Medical University (grant
no. 2015NJMUZD045).
References
|
1
|
Weetman AP: Graves' disease. N Engl J Med.
343:1236–1248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abraham-Nordling M, Törring O, Hamberger
B, Lundell G, Tallstedt L, Calissendorff J and Wallin G: Graves'
disease: A long-term quality-of-life follow up of patients
randomized to treatment with antithyroid drugs, radioiodine, or
surgery. Thyroid. 15:1279–1286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Estcourt S, Vaidya B, Quinn A and Shepherd
M: The impact of thyroid eye disease upon patients' wellbeing: A
qualitative analysis. Clin Endocrinol (Oxf). 68:635–639. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bahn RS: Graves' ophthalmopathy. N Engl J
Med. 362:726–738. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burch HB and Wartofsky L: Graves'
ophthalmopathy: Current concepts regarding pathogenesis and
management. Endocr Rev. 14:747–793. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minakaran N and Ezra DG: Rituximab for
thyroid-associated ophthalmopathy. Cochrane Database Syst Rev.
5:CD0092262013.PubMed/NCBI
|
|
7
|
Reff ME, Carner K, Chambers KS, Chinn PC,
Leonard JE, Raab R, Newman RA, Hanna N and Anderson DR: Depletion
of B cells in vivo by a chimeric mouse human monoclonal antibody to
CD20. Blood. 83:435–445. 1994.PubMed/NCBI
|
|
8
|
Tsokos GC: B cells, be gone-B-cell
depletion in the treatment of rheumatoid arthritis. N Engl J Med.
350:2546–2548. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen S, Chan A, Sfikakis PP, Hsiu Ling AL,
Detorakis ET, Boboridis KG and Mavrikakis I: B-cell targeted
therapy with rituximab for thyroid eye disease: Closer to the
clinic. Surv Ophthalmol. 58:252–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mourits MP, Koornneef L, Wiersinga WM,
Prummel MF, Berghout A and van der Gaag R: Clinical criteria for
the assessment of disease activity in Graves' ophthalmopathy: A
novel approach. Br J Ophthalmol. 73:639–644. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wiersinga WM, Smit T, Schuster-Uittenhoeve
AL, van der Gaag R and Koornneef L: Therapeutic outcome of
prednisone medication and of orbital irradiation in patients with
Graves' ophthalmopathy. Ophthalmologica. 197:75–84. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edwards JC, Szczepanski L, Szechinski J,
Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM and Shaw T:
Efficacy of B-cell-targeted therapy with rituximab in patients with
rheumatoid arthritis. N Engl J Med. 350:2572–2581. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han R and Smith TJ: T helper type 1 and
type 2 cytokines exert divergent influence on the induction of
prostaglandin E2 and hyaluronan synthesis by interleukin-1beta in
orbital fibroblasts: Implications for the pathogenesis of
thyroid-associated ophthalmopathy. Endocrinology. 147:13–19. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krassas GE, Gogakos A and Boboridis K:
Corticosteroids in the medical treatment of thyroid ophthalmopathy:
When and how? Somatostatin analogues: Where we stand today. Pediatr
Endocrinol Rev. 7(Suppl 2): S204–S209. 2010.
|
|
15
|
Rajendram R, Bunce C, Lee RW and Morley
AM: Orbital radiotherapy for adult thyroid eye disease. Cochrane
Database Syst Rev. 7:CD0071142012.PubMed/NCBI
|
|
16
|
Boboridis KG and Bunce C: Surgical orbital
decompression for thyroid eye disease. Cochrane Database Syst Rev.
12:CD0076302011.PubMed/NCBI
|
|
17
|
Salvi M, Vannucchi G, Campi I, Currò N,
Dazzi D, Simonetta S, Bonara P, Rossi S, Sina C, Guastella C, et
al: Treatment of Graves' disease and associated ophthalmopathy with
the anti-CD20 monoclonal antibody rituximab: An open study. Eur J
Endocrinol. 156:33–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bahn RS: Clinical review 157:
Pathophysiology of Graves' ophthalmopathy: The cycle of disease. J
Clin Endocrinol Metab. 88:1939–1946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valyasevi RW, Harteneck DA, Dutton CM and
Bahn RS: Stimulation of adipogenesis, peroxisome
proliferator-activated receptor-gamma (PPARgamma), and thyrotropin
receptor by PPARgamma agonist in human orbital preadipocyte
fibroblasts. J Clin Endocrinol Metab. 87:2352–2358. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao HJ, Wang HS, Zhang Y, Lin HY, Phipps
RP and Smith TJ: Activation of human orbital fibroblasts through
CD40 engagement results in a dramatic induction of hyaluronan
synthesis and prostaglandin endoperoxide H synthase-2 expression.
Insights into potential pathogenic mechanisms of thyroid-associated
ophthalmopathy. J Biol Chem. 273:29615–29625. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El FD, Nielsen CH, Bonnema SJ, Hasselbalch
HC and Hegedüs L: B lymphocyte depletion with the monoclonal
antibody rituximab in Graves' disease: A controlled pilot study. J
Clin Endocrinol Metab. 92:1769–1772. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heemstra KA, Toes RE, Sepers J, Pereira
AM, Corssmit EP, Huizinga TW, Romijn JA and Smit JW: Rituximab in
relapsing Graves' disease, a phase II study. Eur J Endocrinol.
159:609–615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brent GA: Clinical practice. Graves'
disease. N Engl J Med. 358:2594–2605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hasselbalch HC: B-cell depletion with
rituximab-a targeted therapy for Graves' disease and autoimmune
thyroiditis. Immunol Lett. 88:85–86. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang SH and Baker JJ: Targeting B cells in
Graves' disease. Endocrinology. 147:4559–4560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salvi M, Vannucchi G, Campi I, Currò N,
Simonetta S, Covelli D, Pignataro L, Guastella C, Rossi S, Bonara
P, et al: Rituximab treatment in a patient with severe
thyroid-associated ophthalmopathy: Effects on orbital lymphocytic
infiltrates. Clin Immunol. 131:360–365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Khanna D, Chong KK, Afifiyan NF, Hwang CJ,
Lee DK, Garneau HC, Goldberg RA, Darwin CH, Smith TJ and Douglas
RS: Rituximab treatment of patients with severe,
corticosteroid-resistant thyroid-associated ophthalmopathy.
Ophthalmology. 117:133.e2–139.e2. 2010. View Article : Google Scholar
|
|
28
|
Mitchell AL, Gan EH, Morris M, Johnson K,
Neoh C, Dickinson AJ, Perros P and Pearce SH: The effect of B cell
depletion therapy on anti-TSH receptor antibodies and clinical
outcome in glucocorticoid-refractory Graves' orbitopathy. Clin
Endocrinol (Oxf). 79:437–442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Salvi M, Vannucchi G, Currò N, Campi I,
Covelli D, Dazzi D, Simonetta S, Guastella C, Pignataro L, Avignone
S and Beck-Peccoz P: Efficacy of B-cell targeted therapy with
rituximab in patients with active moderate-severe Graves'
orbitopathy: A randomized controlled study. J Clin Endocrinol
Metab. 100:422–431. 2015. View Article : Google Scholar : PubMed/NCBI
|