Introduction
Periodontal disease (PD) is an infection-driven
chronic inflammatory disease characterized by the resorption of the
tooth-supporting alveolar bone (1).
PD is typically induced by bacteria that colonize the tooth surface
and gingival sulcus; however, the host immune response is
hypothesized to serve a crucial function in the breakdown of
connective tissue and bone, and is therefore considered to be a key
feature of the disease process (2).
In addition, an intermediate mechanism involving cytokines,
including chemokines and innate or acquired immune cytokines, has
been hypothesized to link the bacterial stimulation of the immune
system with tissue destruction (3).
Previous studies have reported that macrophage-derived chemokines
were elevated in the destructive and reparative phases of
periodontitis, and that the chemokines were crucially involved in
the recruitment of macrophages (4).
In addition, the cytokines may have directly or indirectly
regulated the alveolar bone loss process (5).
In a previous study, the C-C group chemokine
monocyte chemoattractant protein-1 (MCP-1), which is also known as
chemokine (C-C motif) ligand (CCL) 2, demonstrated chemotactic
effects on lymphocytes and monocytes; suggesting that MCP-1 is a
major signal for triggering the chemotaxis of mononuclear
leukocytes (6). In addition, it has
previously been demonstrated that the activation of the nuclear
factor-κB, mitogen-activated protein kinase (MAPK) and Akt pathways
in bone marrow stem cells (BMSCs), by high glucose levels or
inflammation, induces the expression of interleukin (IL)-1β, IL-6,
CCL2, CCL5, chemokine (C-X-C motif) ligand (CXCL) 1 and CXCL5 via
WNT5A (7).
The spatial organization of tissues and cell-cell
interactions have been disregarded in the majority of available
in vitro models. However, co-culturing techniques have
recently been developed in order to enhance the similarities
between cell cultures and in vivo systems (8,9).
Co-culture permits the free exchange of signaling molecules,
including proteins, carbohydrates and other small molecules
(10–12). Therefore, co-culture may be
considered an alternative approach for mimicking the BMSC
microenvironment in tissue culture. In addition, co-culture may
permit the determination of the intermediate mechanism between
local inflammation caused by bacterial infection and host immune
responses in diabetic periodontitis (DP). The present study aimed
to investigate the mechanisms underlying alveolar bone loss in
patients with DP, using a co-culture system consisting of BMSCs and
macrophages, and a mouse model of DP.
Materials and methods
Reagents
Tissue culture materials were obtained from Corning
Incorporated (Corning, NY, USA). The chemicals and reagents used in
the present study and their suppliers were as follows:
Streptozotocin (STZ) was purchased from Sigma-Aldrich (St. Louis,
MO, USA); the enzyme-linked immunosorbent assay (ELISA) kit for
tumor necrosis factor (TNF)-α was obtained from Cusabio Biotech
Co., Ltd. (Wuhan, China); the ReadyPrep Protein Extraction kit,
nitrocellulose membranes and SuperSignal West Pico Chemiluminescent
Substrate were obtained from Bio-Rad Laboratories, Inc. (Hercules,
CA, USA). The antibodies used in the present study were as follows:
Rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
polyclonal antibody (1:300; sc-25778), mouse anti-c-Jun N-terminal
kinase (JNK) monoclonal antibody (mAb; 1:200; sc-7345), mouse
anti-phosphorylated (p)-JNK mAb (1:200; sc-6254), mouse
anti-extracellular signal-regulated kinase (ERK) mAb (1:200;
sc-376852), mouse anti-p-ERK mAb (1:200; sc-377400), mouse anti-p38
mAb (1:200; sc-81621), mouse anti-p-p38 mAb (1:200; sc-7973), mouse
anti-CCL2 mAb (1:200; sc-1785), mouse anti-CCR2 mAb (1:200;
sc-46862), and goat anti-rabbit (1:3,000; sc-2030) and anti-mouse
(1:2,000; sc-2005) horseradish peroxidase-conjugated secondary
antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Animals and grouping
A total of 30 specific-pathogen-free grade
4-week-old C57BL/6 wild-type female mice, weighing ~22 g, were
obtained from the Experimental Animal Laboratory of Sichuan
University (Chengdu, China). All mice were handled in strict
accordance with the Animal Ethics Procedures and Guidelines of the
People's Republic of China, and the protocol of the present study
was approved by the Institutional Committee for Animal Use at
Sichuan University (SCU-2014-03). All mice were maintained under
standard conditions, including a 12 h light/dark cycle at 18–22°C
and 50–60% humidity, with ad libitum access to standard
laboratory altromin chow (Dossy Co., Chengdu, China) and water.
The mice were randomly divided into three groups
(n=10 per group) as follows: i) Normal control (N) group; ii)
Porphyromonas gingivalis (PG) periodontal infection (P)
group; and iii) hyperglycemia plus PG periodontal infection (HP)
group. All mice were anesthetized using 220 mg/kg sodium
pentobarbital (Anpro Pharmaceuticals, Arcadia, CA, USA) and were
sacrificed via decapitation at 12-weeks-old.
Experimental diabetes induction
Diabetes was induced in 6-week-old HP group mice.
Briefly, the mice in the HP group consumed a high-fat diet (48
kcal% fat) (Dossy Co.) for 7 days, during which the mice received
intraperitoneal injection with 40 mg/kg STZ dissolved in buffer
supplemented with citric acid (pH 4.5) for 5 days, followed by
overnight fasting. After 7 days, the mice in the HP group received
a standard low-fat diet (12.3 kcal% fat; Dossy Co.) for the
remaining experimental period. The mice in the N and P groups
consumed a standard low-fat diet throughout the duration of the
study. At 9 weeks old, all mice in the HP group were rendered
diabetic, according to the standards described in a previous study
(13).
Fasting blood glucose analysis
Blood glucose measurements were conducted when the
mice were aged 9 weeks and upon sacrifice. Briefly, mouse tail
veins were pierced using a needle, and the blood was collected from
the tail vein following a 10-h fast. A glucometer was used in order
to determine the fasting blood glucose levels (OneTouch®
Glucometer; LifeScan, Inc., Wayne, PA, USA).
Quantification of alveolar bone
loss
Following sacrifice, the mandibular jaws of the mice
were separated from the surrounding soft tissues. Alveolar bone
loss at the first and second mandibular molars was monitored using
a stereomicroscope with an attached digital camera (Leica MZ FLIII;
Leica Microsystems GmbH, Wetzlar, Germany). The bone loss level was
determined by measuring the area bordered by the cemento-enamel
junction, alveolar bone crest, and mesial and distal line angles on
the lingual side of the first and second mandibular molars. The
measurements were conducted blindly using 15-fold magnified images
of the bones and run in triplicate. Bone loss per animal was
calculated as the average of both mandibles from each mouse.
Assessment of inflammatory cell
infiltration
Monocyte and lymphocyte infiltration was determined
as the number of cells in the alveolar bone biopsies, which were
histologically prepared from all groups. Mouse maxillas were
harvested following sacrifice and were prepared and examined using
immunohistochemical staining, as previously described (14). Briefly, the maxillas were decalcified
in 10% ethylene diaminetetraacetic acid (EDTA; Bio-Rad
Laboratories, Inc.) for 14 days, and were subsequently embedded in
paraffin. Samples were cut into 5 µm sections and analyzed using
immunohistochemical staining. Both sides of the maxillas were
investigated and five slides were used for each sample at 10
intervals. Cell counts were conducted using images captured using a
camera attached to a light microscope (Nikon Eclipse E600; Nikon
Corporation, Tokyo, Japan) in a square of 45×70 µm.
Isolation of murine meritoneal
macrophages
Peritoneal macrophages were obtained by peritoneal
lavage using 10 ml ice-cold Hank's balanced salt solution
supplemented with 10 U/ml heparin (both Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The cells were washed twice,
and resuspended in Dulbecco's modified Eagles medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), penicillin and
streptomycin (all Gibco; Thermo Fisher Scientific, Inc.).
Subsequently the cells were seeded at a density of 2×106
cells/plate into 35-mm plastic dishes (Sarstedt S.r.l, Verona,
Italy). The plates were incubated in a humidified atmosphere
containing 5% CO2 at 37°C overnight to allow macrophage
adherence.
Isolation and culture of BMSCs
In order to isolate BMSCs, the femurs and tibias of
the mice were harvested, and the muscles and extraosteal tissues
were trimmed. BMSCs were centrifuged at 157 × g at 10°C for 5 min
in a 1.073 g/ml Percoll density gradient (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA). After washing twice with
phosphate-buffered saline (PBS), the cells were seeded into
25-cm2 cell culture flasks containing L-DMEM,
supplemented with 10% FBS and 1% penicillin-streptomycin.
Subsequently, the cultures were incubated at 37°C in 5%
CO2 for 48 h. Following 7–10 days of culture, during
which the original medium was replaced on day 4, the cells were
detached from the culture flask using 0.25% trypsin and 0.02% EDTA,
and concentrated by centrifugation at 157 × g for 5 min.
Subsequently, the suspended BMSCs were seeded into 24-well plates
at a density of 1×106 cells/well and cultured for 2
days.
Transwell migration assay
BMSCs (1×106) were seeded into 24-well
tissue culture plates containing DMEM. Macrophages
(3×105) were seeded into a 24-well Transwell®
culture plate (8-µm pore size filter) containing 300 µl media, and
were exposed to BMSC conditioned media, which allowed the
macrophages and BMSCs to grow in the same medium without direct
contact. In addition, the BMSCs were exposed to macrophage
conditioned media. All cells and the cell culture media were
harvested following 48 h co-culture; the cells on the top of the
porous membrane were manually scraped using a p200 pipette tip. The
cells that had migrated to the underside were stained with crystal
violet staining solution (Winchem Industrial Co., Ltd., Ningbo,
China), which had been prepared as follows: Crystal violet was
dissolved in methanol supplemented with 0.5% stock solution and
further diluted with PBS in 1:4 dilutions for staining.
Subsequently, images of the membrane were captured under the
microscope (Leica Microsystems GmbH) and the number of migrated
cells were counted. Bound antibody was detected using the
Supersignal West Pico Chemiluminescent Substrate system
Cytokine assays
Following 48-h co-culture, the culture supernatants
were harvested, and the TNF-α level in the cell-free supernatants
was quantified using the TNF-α commercial ELISA kit, according to
the manufacturer's protocol. The concentration of TNF-α was
determined by reference to a standard curve constructed with known
amounts of mouse recombinant TNF-α. The sensitivity limit for the
assay was 8.0 pg/ml TNF-α. The analysis was conducted using data
from three independent experiments.
Immunohistochemical analysis
Maxillas were collected from the sacrificed mice and
were used for immunohistochemical staining. Briefly, the maxillas
were decalcified in 10% EDTA solution for 14 days, after which they
were embedded in paraffin. Thin sections (5 µm) were cut and
visualized using immunohistochemical staining, according to the
manufacturer's protocol. Both sides of the maxillae were
investigated and five slides were used for each sample at 10
intervals. For each sample, the average of the measurements from
both sides were calculated. The primary antibodies included: Mouse
anti-CCL2 (1:200), mouse anti-CCR2 (1:200) and anti-TNF-α (1:200).
The secondary antibodies included anti-rabbit and anti-mouse
horseradish peroxidase-conjugated antibodies (1:1,000). Images of
the stained sections were captured using a microscope with an
attached digital camera (Eclipse 80i; Nikon Corporation, Tokyo,
Japan). The staining intensity was quantified using Image-Pro Plus
6.0 image analysis software (Media Cybernetics, Inc., Rockville,
MD, USA), and was presented as the mean optical density.
Western blot analysis
The cells, including the macrophages on the porous
membrane and the BMSCs in 24-well tissue culture plates, were
washed with ice-cold PBS, after which total protein was extracted
using the ReadyPrep Protein Extraction kit. Total protein (~30 µg)
from each sample was separated using 5% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
nitrocellulose membranes by electroblotting. The membranes were
incubated overnight at 4°C with primary antibodies against GAPDH
(1:300), CCL2 (1:200), CCR2 (1:200), JNK (1:200), p-JNK (1:200),
ERK (1:200), p-ERK (1:200), p38 (1:200) and p-p38 (1:200), followed
by incubation with anti-rabbit (1:3,000) or anti-mouse (1:2,000)
horseradish peroxidase-conjugated secondary antibodies for 1 h at
room temperature. Antibody complexes were detected using the Super
Signal West Pico Chemiluminescent Substrate.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS statistical software, version 17.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Differences in parameter
mean values between all groups were analyzed using one-way analysis
of variance, followed by Student-Newman-Keuls test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Alveolar bone loss and inflammatory
cell infiltration
In order to determine the severity of periodontitis,
the present study investigated alveolar bone loss and inflammatory
cell infiltration in three groups by calculating the area
(mm2) bordered by the cemento-enamel junction, alveolar
bone crest and mesial and distal line angles on the lingual sides
of the first and second mandibular molars.
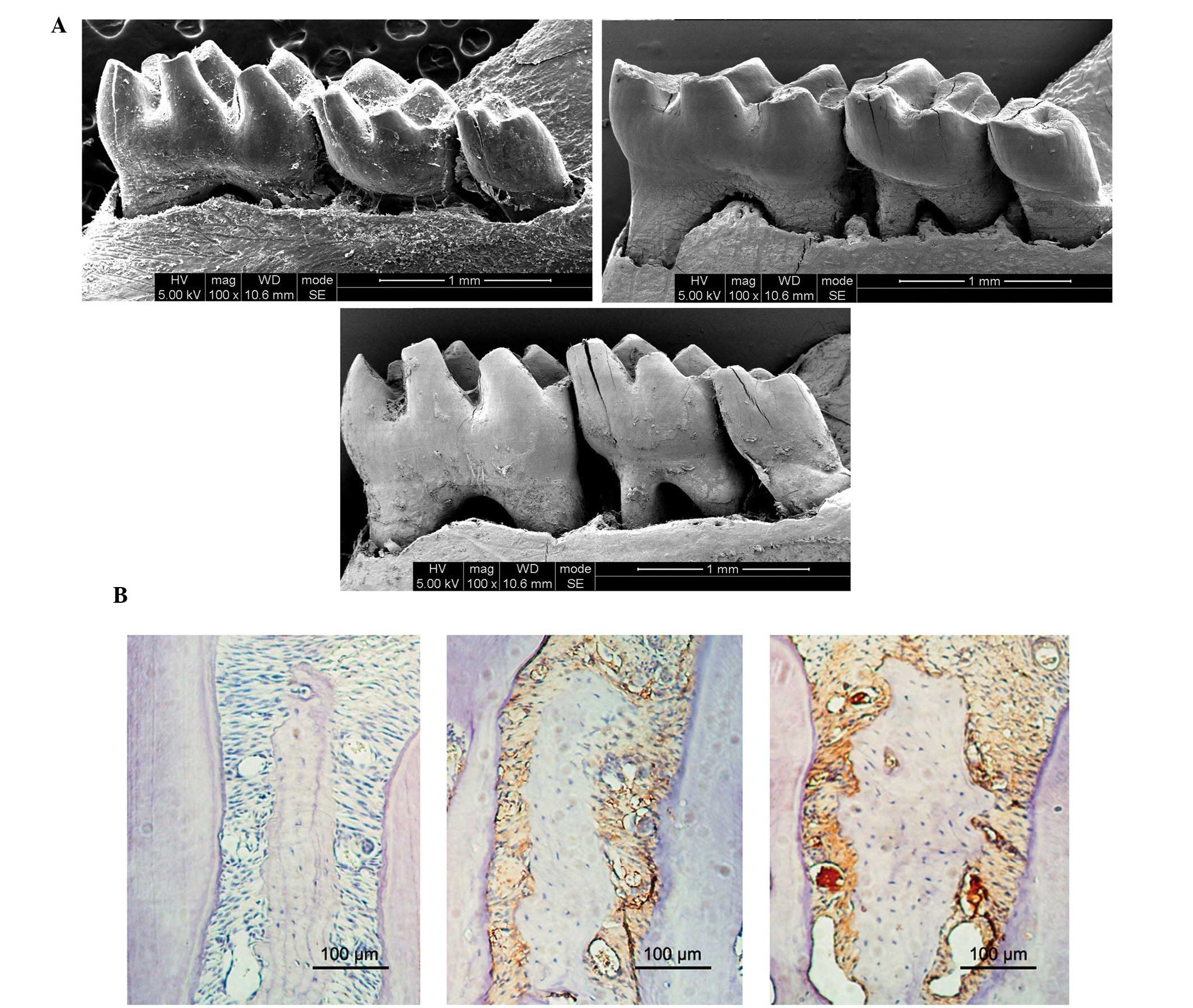
As presented in Fig.
1A, the mice in the HP and P groups exhibited markedly
increased alveolar bone loss, as compared with the mice in the N
group at sacrifice. In addition, the HP mice exhibited markedly
increased alveolar bone loss, as compared with the P mice.
Furthermore, the HP and P mice exhibited increased infiltration of
inflammatory cells, as compared with the N mice (Fig. 1B).
Histomorphometrical analyses demonstrated that the
expression levels of TNF-α in epithelia was significantly increased
in the HP and Pg mice, as compared with the N mice; however, the HP
mice exhibited significantly increased TNF-α expression levels, as
compared with the P mice.
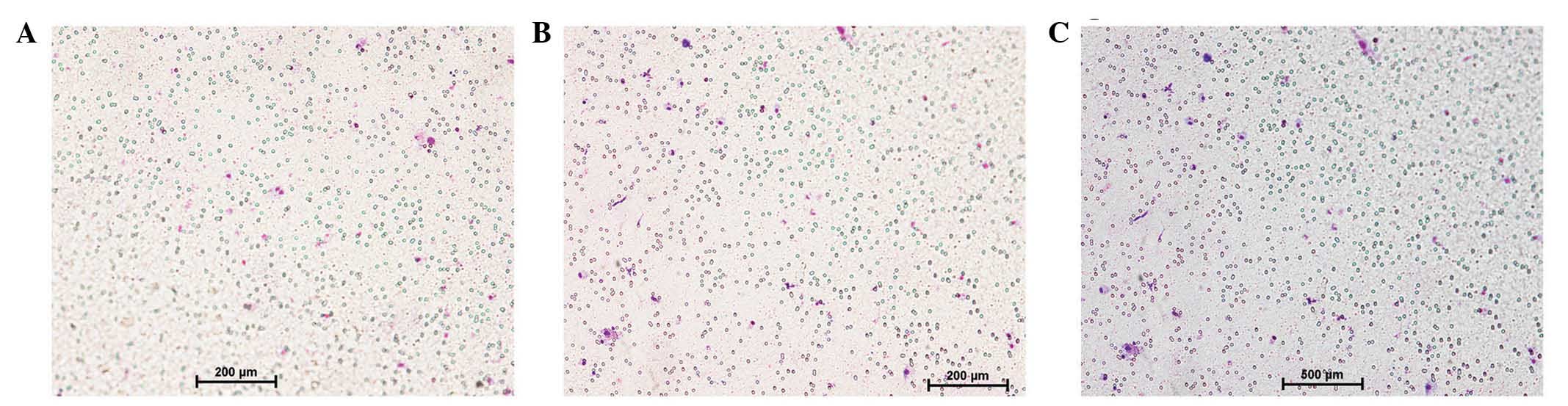
Cell migration analysis
Following 24 h incubation, the cells that had
migrated to the lower side of the porous membrane were counted
using the Transwell migration assay. As presented in Fig. 2, the HP mice exhibited significantly
increased macrophage infiltration, as compared with P mice and the
controls without treatment (P<0.05). No significant differences
were detected between P mice and the normal controls
(P>0.05).
Levels of TNF-α in vivo
The levels of TNF-α in the cell-free supernatants
were determined using an ELISA. As presented in Table I, the levels of TNF-α in the HP and
Pg groups were significantly increased, as compared with the N
group (P<0.05); however, the TNF-α level was significantly
higher in the HP group, as compared with the Pg group.
 | Table I.TNF-α levels in the cell
supernatant. |
Table I.
TNF-α levels in the cell
supernatant.
| Group (n=3) | TNF-α (pg/ml) |
|---|
| HP |
167.3±10.27a |
| P | 68.7±5.9b |
| Normal control | 20.4±3.1c |
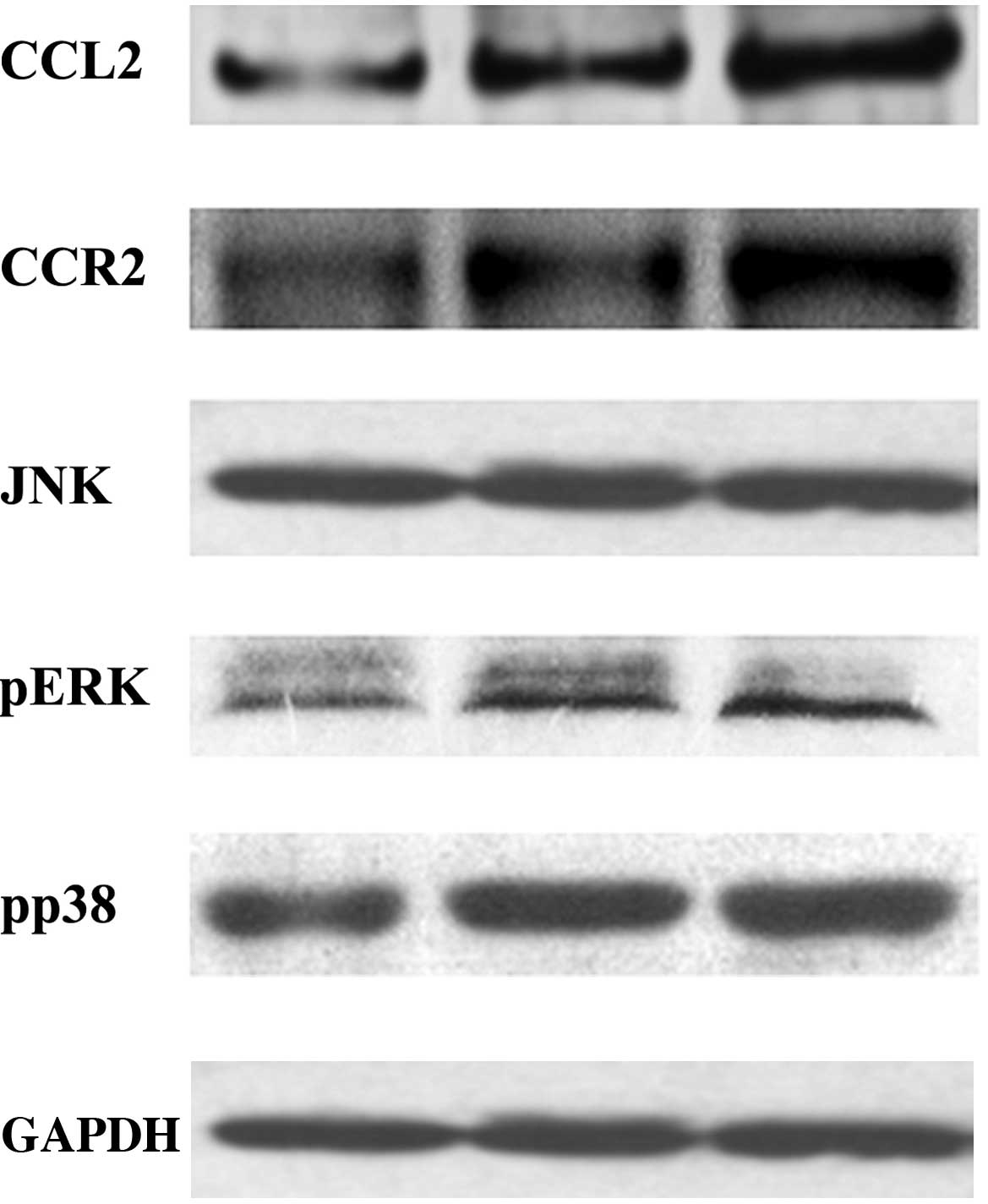
Protein expression levels
In order to investigate the underlying mechanism of
alveolar bone loss in DP, the protein expression levels of CCL2,
ERK, p-ERK, JAK, p-JAK, p38 and p-p38 in the BMSCs, and CCR2 in the
macrophages, were evaluated by western blot. As presented in
Fig. 3, the protein expression
levels of CCL2, p-ERK, p-JAK and p-p38 in the BMSCs were
significantly increased in the HP and Pg groups, as compared with
the N group (P<0.05); however, this increase was most
significant for the HP group. Conversely, there was no significant
difference in the protein expression levels of ERK, JAK, p38
between the three groups (P>0.05). In addition, the protein
expression levels of CCR2 in the macrophages were significantly
increased in the HP and Pg groups, as compared with the N group
(P<0.05), and this increase in CCR2 expression levels was most
significant in the HP group.
Discussion
DP is typically associated with severe clinical
symptoms that make its treatment more challenging, as compared with
PD in the absence of diabetes (13–15).
Disorders of the inflammatory response are thought to be crucially
involved in the progression of DP (16). Furthermore, significant alveolar bone
loss was detected in mice with untreated DP associated with high
TNF-α levels which can negatively regulate insulin tolerance,
pancreatic islets and periodontal tissues (17–19).
Previous studies demonstrated that infection or hyperglycemia
contributed to the increased production of TNF-α by monocytes and
macrophages, and that excessive TNF-α generation in turn promoted a
higher proinflammatory state and tissue damage (20,21). In
the present study, the mice with untreated DP exhibited the most
significantly increased levels of TNF-α; thus suggesting that
periodontal pathogens and hyperglycemia exerted a synergistic
effect on TNF-α production. This is consistent with previous
results of studies involving diabetic patients and patients with
PD, who were shown to exhibit high serum levels of TNF-α (22,23).
CCL2 is a C-C group chemokine that is expressed by
numerous cells, including leukocytes, fibroblasts, kerotinocytes
and endothelial cells, in response to various endogenous and
exogenous stimuli (24,25). The present study demonstrated that
high levels of glucose and inflammation increased the activation of
MAPK signaling and upregulated the expression levels of CCL2 in
BMSCs (a representative chemokine for the migration of macrophages
via the receptor CCR2) (26). These
results, in combination with the increased infiltration of
macrophages into the periodontal of DP mice and histological
analyses, suggested that macrophages were the predominant source of
TNF-α in response to PG infection and high glucose levels. In
addition, these results suggested that the inflamed periodontium
was infiltrated by macrophages.
The migration of monocytes and macrophages to sites
of inflammation may promote inflammatory tissue destruction due to
the requirement of dynamic integrin-dependent adhesion. The present
study demonstrated that PG infection and high glucose levels
stimulated BMSC-mediated synthesis of CCL2, which may have
contributed to the migration of macrophages into the inflamed
tissue via the CCL2/CCR2 axis; thus suggesting that the increased
infiltration of macrophages and higher levels of TNF-α in the DP
mice may have led to the severe alveolar bone loss.
In conclusion, the results of the present study
suggested that DP may be characterized as an interaction between
local inflammation and the host immune responses, in particular
involving the upregulation of CCL2 and TNF-α expression levels in
periodontal tissues. In addition, it may be characterized by the
recruitment of macrophages via the CCL2/CCR2 axis, which in turn
may lead to the alveolar bone loss associated with DP.
References
|
1
|
Papadopoulos G, Weinberg EO, Massari P,
Gibson FC III, Wetzler LM, Morgan EF and Genco CA:
Macrophage-specific TLR2 signaling mediates pathogen-induced
TNF-dependent inflammatory oral bone loss. J Immunol.
190:1148–1157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Veronica L, Saviuc CM and Chifiriuc MC:
Periodontitis and Periodontal Disease - innovative strategies for
reversing the chronic infectious and inflammatory condition by
natural products. Curr Pharm Des. Nov 12–2015.(Epub ahead of
print).
|
|
3
|
Graves D: Cytokines that promote
periodontal tissue destruction. J Periodontol. 79(Suppl):
1585–1591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herrera BS, Bastos AS, Coimbra LS,
Teixeira SA, Rossa C Jr, Van Dyke TE, Muscara MN and Spolidorio LC:
Peripheral blood mononuclear phagocytes from patients with chronic
periodontitis are primed for osteoclast formation. J Periodontol.
85:e72–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hernández M, Gamonal J, Salo T,
Tervahartiala T, Hukkanen M, Tjäderhane L and Sorsa T: Reduced
expression of lipopolysaccharide-induced CXC chemokine in
Porphyromonas gingivalis-induced experimental periodontitis
in matrix metalloproteinase-8 null mice. J Periodontal Res.
46:58–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakallioğlu EE, Ayas B, Lütfioğlu M, Keleş
GC, Açikgöz G and Firatli E: Gingival levels of monocyte
chemoattractant protein-1 (MCP-1) in diabetes mellitus and
periodontitis: An experimental study in rats. Clin Oral Investig.
12:83–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rauner M, Stein N, Winzer M, Goettsch C,
Zwerina J, Schett G, Distler JH, Albers J, Schulze J, Schinke T, et
al: WNT5A is induced by inflammatory mediators in bone marrow
stromal cells and regulates cytokine and chemokine production. J
Bone Miner Res. 27:575–585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shin Y, Han S, Jeon JS, Yamamoto K,
Zervantonakis IK, Sudo R, Kamm RD and Chung S: Microfluidic assay
for simultaneous culture of multiple cell types on surfaces or
within hydrogels. Nat Protoc. 7:1247–1259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu J, Chen Q, Liu W, Zhang Y and Lin JM:
Cytotoxicity of quantum dots assay on a microfluidic 3D-culture
device based on modeling diffusion process between blood vessels
and tissues. Lab Chip. 12:3474–3480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao D, Liu H, Lin JM, Wang Y and Jiang Y:
Characterization of drug permeability in Caco-2 monolayers by mass
spectrometry on a membrane-based microfluidic device. Lab Chip.
13:978–985. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huh D, Matthews BD, Mammoto A,
Montoya-Zavala M, Hsin HY and Ingber DE: Reconstituting organ-level
lung functions on a chip. Science. 328:1662–1668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Achyuta AK, Conway AJ, Crouse RB,
Bannister EC, Lee RN, Katnik CP, Behensky AA, Cuevas J and Sundaram
SS: A modular approach to create a neurovascular unit-on-a-chip.
Lab Chip. 13:542–553. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Graves DT and Kayal RA: Diabetic
complications and dysregulated innate immunity. Front Biosci.
13:1227–1239. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sulniute R, Lindh T, Wilczynska M, Li J
and Ny T: Plasmin is essential in preventing periodontitis in mice.
Am J Patho. 179:819–828. 2011. View Article : Google Scholar
|
|
15
|
Lamster IB, Lalla E, Borgnakke WS and
Taylor GW: The relationship between oral health and diabetes
mellitus. J Am Dent Assoc. 139(Suppl): 19S–24S. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Preshaw PM: Periodontal disease and
diabetes. J Dent. 37:S575–S577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ekuni D, Tomofuji T, Irie K, Kasuyama K,
Umakoshi M, Azuma T, Tamaki N, Sanbe T, Endo Y, Yamamoto T, et al:
Effects of periodontitis on aortic insulin resistance in an obese
rat model. Lab Invest. 90:348–359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gurzov EN and Eizirik DL: Bcl-2 proteins
in diabetes: Mitochondrial pathways of β-cell death and
dysfunction. Trends Cell Biol. 21:424–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Graves DT, Oskoui M, Volejnikova S, Naguib
G, Cai S, Desta T, Kakouras A and Jiang Y: Tumor necrosis factor
modulates fibroblast apoptosis, PMN recruitment, and osteoclast
formation in response to P. gingivalis infection. J Dent
Res. 80:1875–1879. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gonzalez Y, Herrera MT, Soldevila G,
Garcia-Garcia L, Fabián G, Pérez-Armendariz EM, Bobadilla K,
Guzmán-Beltrán S, Sada E and Torres M: High glucose concentrations
induce TNF-α production through the down-regulation of CD33 in
primary human monocytes. BMC Immunol. 13:192012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amar S, Oyaisu K, Li L and Van Dyke T:
Moesin: A potential LPS receptor on human monocytes. J Endotoxin
Res. 7:281–286. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lechleitner M, Koch T, Herold M, Dzien A
and Hoppichler F: Tumour necrosis factor-alpha plasma level in
patients with type 1 diabetes mellitus and its association with
glycaemic control and cardiovascular risk factors. J Intern Med.
248:67–76. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Górska R, Gregorek H, Kowalski J,
Laskus-Perendyk A, Syczewska M and Madaliński K: Relationship
between clinical parameters and cytokine profiles in inflamed
gingival tissue and serum samples from patients with chronic
periodontitis. J Clin Periodontol. 30:1046–1052. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sassy-Prigent C, Heudes D, Mandet C,
Bélair MF, Michel O, Perdereau B, Bariéty J and Bruneval P: Early
glomerular macrophage recruitment in streptozotocin-induced
diabetic rats. Diabetes. 49:466–475. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nomura S, Shouzu A, Omoto S, Nishikawa M
and Fukuhara S: Significance of chemokines and activated platelets
in patients with diabetes. Clin Exp Immunol. 121:437–443. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saeki K, Kanai T, Nakano M, Nakamura Y,
Miyata N, Sujino T, Yamagishi Y, Ebinuma H, Takaishi H, Ono Y, et
al: CCL2-induced migration and SOCS3-mediated activation of
macrophages are involved in cerulein-induced pancreatitis in mice.
Gastroenterology. 142:1010–1020.e9. 2012. View Article : Google Scholar : PubMed/NCBI
|