Introduction
Prostate cancer (PCa) is the most common cancer
among European and American men, and accounts for 27% (233,000) of
cancer incidences in men in the USA (1). It has a high mortality rate as a result
of its high propensity for metastasis (2,3). PCa has
been shown to preferentially metastasize to the bone marrow stroma
of the axial skeleton (4); however,
the precise mechanism underlying PCa metastasis is currently
unclear. Therefore, the identification of specific metastasis
biomarkers and novel diagnostic targets is required in order to
improve the prognosis and treatment of the disease.
Previous studies have made considerable progress in
identifying the key regulators in the PCa metastatic process.
E-cadherin, which is attached to the actin cytoskeleton via
intracellular catenin, has been implicated in the process of PCa
metastasis; in primary PCa, reduced E-cadherin expression was
associated with bone metastasis and a poor prognosis (5). In addition, the expression of the
DLC1 tumor-suppressor gene in metastatic PCa cells has been
shown to upregulate the expression of E-cadherin, resulting in the
suppression of highly metastatic PCa cell invasion by inhibiting
the activity of RhoA-GTP and RhoC-GTP (6). The activation of Rho GTPases is
dependent on the downstream Ras protein, which has a major
influence on cell signaling (7).
Members of the Rho GTPase family are involved in cancer cell
motility by regulating actin dynamics and controlling morphological
changes (8). A previous study
demonstrated that the suppression of the farnesyl and
geranyl-geranyl prenylation pathways markedly reduced the migration
and motility of PCa cells by inhibiting Ras prenylation and
concurrent Rho activation (9).
Furthermore, activation of the phosphoinositide 3-kinase/protein
kinase B (AKT) signaling pathway has been more frequently observed
in resistant and metastatic PCa compared with primary PCa, and thus
targeting this signaling pathway may improve the outcome of
patients with aggressive PCa (10).
Previous studies have reported various genes able to promote PCa
tumorigenesis and metastasis, including CCL2 (11), SERPINB5 (12), SRC (13), TMPRSS2-ERG gene fusion and
PCA3 (14). In addition,
microRNAs (miRNAs), which are considered to be important regulators
of gene expression, have been associated with the development of
metastatic PCa. For instance, miR-203 (15), miR-16 (16), miR-205 (17), miR-24 (18), miR-29a (19) and miR-145 (16) have all been implicated in PCa
metastasis.
Varambally et al (20) performed an integrative genomic and
proteomic analysis of benign prostate and metastatic PCa; they
reported 48–64% concordance between protein and transcript levels
and demonstrated that proteomic alterations between metastatic and
clinically localized PCa, which map concordantly to gene
transcripts, can serve as predictors of clinical outcome in PCa as
well as other solid tumors. However, to the best of our knowledge,
the potential miRNAs involved in metastatic PCa, and the
interactions of differentially-expressed genes (DEGs) targeted by
miRNAs, have yet to be investigated. Therefore, the present study
aimed to further elucidate the molecular mechanisms underlying the
metastasis of PCa by analyzing the microarray data of benign
prostate, clinically localized and metastatic PCa deposited by
Varambally et al (20) in the
Gene Expression Omnibus (GEO) database. Initially a hierarchical
cluster analysis for DEGs was performed, followed by a Gene
Ontology (GO) functional enrichment analysis. Furthermore,
potential miRNAs in metastatic PCa were identified and a miRNA-DEG
regulatory network was constructed. Finally, a pathway enrichment
analysis for DEGs in the regulatory network was performed. The
results of this bioinformatics analysis may shed light on the
molecular mechanisms underlying the metastasis of PCa and provide
novel diagnostic biomarkers.
Materials and methods
Affymetrix microarray data
The GSE3325 gene expression profile data (20) was downloaded from the GEO (http://www.ncbi.nlm.nih.gov/geo/) and was based
on the GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus
2.0 Array platform. A total of 19 human prostate tissue samples
were available for further analysis, including seven clinically
localized PCa samples, six hormone-refractory metastatic PCa
samples and six benign prostate tissue samples.
CEL and probe annotation files were downloaded from
GEO, and the gene expression data for all samples were preprocessed
via Robust Multichip Averaging background correction, quantile
normalization and probe summarization (21) in the affy software package (version
1.34.0; http://bioconductor.org/packages/release/bioc/html/affy.html),
as described previously (22).
DEGs screening
The Linear Models for Microarray Data package of R
(https://bioconductor.org/packages/release/bioc/html/limma.html)
was used to identify genes that were differentially expressed in
the primary PCa and metastatic PCa groups, as compared with the
benign prostate group, as described previously (23). The raw P-value was adjusted according
to the false discovery rate (FDR) using the Benjamin and Hochberg
method (24). Only genes with a
cut-off criteria of |log2fold change| >1 and
FDR<0.01 were considered to be differentially expressed.
Hierarchical cluster analysis for
DEGs
Hierarchical clustering is a common method used to
determine clusters of similar data points in a multidimensional
space (25). The pheatmap package
(version 1.0.2; https://cran.r-project.org/web/packages/pheatmap/index.html)
was used to perform hierarchical clustering of the DEGs via joint
between-within distances, as described previously (26). Expression values from multiple clones
or probe sets mapping to the same Unigene Cluster ID were
averaged.
GO functional enrichment analysis for
DEGs
The Database for Annotation, Visualization and
Integrated Discovery (DAVID; https://david.ncifcrf.gov/) provides a comprehensive
set of novel and powerful tools for assigning biological meaning to
a set of genes (27). FDR<0.05
was used as the cut-off criterion for GO functional enrichment
analysis by DAVID.
Integrated miRNA-DEG regulatory
network construction
The common miRNAs in Gene set B, as predicted by the
databases of miRecords (http://c1.accurascience.com/miRecords/), TarBase
(http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=tarbase/index)
and TargetScan (http://www.targetscan.org/), were selected using
WEB-based GEne SeT AnaLysis Toolkit software (update 2013;
http://bioinfo.vanderbilt.edu/webgestalt/), and
P<0.05 was used as the cut-off criterion. Subsequently, the
Search Tool for the Retrieval of Interacting Genes (http://string-db.org/) was used to analyze the
interactions between the DEGs targeted by miRNAs by calculating
their combined score; a score of >0.4 was set as the cut-off
criterion. Finally, the integrated miRNA-DEG regulatory network was
visualized using Cytoscape (http://cytoscape.org/).
Pathway enrichment analysis for DEGs
in the regulatory network
Pathway enrichment analysis was conducted as
described previously (28) to
identify significant metabolic pathways for the DEGs. P<0.05 was
used as the cut-off criterion for the Kyoto Encyclopedia of Genes
and Genomes pathway enrichment analysis using DAVID.
Results
Identification of DEGs
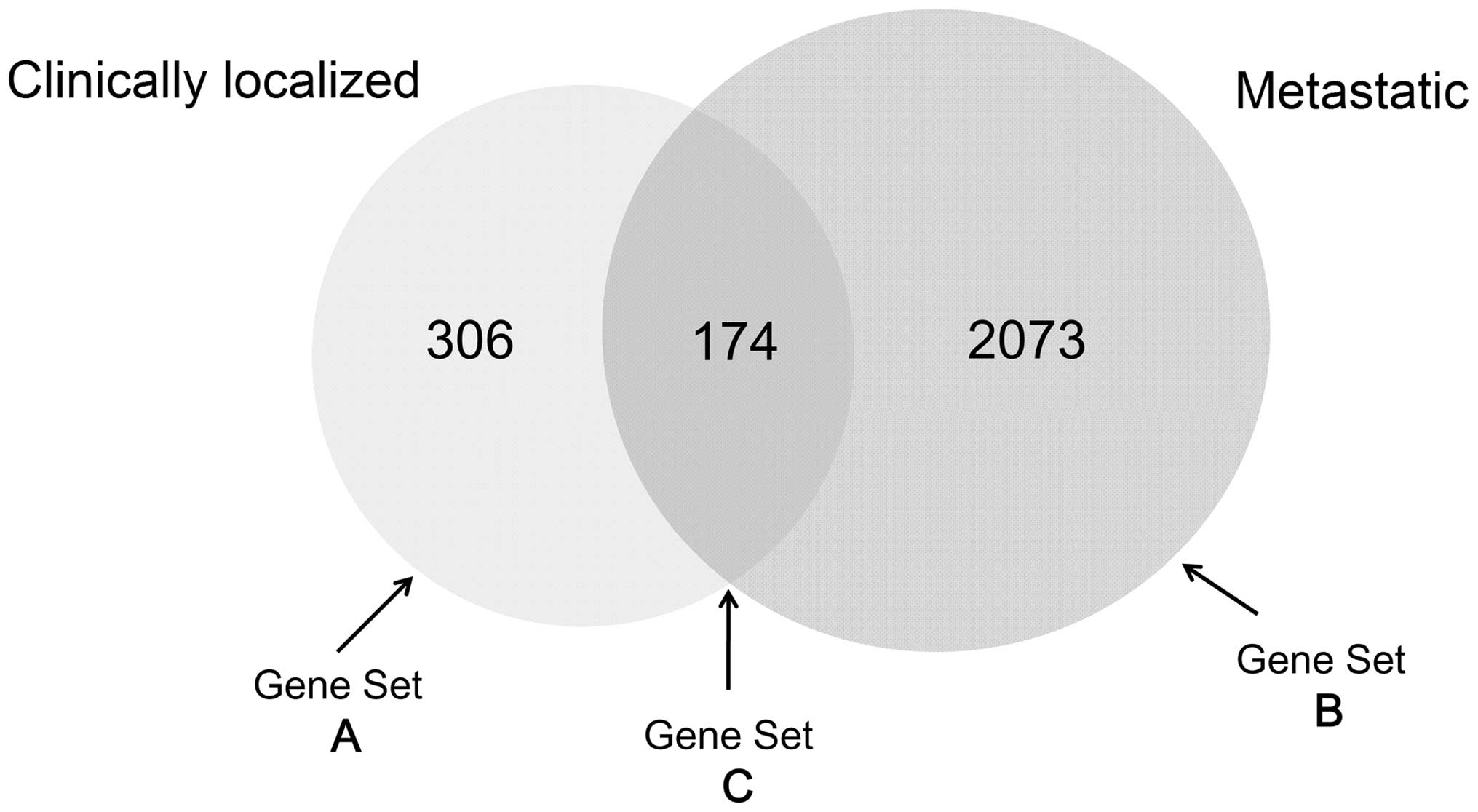
Based on the cut-off criteria, 2,727 DEGs were
identified for the clinically localized PCa and metastatic PCa
groups, of which 306 were differentially expressed in the
clinically localized PCa group only (Gene set A). A total of 2,073
genes were differentially expressed in the metastatic PCa group
only (Gene set B) and 174 genes were differentially expressed in
both groups (Gene set C; Fig. 1), as
compared with the benign prostate group.
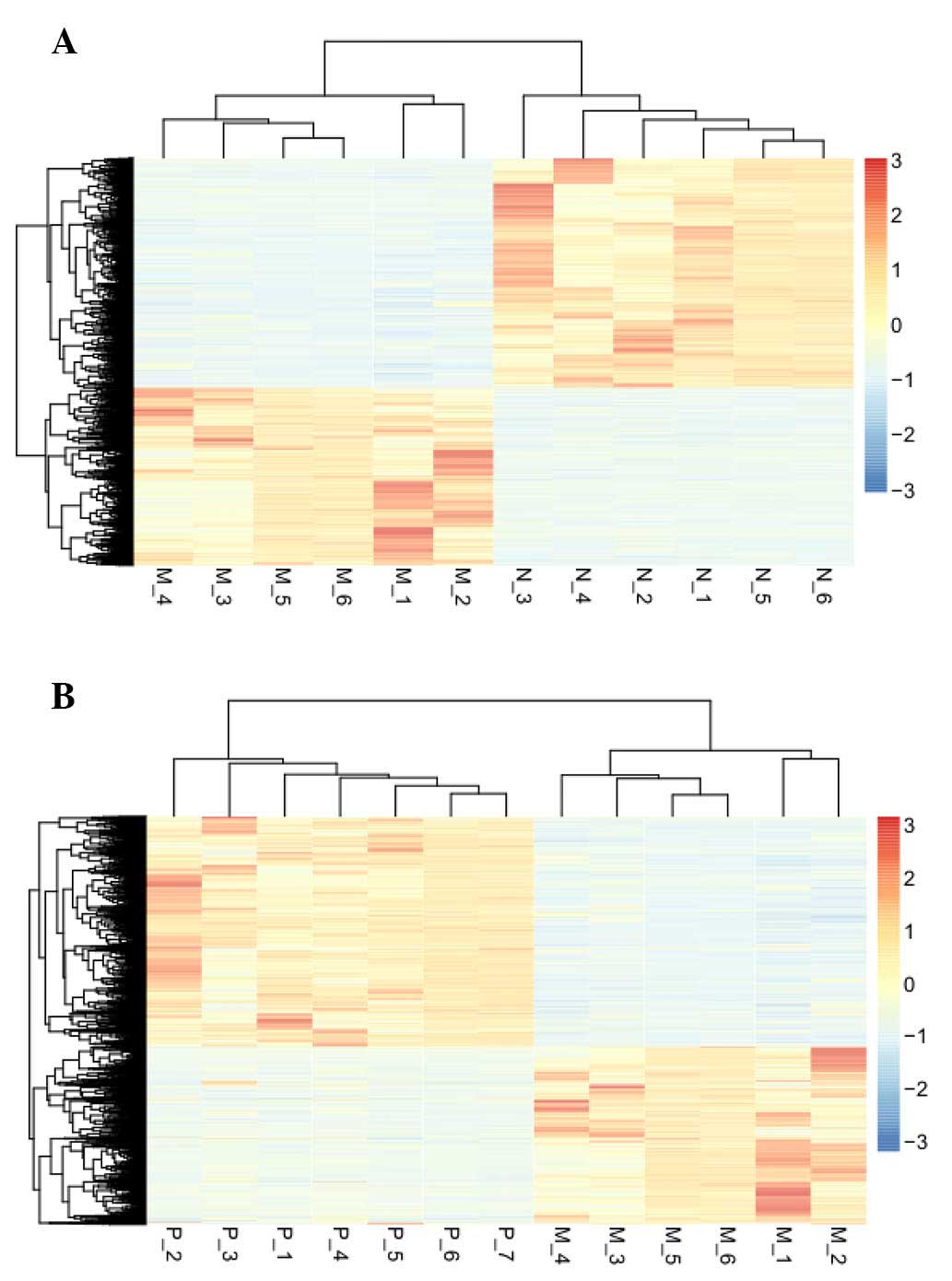
Hierarchical cluster analysis
An unsupervised hierarchical cluster analysis of the
data revealed that the DEGs could be used to accurately classify
prostate samples as benign, clinically localized prostate cancer or
metastatic disease (Fig. 2).
GO functional enrichment analysis for
Gene sets A, B and C
In Gene set A, DLX2, DLX1,
HOXD10 and HOXD11 DEGs were associated with
proximal/distal pattern formation (FDR=3.55E-04), whereas
RBP4, PDE3B and PPARG were implicated in the
response to insulin (FDR=7.8400), homeostatic processes
(FDR=9.6200), chemical homeostasis (FDR=0.0019) and responses to
peptide hormones (FDR=0.0023) and organic substances (FDR=0.0029)
(Table I).
 | Table I.Enriched terms for Gene sets A, B and
C. |
Table I.
Enriched terms for Gene sets A, B and
C.
| Category | Term | No. of genes | FDR | Genes |
|---|
| Gene set A |
GO:0009954~proximal/distal pattern
formation |
5 | 3.5500 | DLX2, DLX1,
GREM1, HOXD10, HOXD11 |
|
| GO:0032868~response
to insulin stimulus |
8 | 7.8400 | RBP4, EIF4EBP1,
FADS1, PPARG, PDE3B, STXBP4, GAL, VLDLR |
|
|
GO:0042592~homeostatic process | 24 | 9.6200 | RBP4, SLC12A2,
PPARG, F2RL1, PRDX4, PDE3B, CACNG2, ITPR3, PPARGC1A, MUC6… |
|
| GO:0001501~skeletal
system development | 14 | 0.0011 | RBP4, HOXD10,
HOXD11, MSX2, DLX2, DLX1, COL9A2, BCL2, CLEC3A, NAB1… |
|
| GO:0048878~chemical
homeostasis | 18 | 0.0019 | RBP4, F2RL1,
NOX1, PPARG, PDE3B, PPARGC1A, PRKCB, CCL11, MALL, ATP7B… |
|
|
GO:0021877~forebrain neuron fate
commitment |
3 | 0.0022 | DLX2, DLX1,
LHX6 |
|
| GO:0043434~response
to peptide hormone stimulus |
9 | 0.0023 | RBP4, EIF4EBP1,
FADS1, BCL2, PPARG, PDE3B, STXBP4, GAL, VLDLR |
|
| GO:0010033~response
to organic substance | 22 | 0.0029 | RBP4, ADCY1,
GNRH1, FADS1, LOC646626, PPARG, PTGS1, PDE3B, COLEC12,
STXBP4… |
|
| GO:0009725~response
to hormone stimulus | 14 | 0.0038 | RBP4, ADCY1,
GNRH1, FADS1, PTGS1, PPARG, PDE3B, STXBP4, GAL, EIF4EBP1… |
|
| GO:0034637~cellular
carbohydrate biosynthetic process |
6 | 60.0038 | RBP4, ISYNA1,
UAP1, GNE, PPARGC1A, ACN9 |
| Gene set B | GO:0022402~cell
cycle process | 102 | 5.2300 | PRC1, ZAK, AIF1,
BTRC, CDCA8, CDC6, CENPF, PTTG1, AURKB, TGFB2… |
|
|
GO:0051726~regulation of cell cycle | 68 | 1.1100 | E2F2, PTGS2,
ZAK, FAM175A, PKMYT1, PDCD4, PTEN, GTSE1, TGFB2, MYC… |
|
| GO:0007049~cell
cycle | 128 | 1.8200 | ZAK, PRC1, AIF1,
BTRC, PKMYT1, RBM7, AURKA, AURKB, PTTG1, TGFB2… |
|
| GO:0051301~cell
division | 61 | 4.6100 | PRC1, PTTG1,
CCNE1, CDCA2, CDC6, CABLES2, CDCA5, CCNA2, ASPM, CDK1… |
|
| GO:0022403~cell
cycle phase | 78 | 4.7700 | E2F1, PRC1,
PKMYT1, RBM7, AURKA, AURKB, PTTG1, GTSE1, CCNE1, CDCA8… |
|
| GO:0010035~response
to inorganic substance | 47 | 6.4600 | CAV1, GCLC,
PTGS2, PDGFA, SNCA, TPM1, PTEN, KCNMB1, FOS, GSN… |
|
|
GO:0007346~regulation of mitotic cell
cycle | 38 | 0.0011 | CAV2, HOXA13,
PML, PKMYT1, ASNS, ANLN, ZNF655, RCC1, SCRIB, MYC… |
|
|
GO:0042127~regulation of cell
proliferation | 126 | 0.0012 | HRAS, CD38,
IL6ST, PDGFA, TP63, MAF, TGFB3, STRN, PNP, TGFB2… |
|
| GO:0030030~cell
projection organization | 70 | 0.0015 | CAV2, HOXA13,
PML, PKMYT1, ANLN, ZNF655, RCC1, SCRIB, GTSE1, MYC… |
|
| GO:0000278~mitotic
cell cycle | 70 | 0.0018 | E2F1, PRC1,
BTRC, PKMYT1, AURKA, AURKB, PTTG1, GTSE1, CCNE1, NDE1… |
| Gene set C | GO:0022402~cell
cycle process | 21 | 3.3000 | MKI67, DLGAP5,
SGOL1, NUSAP1, BIRC5, PBK, CDKN3, CCNB1, CENPA, CAMK2D… |
|
| GO:0022403~cell
cycle phase | 18 | 3.5700 | MKI67, DLGAP5,
SGOL1, NUSAP1, TTK, BIRC5, PBK, CCNB1, CAMK2D, ID4… |
|
| GO:0000278~mitotic
cell cycle | 17 | 4.0700 | DLGAP5, SGOL1,
NUSAP1, TTK, BIRC5, PBK, CDKN3, CCNB1, CENPA, CAMK2D… |
|
| GO:0000279~M
phase | 15 | 2.7400 | MKI67, DLGAP5,
SGOL1, NUSAP1, TTK, BIRC5, PBK, UBE2C, CCNB1, KIF2C… |
|
| GO:0007049~cell
cycle | 22 | 1.1800 | DLGAP5, SGOL1,
NUSAP1, BIRC5, PBK, CCNB1, SH3BP4, KIF2C, CENPA, CAMK2D… |
|
| GO:0000280~nuclear
division | 11 | 4.5000 | CCNB1, KIF2C,
CCNB2, DLGAP5, SGOL1, NUSAP1, BIRC5, PBK, UBE2C, ERCC6L… |
|
|
GO:0007067~mitosis | 11 | 4.5000 | CCNB1, KIF2C,
CCNB2, DLGAP5, SGOL1, NUSAP1, BIRC5, PBK, UBE2C, ERCC6L… |
|
| GO:0000087~M phase
of mitotic cell cycle | 11 | 5.2300 | CCNB1, KIF2C,
CCNB2, DLGAP5, SGOL1, NUSAP1, BIRC5, PBK, UBE2C, ERCC6L… |
|
|
GO:0048285~organelle fission | 11 | 6.3000 | CCNB1, KIF2C,
CCNB2, DLGAP5, SGOL1, NUSAP1, BIRC5, PBK, UBE2C, ERCC6L… |
|
|
GO:0007346~regulation of mitotic cell
cycle |
9 | 9.6800 | DLGAP5, CAMK2D,
NUSAP1, TTK, BIRC5, AFAP1L2, GAS1, UBE2C, MYC |
In Gene set B, the DEGs were predominantly
associated with the cell cycle: PRC1, ZAK,
PTTG1, TGFB2, CDCA8, CDC6 and
CENPF were associated with the cell cycle process
(FDR=5.2300); PRC1, PTTG1, CCNE1, CDCA2
and CDC6 were involved in cell division (FDR=4.6100); and
HRAS, CD38, IL6ST, PDGFA, TP63,
MAF and TGFB3 were associated with the regulation of
cell proliferation (FDR=0.0012) (Table
I).
In Gene set C, the DEGs were also predominantly
associated with the cell cycle. DLGAP5, SGOL1,
NUSAP1, PBK, BIRC5 and CCNB1 were
associated with the cell cycle process (FDR=3.3000), M phase
(FDR=2.7400), mitosis (FDR=4.5000) and organelle fission
(FDR=6.3000), whereas SH3BP4, KIF2C, CCNB2,
CENPA and CAMK2D were associated with the cell cycle
only (FDR=1.1800) (Table I).
Analysis of the miRNA-DEG regulatory
network
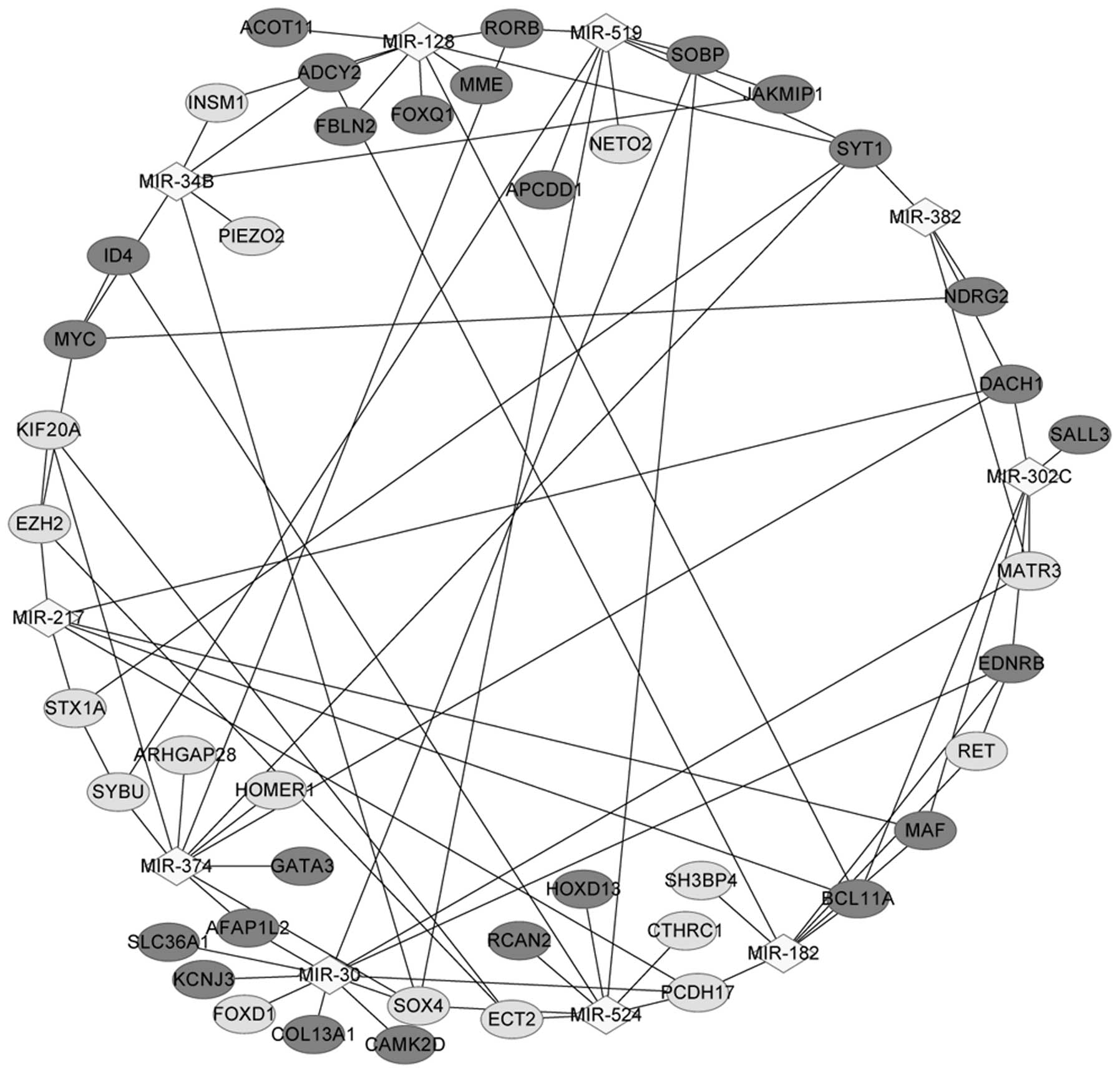
A total of 10 miRNAs were identified in Gene set B,
including miR-374, miR-128, miR-182, miR-30, miR-302c and miR-524.
Notably, miR-30 targeted the majority of the DEGs (11 DEGs,
including CAMK2D, PCDH17, EDNRB, KCNJ3
and SOX4), and miR-182 targeted seven DEGs, including
EDNRB, MAF, ADCY2, PCDH17, RET,
SH3BP4 and BCL11A (Table
II).
 | Table II.Enriched microRNAs in Gene set B. |
Table II.
Enriched microRNAs in Gene set B.
| microRNA | P-value | Count | Genes targeted by
microRNA |
|---|
| hsa_TATTATA,
MIR-374 | 2.1100 | 10 | RORB,
HOMER1, KIF20A, SYBU, DACH1,
GATA3, ARHGAP28, AFAP1L2, SOX4,
SYT1 |
| hsa_CACTGTG,
MIR-128 | 0.0003 | 9 | RORB, FBLN2,
ADCY2, ACOT11, INSM1, SYT1, FOXQ1, MME, BCL11A |
| hsa_ATGCAGT,
MIR-217 | 0.0003 | 6 | STX1A, MAF,
PCDH17, DACH1, EZH2, BCL11A |
| hsa_TGTTTAC,
MIR-30 | 0.0005 | 11 | SOBP, CAMK2D,
COL13A1, SLC36A1, PCDH17, AFAP1L2, EDNRB, KCNJ3, SOX4,
MATR3…… |
| hsa_ACAACTT,
MIR-382 | 0.0032 | 4 | NDRG2, SYT1,
MATR3, DACH1 |
| hsa_ACTGCCT,
MIR-34B | 0.0032 | 6 | INSM1, SOX4,
MYC, ADCY2, PIEZO2, JAKMIP1 |
| hsa_TTGCCAA,
MIR-182 | 0.0036 | 7 | EDNRB, MAF,
ADCY2, PCDH17, RET, SH3BP4, BCL11A |
| hsa_CTTTGTA,
MIR-524 | 0.0036 | 8 | SOBP, CTHRC1,
PCDH17, ECT2, ID4, RCAN2, HOXD13, SOX4 |
| hsa_ATGTTAA,
MIR-302C | 0.0038 | 6 | EDNRB, SALL3,
MAF, MATR3, DACH1, BCL11A |
| hsa_TGCACTT,
MIR-519 | 0.0038 | 8 | SOBP, RORB,
SYBU, NETO2, SOX4, SYT1, APCDD1, JAKMIP1 |
The miRNA-DEG regulatory network in Fig. 3 contained 10 miRNAs and 43
corresponding DEGs. ADCY2 was regulated by miR-128, miR-34B
and miR-182; EDNRB was regulated by miR-30, miR-182 and
miR-302C; CAMK2D was regulated by miR-30; PCDH17 was
modulated by miR-217, miR-30, miR-182 and miR-524; SH3BP4
was modulated by miR-182; and MAF interacted with miR-182,
miR-302c and BCL11A.
Pathway enrichment analysis for the
DEGs in the regulatory network
The DEGs in the regulatory network were enriched in
two pathways, including the calcium signaling pathway
(EDNRB, ADCY2 and CAMK2D), and thyroid cancer
(RET and MYC; Table
III).
 | Table III.Enriched pathways for the
differentially-expressed genes in the regulatory network. |
Table III.
Enriched pathways for the
differentially-expressed genes in the regulatory network.
| Term | Description | Count | P-value | Genes |
|---|
| hsa04020 | Calcium signaling
pathway | 3 | 0.02231 | EDNRB, ADCY2,
CAMK2D |
| hsa05216 | Thyroid cancer | 2 | 0.03927 | RET,
MYC |
Discussion
The present study identified 306 and 2,073 genes
that were differentially expressed in the clinically localized PCa
group and the metastatic PCa group, respectively, as compared with
the benign prostate group. Of these, 174 genes were differentially
expressed in both the clinically localized PCa and metastatic PCa
groups.
ADCY2, which encodes adenylate cyclase 2, and
CAMK2D, which encodes calcium/calmodulin-dependent protein
kinase II δ (29,30), were shown to be enriched in the
calcium signaling pathway. Metastasis is the predominant cause of
mortality in patients with PCa, and Ca2+ is a crucial
regulator of cell migration (31).
Elevated intracellular concentrations of Ca2+ may
facilitate the metastasis of PCa by triggering the activation of
the Akt signaling pathway and promoting PCa cell (PC3) attachment
(32). CAMK2D encodes
components of the Wnt/β-catenin-signaling pathway, the inhibition
of which delays metastatic PCa cell cycle progression and
proliferation (33). In the present
study, CAMK2D was associated with the cell cycle, which is
known to be a critical event in tumor growth and metastasis
(34). Furthermore, CAMK2D
was observed to be regulated by miR-30. As a tumor suppressor,
miR-30 has been shown to be downregulated by oncogenic signals,
such as hepatocyte growth factor and epidermal growth factor, in
PCa samples (35), and
overexpression of miR-30 in PCa cells was reported to suppress the
epithelial-to-mesenchymal transition and inhibit cell migration and
invasion (36).
ADCY2 was observed to be modulated by
miR-182. A previous study demonstrated that ectopic expression of
miR-182 in PC3 significantly reduced protein expression levels of
GNA13, GNA13-3′-untranslated region (UTR)-reporter activity and
extracorporeal invasion of these cells (37). In addition, aberrant overexpression
of miR-182 was shown to promote the proliferation, increase the
invasion, facilitate the G1/S cell cycle transition and reduce
early apoptosis of PC3 cells; and, miR-182 was able to suppress the
expression of the NDRG1 tumor suppressor gene by directly
targeting the NDRG1 3′-UTR (38). Therefore, CAMK2D and
ADCY2 may be involved in the metastasis of PCa via calcium
signaling and regulation by miR-30 and miR-182, respectively.
MAF, which was also modulated by miR-182 in
the present study, was associated with the regulation of cell
proliferation. MAF acts as a macrophage-activating factor and is
generated from a precursor protein termed the Gc protein (39). Deglycosylation of the Gc protein
prevents its conversion to MAF, inhibiting macrophage activation
and resulting in immunosuppression (40). In a previous study, patients with
metastatic PCa were administered Gc protein with MAF precursor
activity (100 ng/week), and were shown to have serum activity
levels of Nagalase equivalent to those of healthy controls, thus
suggesting that these patients were tumor-free (41). Furthermore, MAF expression has
been associated with the receptor tyrosine kinase, platelet-derived
growth factor receptor (PDGFR)-β status (42). In the miRNA-DEG regulatory network,
MAF was also modulated by miR-302c, and it has been reported
that miR-302c is downregulated in clinical PCa samples (43). In addition, MAF interacted
with BCL11A, which was observed to be upregulated in PC3
holoclones (44). Therefore,
MAF may have an important role in the metastasis of PCa by
interacting with miR-182, miR-302c and BCL11A.
In the present study, the downregulated DEG
SH3BP4 was shown to be associated with the cell cycle and
was also regulated by miR-182. SH3BP4 encodes SH3-domain
binding protein 4 (45). SH3 domains
are found in a variety of proteins, including tyrosine kinases,
such as Abl and Src, and are involved in endocytosis, intracellular
sorting and the cell cycle (46).
Another downregulated DEG PCDH17, which encodes
protocadherin 17, was shown to interact with miR-182 and miR-30.
PCDH17 methylation is a common tumor-specific event in PCa
and has been associated with a shorter biochemical recurrence-free
survival rate and a reduced overall survival rate of patients with
PCa following a radical prostatectomy (47). Therefore, SH3BP4 and
PCDH17 may be responsible for the metastasis of PCa via
their interactions with miR-182 and/or miR-30. Furthermore, miR-374
was significantly enriched in Gene set B. Previous studies have
reported that miR-374 is markedly downregulated in PCa (48,49).
Furthermore, miR-374b, which is a subtype of miR-374, has been
shown to be downregulated in prostate fluid or serum samples from
prostate cancer patients, and thus may serve as a PCa biomarker in
clinical diagnosis (50).
In conclusion, the present study identified numerous
important DEGs, including ADCY2, CAMK2D, MAF,
SH3BP4 and PCDH17, that may be involved in the
metastasis of PCa. However, the results of the present study
require validation by further experiments, and the molecular
mechanisms underlying metastatic PCa require further
investigation.
Acknowledgements
The present study was supported by the National
Science Foundation of China (grant no. 81470923).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: Estimates of cancer incidence and mortality in Europe in 2008.
Eur J Cancer. 46:765–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Watahiki A and Wang Y, Morris J, Dennis K,
O'Dwyer HM, Gleave M, Gout PW and Wang Y: MicroRNAs associated with
metastatic prostate cancer. PLoS One. 6:e249502011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun YX, Schneider A, Jung Y, Wang J, Dai
J, Wang J, Cook K, Osman NI, Koh-Paige AJ, Shim H, et al: Skeletal
localization and neutralization of the SDF-1 (CXCL12)/CXCR4 axis
blocks prostate cancer metastasis and growth in osseous sites in
vivo. J Bone Miner Res. 20:318–329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng L, Nagabhushan M, Pretlow TP, Amini
SB and Pretlow TG: Expression of E-cadherin in primary and
metastatic prostate cancer. Am J Pathol. 148:1375–1380.
1996.PubMed/NCBI
|
|
6
|
Tripathi V, Popescu NC and Zimonjic DB:
DLC1 induces expression of E-cadherin in prostate cancer cells
through Rho pathway and suppresses invasion. Oncogene. 33:724–733.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu L, Shi Y, Hsu JH, Gera J, Van Ness B
and Lichtenstein A: Downstream effectors of oncogenic ras in
multiple myeloma cells. Blood. 101:3126–3135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hall A: Rho family GTPases. Biochem Soc
Trans. 40:1378–1382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khafagy R, Stephens T, Hart C, Ramani V,
Brown M and Clarke N: In vitro effects of the prenyl transferase
inhibitor AZD3409 on prostate cancer epithelial cells. J Clin
Oncol. 22:47442004.
|
|
10
|
Toren P and Zoubeidi A: Targeting the
PI3K/Akt pathway in prostate cancer: Challenges and opportunities
(review). Int J Oncol. 45:1793–1801. 2014.PubMed/NCBI
|
|
11
|
Zhang J, Patel L and Pienta KJ: CC
chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis
and metastasis. Cytokine Growth Factor Rev. 21:41–48. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo JL, Tan W, Ricono JM, Korchynskyi O,
Zhang M, Gonias SL, Cheresh DA and Karin M: Nuclear
cytokine-activated IKKalpha controls prostate cancer metastasis by
repressing Maspin. Nature. 446:690–694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai H, Smith DA, Memarzadeh S, Lowell CA,
Cooper JA and Witte ON: Differential transformation capacity of Src
family kinases during the initiation of prostate cancer. Proc Natl
Acad Sci USA. 108:6579–6584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salagierski M and Schalken JA: Molecular
diagnosis of prostate cancer: PCA3 and TMPRSS2: ERG gene fusion. J
Urol. 187:795–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saini S, Majid S, Yamamura S, Tabatabai L,
Suh SO, Shahryari V, Chen Y, Deng G, Tanaka Y and Dahiya R:
Regulatory role of miR-203 in prostate cancer progression and
metastasis. Clin Cancer Res. 17:5287–5298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schaefer A, Jung M, Mollenkopf HJ, Wagner
I, Stephan C, Jentzmik F, Miller K, Lein M, Kristiansen G and Jung
K: Diagnostic and prognostic implications of microRNA profiling in
prostate carcinoma. Int J Cancer. 126:1166–1176. 2010.PubMed/NCBI
|
|
17
|
Gandellini P, Folini M and Zaffaroni N:
Towards the definition of prostate cancer-related microRNAs: Where
are we now? Trends Mol Med. 15:381–390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szczyrba J, Löprich E, Wach S, Jung V,
Unteregger G, Barth S, Grobholz R, Wieland W, Stöhr R, Hartmann A,
et al: The microRNA profile of prostate carcinoma obtained by deep
sequencing. Mol Cancer Res. 8:529–538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Varambally S, Yu J, Laxman B, Rhodes DR,
Mehra R, Tomlins SA, Shah RB, Chandran U, Monzon FA, Becich MJ, et
al: Integrative genomic and proteomic analysis of prostate cancer
reveals signatures of metastatic progression. Cancer Cell.
8:393–406. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gautier L, Cope L, Bolstad BM and Irizarry
RA: affy-analysis of Affymetrix GeneChip data at the probe level.
Bioinformatics. 20:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smyth GK: Linear models and empirical
bayes methods for assessing differential expression in microarray
experiments. Stat Appl Genet Mol Biol. 3:2004.PubMed/NCBI
|
|
24
|
Verhoeven KJ, Simonsen KL and McIntyre LM:
Implementing false discovery rate control: Increasing your power.
Oikos. 108:643–647. 2005. View Article : Google Scholar
|
|
25
|
Olson CF: Parallel algorithms for
hierarchical clustering. Parallel Computing. 21:1313–1325. 1995.
View Article : Google Scholar
|
|
26
|
Kolde R: Pheatmap: Pretty Heatmaps. R
package version 0.7. 7. Journal. 2012.
|
|
27
|
Huang DW, Sherman BT, Tan Q, Collins JR,
Alvord G, Roayaei J, Stephens R, Baseler MW, Lane HC and Lempicki
RA: The DAVID Gene functional classification tool: A novel
biological module-centric algorithm to functionally analyze large
gene lists. Genome Biol. 8:R1832007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang DW, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Visel A, Alvarez-Bolado G, Thaller C and
Eichele G: Comprehensive analysis of the expression patterns of the
adenylate cyclase gene family in the developing and adult mouse
brain. J Comp Neurol. 496:684–679. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hagemann D, Bohlender J, Hoch B, Krause EG
and Karczewski P: Expression of
Ca2+/calmodulin-dependent protein kinase II
delta-subunit isoforms in rats with hypertensive cardiac
hypertrophy. Mol Cell Biochem. 220:69–76. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Prevarskaya N, Skryma R and Shuba Y:
Calcium in tumour metastasis: New roles for known actors. Nat Rev
Cancer. 11:609–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liao J, Schneider A, Datta NS and McCauley
LK: Extracellular calcium as a candidate mediator of prostate
cancer skeletal metastasis. Cancer Res. 66:9065–9073. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rajan P, Sudbery IM, Villasevil ME, Mui E,
Fleming J, Davis M, Ahmad I, Edwards J, Sansom OJ, Sims D, et al:
Next-generation sequencing of advanced prostate cancer treated with
androgen-deprivation therapy. Eur Urol. 66:32–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Whitfield ML, Sherlock G, Saldanha AJ,
Murray JI, Ball CA, Alexander KE, Matese JC, Perou CM, Hurt MM,
Brown PO and Botstein D: Identification of genes periodically
expressed in the human cell cycle and their expression in tumors.
Mol Biol Cell. 13:1977–2000. 2002. View Article : Google Scholar PubMed/NCBI
|
|
35
|
White R and Kung H: miR-30 as a tumor
suppressor connects EGF/Src signal to ERG and EMT. Oncogene.
33:2495–2503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kao C, Martiniez A, Shi X, Yang J, Evans
C, Dobi A, Devere White R and Kung H: miR-30 as a tumor suppressor
connects EGF/Src signal to ERG and EMT. Oncogene. 33:2495–2503.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rasheed SA, Teo CR, Beillard EJ, Voorhoeve
PM and Casey PJ: MicroRNA-182 and microRNA-200a control G-protein
subunit α-13 (GNA13) expression and cell invasion synergistically
in prostate cancer cells. J Biol Chem. 288:7986–7995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu R, Li J, Teng Z, Zhang Z and Xu Y:
Overexpressed microRNA-182 promotes proliferation and invasion in
prostate cancer PC-3 cells by down-regulating N-myc downstream
regulated gene 1 (NDRG1). PLoS One. 8:e689822013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nagasawa H, Uto Y, Sasaki H, Okamura N,
Murakami A, Kubo S, Kirk KL and Hori H: Gc protein (vitamin
D-binding protein): Gc genotyping and GcMAF precursor activity.
Anticancer Res. 25:3689–3695. 2005.PubMed/NCBI
|
|
40
|
Yamamoto N, Naraparaju VR and Asbell SO:
Deglycosylation of serum vitamin D3-binding protein leads to
immunosuppression in cancer patients. Cancer Res. 56:2827–2831.
1996.PubMed/NCBI
|
|
41
|
Yamamoto N, Suyama H and Yamamoto N:
Immunotherapy for prostate cancer with Gc protein-derived
macrophage-activating factor, GcMAF. Transl Oncolo. 1:65–72. 2008.
View Article : Google Scholar
|
|
42
|
Sharad S, Srivastava A, Ravulapalli S,
Parker P, Chen Y, Li H, Petrovics G and Dobi A: Prostate cancer
gene expression signature of patients with high body mass index.
Prostate Cancer Prostatic Dis. 14:22–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Coppola V, De Maria R and Bonci D:
MicroRNAs and prostate cancer. Endocr Relat Cancer. 17:F1–F17.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang K and Waxman DJ: PC3 prostate
tumor-initiating cells with molecular profile
FAM65Bhigh/MFI2low/LEF1low increase tumor angiogenesis. Mol Cancer.
9:3192010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dunlevy JR, Koppelman ED and Kolberg JB:
The expression of a SH3BP4-related protein in retinal cells. Invest
Ophthalmol Vis Sci. 46:2996. 2005.
|
|
46
|
Dunlevy JR, Berryhill BL, Vergnes JP,
SundarRaj N and Hassell JR: Cloning, chromosomal localization and
characterization of cDNA from a novel gene, SH3BP4, expressed by
human corneal fibroblasts. Genomics. 62:519–524. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin YL, Xie PG, Wang L and Ma JG: Aberrant
methylation of protocadherin 17 and its clinical significance in
patients with prostate cancer after radical prostatectomy. Med Sci
Monit. 20:1376–1382. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tang X, Tang X, Gal J, Kyprianou N, Zhu H
and Tang G: Detection of microRNAs in prostate cancer cells by
microRNA array. Methods Mol Biol. 732:69–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ma S, Chan YP, Kwan PS, Lee TK, Yan M,
Tang KH, Ling MT, Vielkind JR, Guan XY and Chan KW: MicroRNA-616
induces androgen-independent growth of prostate cancer cells by
suppressing expression of tissue factor pathway inhibitor TFPI-2.
Cancer Res. 71:583–592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
He HC, Han ZD, Dai QS, Ling XH, Fu X, Lin
ZY, Deng YH, Qin GQ, Cai C, Chen JH, et al: Global analysis of the
differentially expressed miRNAs of prostate cancer in Chinese
patients. BMC Genomics. 14:7572013. View Article : Google Scholar : PubMed/NCBI
|