Introduction
Cyanobacterial blooms remain a global burden, due to
the production of cyanotoxins (1).
An outbreak of cyanobacterial blooms induces the release of
microcystins (MCs) into water, and is a serious threat to aquatic
organisms, wildlife and humans that ingest the toxins from
cyanobacteria or water aquatic ecosystems (2). MCs are a group of >100
cyanobacterial toxin variants, of which MC-LR is the most common
variant and the most potently toxic peptide (3). Furthermore, it has previously been
reported that MC-LR is highly hepatotoxic and is a liver
tumor-specific promoter (4).
MCs are a group of highly stable environmental
pollutants that are not readily hydrolyzed or oxidized at normal
pH, thus, they may survive for months to several years (5). Toxins released into the water from
broken algal cells are a threat to human health through skin
contact, inhalation, hemodialyses and oral ingestion. It has been
reported that MCs may cause damage to the respiratory system
(6); however, the associated
mechanism has yet to be elucidated. Incidents involving poisoning
of the respiratory system have been reported in several countries
and regions as a result of contact with poisonous algae since the
20th century (7–9). In 1916, respiratory system symptoms
were reported in patients following algal poisoning on the West
Coast of Florida in the United States (8). Furthermore, in Britain in 1989,
pneumonia was detected in patients after direct contact with
MCs-contaminated water as a result of swimming or boating (7,9). Toxic
cyanobacteria present in water entertainment parks can also
generate atomized microcystins that enter the respiratory tract,
which is the predominant route leading to disease of the
respiratory system (10). Pilotto
et al (11) reported that
participants exposed to >5,000 cyanobacteria cells/ml for >1
h had a significant increase in flu-like symptoms, such as fever
and skin rashes, as compared with unexposed participants over the
course of 7 days (11). In lakes
with a high concentration of cyanobacteria (cell surface area
>12.0 mm2/ml), the probability of individuals
developing respiratory symptoms is 2.1 times that of individuals
who are exposed to a low concentration of cyanobacteria (cell
surface area <2.4 mm2/ml) (12). Water-based recreational activities
can expose participants to low concentrations of microcystins via
the aerosol; Backer et al (13) recruited 104 participants planning
recreational activities in a lake containing cyanobacteria, as well
as a nearby cyanobacteria-free lake, and demonstrated that low
levels of microcystins were detected in the blood of all
participants (13).
Apoptosis is a key pathophysiological mechanism
associated with pneumonia. When pneumonia occurs, pneumococci
induce the apoptosis of human alveolar and bronchial epithelial
cells (14). Bronchial epithelial
cells are the first-line defense and are therefore the first cells
to be damaged (15). The damage and
proliferation of bronchial epithelial cells has an important role
in the repair and regeneration of lung tissues, pulmonary fibrosis
and cancer (16–18). When bronchial epithelial cells are
exposed to adverse factors, molecular events may occur, including
oxidative stress, damage of genes, activation of proto-oncogenes or
the inhibition of tumor suppressor genes in cells. These events may
subsequently alter the expression levels of apoptosis-regulatory
genes, leading to proliferation or damage and malignant
transformation of alveolar epithelial cells, culminating in their
development into lung cancer cells (19,20).
Several studies have proposed that MC-LR induces
apoptosis (21,22), and it has been demonstrated that
oxidative stress is an important mechanism of MCs toxicity
(23). Oxidative stress may be
induced by the imbalance between reactive oxygen species (ROS)
formation and antioxidants (24).
MC-LR may cause oxidative stress by increasing intracellular ROS
production and diminishing glutathione in mouse hepatocytes
(25). Furthermore, it has also been
reported that MC-LR is capable of inducing mitochondrial damage
(26) and MC-LR has been shown to
persistently decrease B-cell lymphoma-2 (Bcl-2) expression levels
and increase the expression levels of p53, Bcl-2-associated X
protein (Bax) and caspase-3 (23,27).
These findings indicated that oxidative stress and mitochondrial
damage have an important role in MC-LR-induced apoptosis.
In the present study, human bronchial epithelial
(HBE)cells were used to assess MC-LR-induced toxicity and its
potential mechanisms. Cell viability, ROS, mitochondrial membrane
potential (MMP), apoptosis rate, and protein expression levels of
caspase-3, caspase-9, cytochrome c (Cyt c), Bax and
Bcl-2 were determined to investigate MC-LR toxicity, and to explore
the role of the mitochondrial pathway in MC-LR-induced apoptosis of
HBE cells. The present study aimed to investigate the toxicity of
MC-LR on the respiratory system.
Materials and methods
Cell culture
HBE cells were kindly provided by Dr. XiuliAn in the
New York Blood Center (New York, NY, USA). Cells were maintained in
RPMI 1640 medium supplemented with 10% fetal calf serum (FCS;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in an
atmosphere containing 5% CO2. When the cells reached
>90% confluence, they were trypsinized (Beyotime Institute of
Biotechnology, Inc., Haimen, China) and subcultured. The cells were
generally used between passages 20–30 to avoid variation.
Chemicals and reagents
MC-LR with purity of ≥95% was obtained from Beijing
Express Technology Co., Ltd., (Beijing, China). RPMI-1640 medium,
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
assay kit and trypsin were purchased from Beijing Solarbio Science
& Technology Co., Ltd., (Beijing, China). ROS Assay kit and
Mitochondrial Membrane Potential Assay kit were purchased from
Beyotime Institute of Biotechnology and an MTT assay kit was
purchased from Sigma-Aldrich (St. Louis, MO, USA). Furthermore,
rabbit anti-human caspase-3 (cat. no. KGYT0656-7), rabbit
anti-human caspase-9 (cat. no. KGYT0661-7), goat anti-rabbit Cyt
c (cat. no. KG22230-2), rabbit anti-human Bax (cat. no.
KGYT0459-7) and rabbit anti-human Bcl-2 (cat. no. KGYT0469-7)
polyclonal antibodies, goat anti-rabbit horseradish peroxidase
(HRP)-conjugated secondary antibodies (cat. no. KGAA35; all Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) and fetal calf serum
(Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.,
Hangzhou, China) were used in the present study. HBE cells were
maintained in RPMI-1640 medium supplemented with 10% FCS at 37°C in
an atmosphere containing 5% CO2. When the cells reached
>90% confluence, they were trypsinized (Beyotime Institute of
Biotechnology, Inc.) and subcultured. The cells were generally used
between passages 20–30 to avoid the generation of variation. All
other reagents were of analytical grade.
Cell viability assay
Cell viability was assessed by MTT assay as
described previously (28). Briefly,
HBE cells were seeded into 96-well plates at a density of
1×104 cells/ml and, after 24 h, cells were treated with
various concentrations of MC-LR (1, 10, 20, 30 and 40 µg/ml) for 24
h. The cells were maintained in RPMI-1640 medium supplemented with
10% FCS at 37°C in an atmosphere containing 5% CO2.
Following this, each well was supplemented with 20 µl MTT solution
(500 µg/ml) and incubated at 37°C for 4 h, prior to incubation with
150 µl dimethyl sulfoxide at 37°C for 10 min. Absorbance was
measured at 490 nm using a microplate reader (Multiskan MK3; Thermo
Electric (Shanghai) Technology Instrument Co., Ltd., Shanghai,
China). EC50 was defined as the concentration of MC-LR
at which 50% of cell growth was inhibited when compared with the
control group.
Cell viability was also assessed following
pretreatment with Z-VAD-FMK. Briefly, HBE cells were seeded into
96-well plates at a density of 1×104 cells/ml. The cells
were grown for 24 h prior to treatment. After 24 h, cells were
pretreated with Z-VAD-FMK at various concentrations (0, 10, 20, 40,
60, 80, 100, 120 and 140 µM) for 30 min prior to treatment with
various concentrations of MC-LR (2.5, 5 and 10 µg/ml) for 24 h. An
MTT assay was performed to detect cell viability, as described for
MC-LR alone.
Detection of ROS and MMP
2′,7′-dichlorofluorescein diacetate (DCFH-DA;
Beyotime Institute of Biotechnology, Inc.) was used for the
detection of intracellular ROS. DCFH-DA is deacetylated to DCFH,
and ROS then converts DCFH into oxidized DCF, which fluoresces
(29). Fluorescence intensity is
proportional to oxidant production (29). JC-1 staining (Beyotime Institute of
Biotechnology, Inc.) was used to detect MMP, according to the
previously reported protocol (30).
ROS and MMP levels were determined by flow cytometry (BD Accuri™
C6 Flow Cytometer; BD Biosciences, Franklin Lakes, NJ,
USA). HBE cells (3×105 cells/ml) were seeded into 6-well
plates. After 24 h the cells were treated with various
concentrations of MC-LR (2.5, 5 and 10 µg/ml) for 24 and 48 h,
washed twice with phosphate buffered saline (PBS) and stained with
DCFH-DA and JC-1 for 20 min at 37°C in darkness prior to washing
twice with PBS.
Detection of cell apoptosis via
Annexin V/PI assay
Annexin-V FITC / PI double staining assay was used
to detect cell apoptosis, according to the manufacturer's protocol.
HBE cells (1×105 cells/ml) were seeded into 12-well
plates and treated with various concentrations of MC-LR (2.5, 5 and
10 µg/ml) for 24 and 48 h. In addition, the cells were pretreated
with 50 µM zVADfmk for 1 h prior to the addition of 10 µg/ml MC-LR.
Cells were then washed twice with PBS and, after re-suspension in
500 µl binding buffer (Nanjing KeyGen Biotech Co., Ltd.), were
incubated with 5 µl Annexin V-FITC and 5 µl PI for 15 min at room
temperature in darkness. The percentage of apoptotic cells was
determined by flow cytometry. All experiments were repeated three
times.
Western blot assay
MC-LR-treated cells were washed with PBS, lysed in
lysis buffer (Beyotime Institute of Biotechnology, Inc.) for 30 min
on ice for protein extraction, centrifuged at 12,000 × g at 4°C for
5 min and the supernatants were collected. Protein concentrations
were determined using the BCA method and ~40 µg of extracted
protein from each sample was separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and electrophoretically
transferred onto polyvinylidene difluoride membranes (Beyotime
Institute of Biotechnology, Inc.) using electroblotting apparatus.
Membranes were subsequently blocked in Tris-buffered saline and
Tween 20 (TBS-T; Beijing ComWin Biotech Co., Ltd. Beijing, China)
supplemented with 5% non-fat milk for 2 h at room temperature,
prior to incubation with anti-caspase-3 (1:200), anti-caspase-9
(1:200), anti-Cyt c (1:200), anti-Bax (1:200) anti-Bcl-2
(1:200) and rabbit anti-β-actin (1:500; cat. no. KGAA006-2; Nanjing
KeyGen Biotech Co., Ltd.) antibodies, respectively, at 4°C
overnight. Membranes were then washed three times in TBS-T (5 min
each) and incubated with HRP-conjugated secondary antibody
(1:5,000) for 1 h at room temperature. Membranes were washed three
times with TBS-T for 15 min and visualized with chemiluminescent
substrates (Beijing ComWin Biotech Co., Ltd.). The immunoreactive
protein was visualized using electrochemiluminescence reagents kit
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and Image J
version 1.49 was used to analyze the immunoblots.
Statistical analysis
Data were obtained from three independent
experiments and are presented as the mean ± standard deviation.
Comparisons were performed using one-way analysis of variance
following the appropriate transformation to achieve a normal
distribution and equalized variance where necessary. Further to
this, a Student-Newman-Keuls test was used for multiple comparison
of variances with homogeneity and a Games-Howell test in variances
with no homogeneity. SPSS 21.0 (SPSS, Inc., Chicago, IL, USA) was
used for data analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
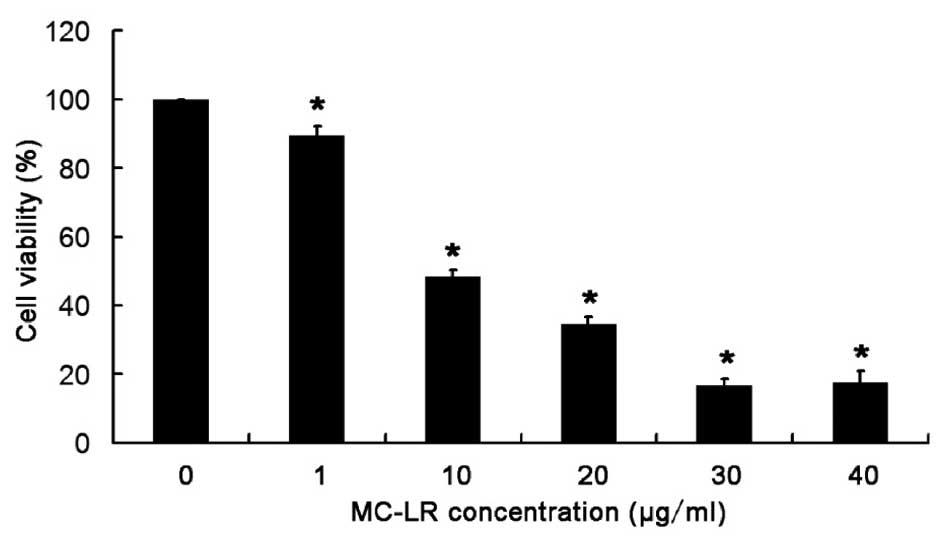
MC-LR treatment significantly
decreases the viability of HBE cells in a concentration-dependent
manner
An MTT assay was used to detect the viability of HBE
cells following MC-LR treatment. As displayed in Fig. 1, cell viability reduced with the
increase in MC-LR concentration, and a significant reduction in
cell viability (P<0.05) was observed in cells after MC-LR
treatment (1, 10, 20, 30 and 40 µg/ml), when compared with the
control group (0 µg/ml). The EC50 value of MC-LR was 10 µg/ml in
HBE cells following 24 h treatment. Thus, the viability of HBE
cells significantly decreases with the increase in MC-LR
concentration.
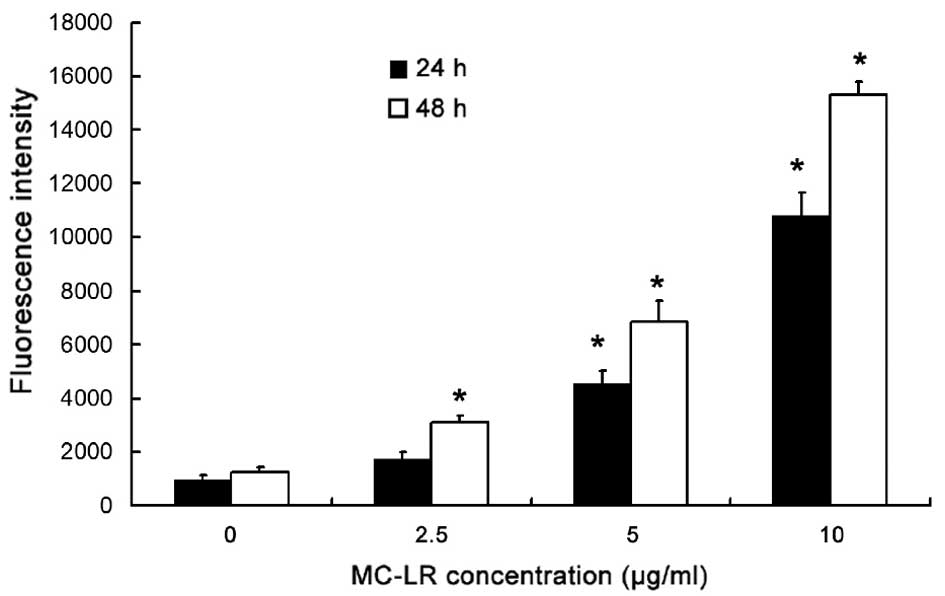
MC-LR treatment increases ROS
production in a concentration- and time-dependent manner
ROS in HBE cells were assessed by DCF assay. As
displayed in Fig. 2, when the
treatment time was constant at 24 or 48 h, ROS levels increased
with the increase in MC-LR concentration, when compared with the
control group (0 µg/ml MC-LR). An MC-LR concentration of 5 or 10
µg/ml resulted in a significant increase in the fluorescence
intensity in association with the increase in treatment time
(P<0.05). As compared with the control, ROS increased
significantly (P<0.05) in cells treated with 2.5 µg/ml MC-LR for
48 h, whereas no significant change in ROS was observed following
MC-LR treatment for 24 h. Therefore, MC-LR may increase ROS
production in a concentration- and time-dependent manner.
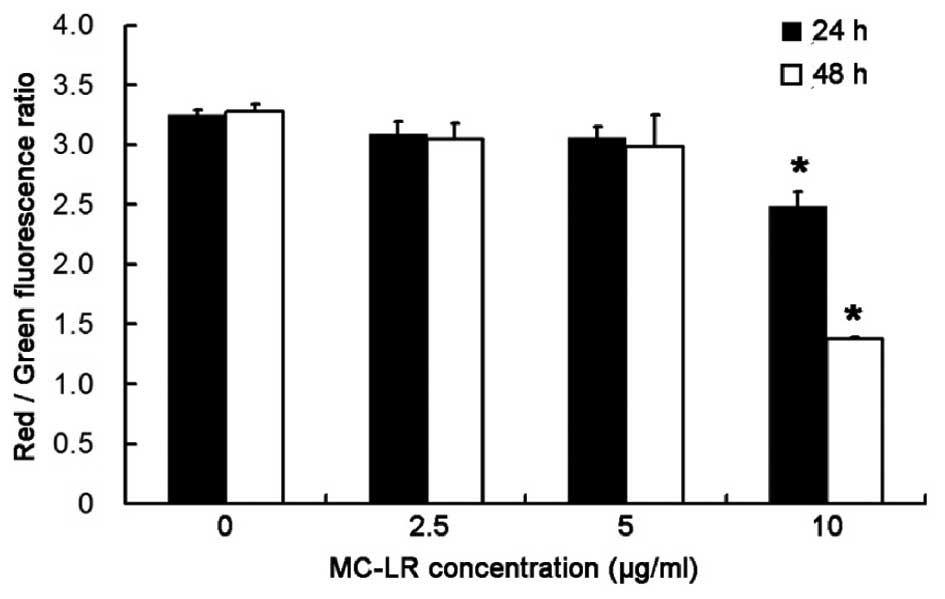
Treatment with 10 µg/ml MC-LR
significantly decreases MMP in a time-dependent manner
To investigate the alterations in MMP following
MC-LR treatment, the ratio of red:green fluorescence was determined
following staining with JC-1. As demonstrated in Fig. 3, after exposure to 10 µg/ml MC-LR for
24 and 48 h, a significant decrease in MMP was observed in HBE
cells in a time-dependent manner (P<0.05). Therefore, MMP in HBE
cells decreases with the increase of the treatment time.
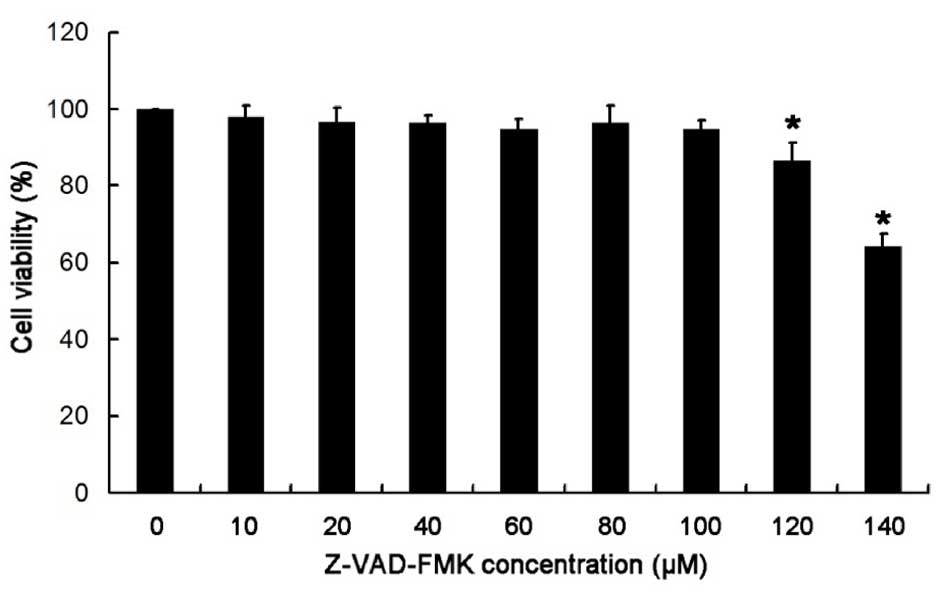
Treatment with >100 µM Z-VAD-FMK
significantly reduces the viability of HBE cells
As demonstrated in Fig.
4, after treatment with Z-VAD-FMK at various concentrations (0,
10, 20, 40, 60, 80, 100, 120 and 140 µM) for 24 h, no significant
difference in cell viability was observed when Z-VAD-FMK
concentration were ≤100 µM; however, cell viability significantly
reduced when treated with >100 µM Z-VAD-FMK (P<0.05) when
compared with the control group (0 µM). Thus, Z-VAD-FMK
concentrations ≤100 µM (2.5, 5, 10, 50 and 100 µM) were selected
for all subsequent experiments. When the concentration of Z-VAD-FMK
>100 µM, the viability of HBE cells was significantly
reduced.
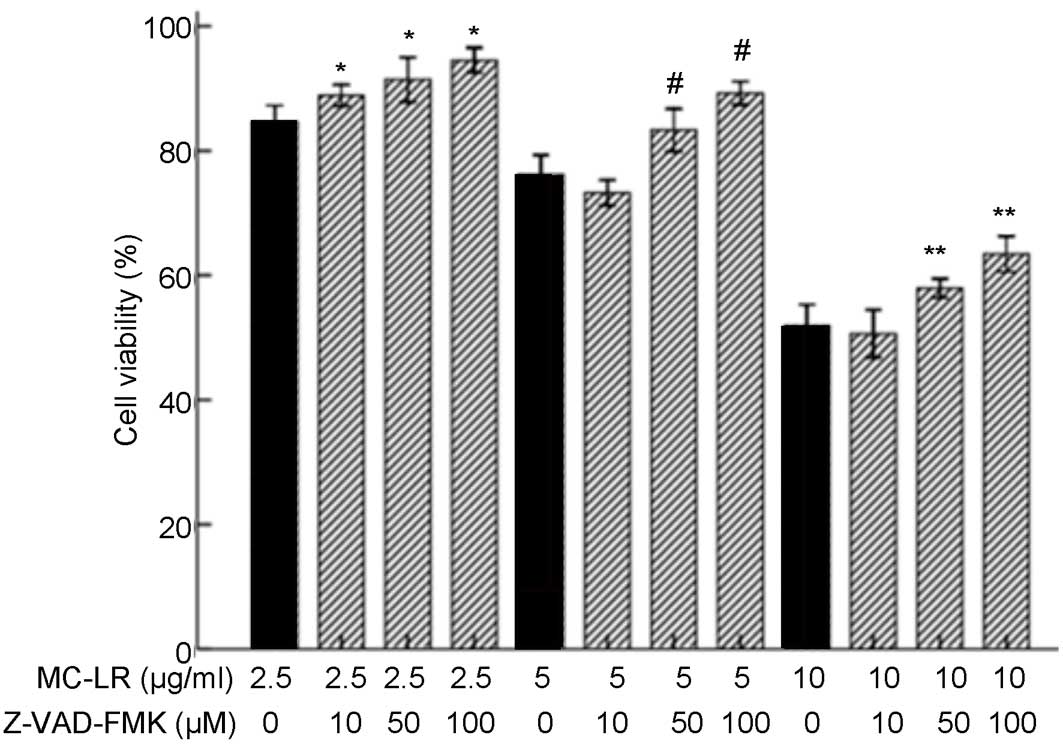
Treatment with 50 or 100 µM Z-VAD-FMK
inhibits the effect of MC-LR on the viability of HBE cells
The viability of HBE cells was determined by MTT
assay. As demonstrated in Fig. 5,
treatment with 2.5 µg/ml MC-LR induced significantly increased cell
viability following pretreatment with 10, 50 and 100 µM Z-VAD-FMK
(P<0.05) when compared with the non-pretreatment group.
Treatment with 5 or 10 µg/ml MC-LR significantly increased cell
viability following pretreatment with 50 and 100 µM Z-VAD-FMK
(P<0.05), whereas a decrease was detected following pretreatment
with 10 µM Z-VAD-FMK when compared with the non-pretreatment group
(0 µM Z-VAD-FMK). Therefore, 10 µM Z-VAD-FMK was not selected for
use in subsequent experiments. As concentrations of 50 or 100 µM
Z-VAD-FMK inhibited the effect of MC-LR on the viability of HBE
cells, 50 µM Z-VAD-FMK was selected for subsequent experiments. The
viability of HBE cells exposed to MC-LR significantly increased
when treated with 100 µM Z-VAD-FMK and when the concentration of
Z-VAD-FMK >100 µM, whereas the viability of HBE cells unexposed
to MC-LR was significantly reduced.
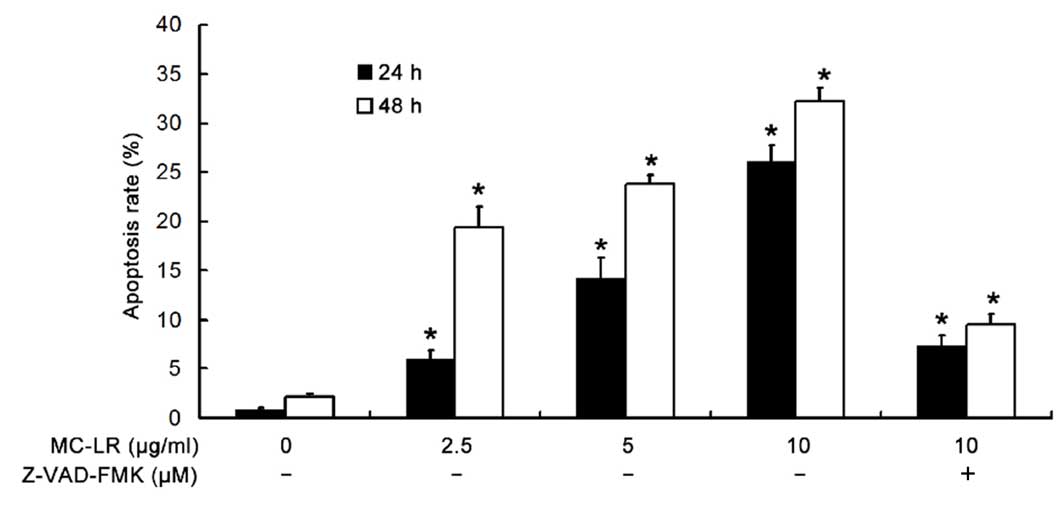
MC-LR treatment significantly
increases the apoptotic rate of HBE cells in a time- and
dose-dependent manner
To determine the apoptotic rate of HBE cells, flow
cytometry was performed following Annexin V FITC and PI double
staining. As demonstrated in Fig. 6,
MC-LR significantly increased the apoptotic rate of HBE cells in a
time- and dose-dependent manner (P<0.05). In addition, when
MC-LR was administered at a concentration of 10 µg/ml, the
apoptotic rate was significantly inhibited by pretreatment with
Z-VAD-FMK (P<0.05) when compared with the non-pretreatment
group.
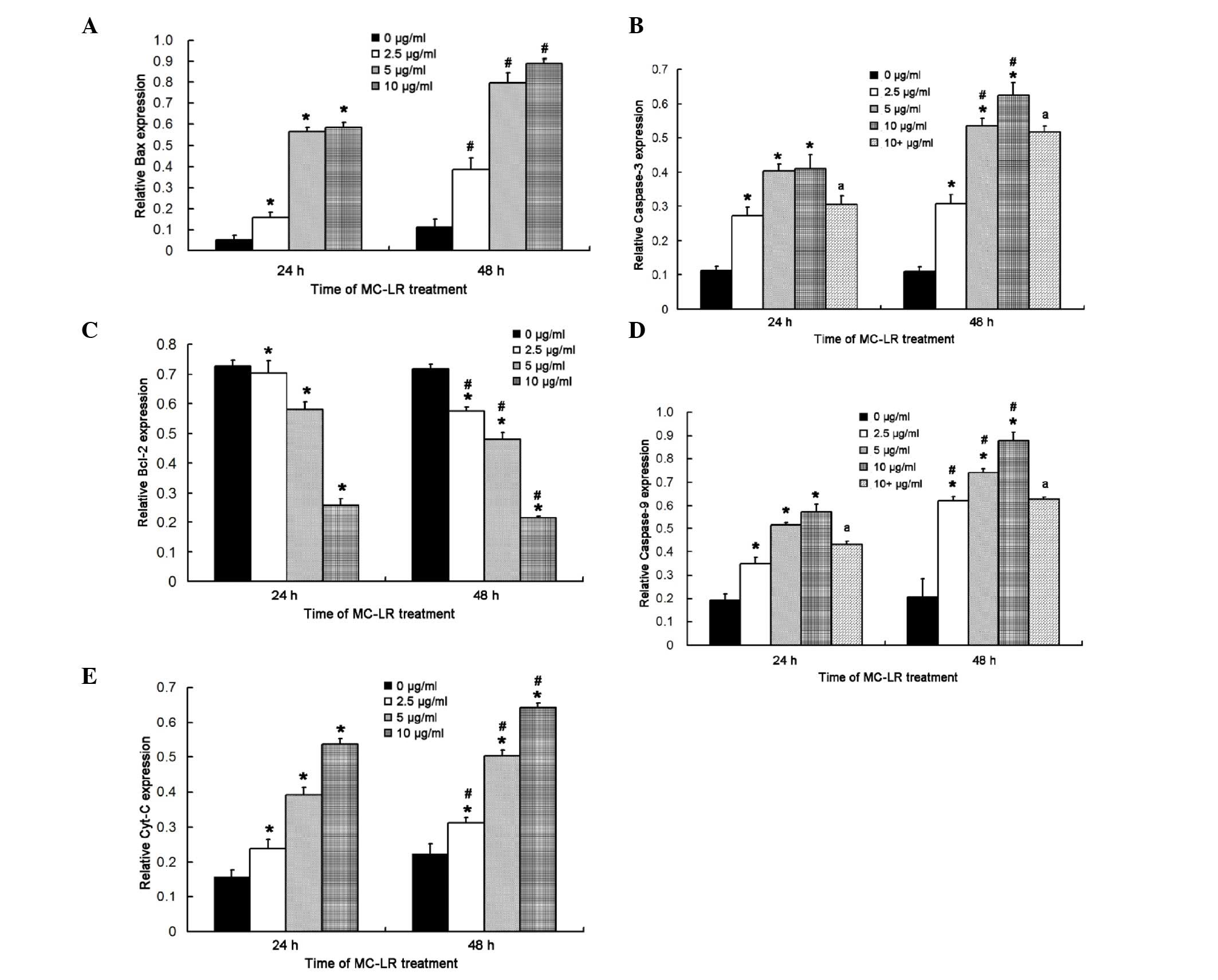
MC-LR treatment significantly
increases the expression levels of caspase-3, caspase-9, Cyt c and
Bax and reduces Bcl-2 expression
Quantification of the western blot assay indicated
that the expression levels of caspase-3, caspase-9, Cyt c and Bax
significantly increased in HBE cells after exposure to MC-LR for 24
h (P<0.05), whereas Bcl-2 expression levels significantly
decreased (P<0.05), when compared with the control group
(Fig. 7). Expression level trends of
the aforementioned proteins were consistent with those at 24 h when
the duration of MC-LR exposure was increased to 48 h. In addition,
when HBE cells were exposed to MC-LR at the same concentration, the
expression levels of caspase-3, caspase-9, Cyt c and Bax
increased over time, however, those of Bcl-2 decreased, indicating
a time- and concentration-dependent association. When HBE cells
were exposed to MC-LR for 48 h at the same concentration, the
expression levels of caspase-3, caspase-9, Cyt c and Bax
increased; however, the expression levels of Bcl-2 decreased, as
compare with those of HBE cells exposed to MC-LR for 24 h,
indicating a time- and concentration-dependent association.
Furthermore, pretreatment with Z-VAD-FMK significantly inhibited
the expression levels of caspase-3 and caspase-9 at 24 and 48 h
after (P<0.05), when compared with the non-pretreatment group
(Fig. 7B and D, respectively).
Discussion
MC-LR is a potent inhibitor of protein phosphatase 1
and protein phosphatase 2A (31).
The aforementioned phosphatases are critical regulators of
embryonic development (32). Our
previous in vitro studies revealed that MC-LR exerts toxic
effects on Sertoli and CHO cells, which are associated with the
reproductive system (27,33). In recent years, the toxicity of MCs
has been investigated, and several studies have assessed the effect
of MC-LR on the apoptosis of liver cells, kidney cells, cells in
the lymph nodes and germ cells, in addition to the potential
mechanisms of MC-LR-induced toxicity (27,34,35).
It has been reported that cyanobacterial toxins may
be inhaled into the body via spindrifts produced in water, which
may induce respiratory diseases (26). Under these conditions, patients
typically present with symptoms of the respiratory system including
polypnea, cyanosis, asphyxia, which may even be fatal (8). Although previous studies have reported
that MCs are capable of inducing damage to the respiratory system,
little is known about the mechanism of MC toxicity to the
respiratory system (8,26). In the present study, HBE cells were
used to investigate MC toxicity and its potential mechanism.
It is well-established that ROS and oxidative stress
may trigger an apoptotic cascade (36). Previous studies have suggested that
MC-LR may induce excessive ROS production (37,38). For
example, Chen et al(37)
demonstrated that MC-LR is able to damage mitochondrial respiratory
chains and oxidative phosphorylation systems by inducing ROS
formation and oxidative stress. The effects of MC-LR on ROS
generation are dependent on time and concentration, and
N-acetylcysteine significantly decreases MC-LR-induced ROS
generation (38). Ding et
al(39) reported significant and
rapid increases in ROS production and the apoptosis of hepatocytes
following MC-LR treatment, indicating that ROS have a critical role
in MC-LR-induced apoptosis. The results of the present study
demonstrated that ROS production increased with increasing MC-LR
concentration, and that when the MC-LR concentration was constant,
ROS increased over time, suggesting a concentration and
time-dependent association. These findings indicated that MC-LR may
induce ROS generation and oxidative stress in HBE cells, resulting
in their apoptosis.
As a key process for eliminating unwanted or
defective cells, apoptosis is an orderly process of cellular
disintegration which is critical for the development and
homeostasis of normal tissues. The majority of apoptotic signaling
processes are associated with the alteration of apoptosis-related
molecules, including Bcl-2/Bax and Cyt c (40). Bax is a pro-apoptotic member of the
Bcl-2 family which is located in the outer membrane of mitochondria
(41); whereas Bcl-2 is an
anti-apoptotic member of the Bcl-2 family that is present in the
outer mitochondrial membrane, where it is able to suppress
apoptosis via blocking Cyt c release and binding to
apoptotic protease-activating factor 1 (42,43).
Furthermore, previous studies have indicated that Bax expression is
upregulated and Bcl-2 expression is downregulated following
prolonged exposure to MC-LR, and a decrease in the Bcl-2/Bax ratio
has been revealed to be associated with apoptosis or cell death
(44,45). In addition, it has been demonstrated
that proteins of the Bcl-2 family are able to regulate the
mitochondrial apoptotic pathway (46). The results of the present study
indicated that, after exposure to MC-LR for 24 h, the expression
levels of Bax significantly increased and those of Bcl-2
significantly decreased. Following exposure to MC-LR for 48 h, a
similar change in the expression levels of the aforementioned
proteins was observed. In addition, upon exposure to MC-LR at
specific concentrations for 24 and 48 h in HBE cells, Bax
expression levels increased over time whereas those of Bcl-2
decreased. The aforementioned findings indicated that MC-LR
administration may increase Bax expression and decreases Bcl-2
expression in a time- and concentration-dependent manner.
It is widely recognized that apoptosis is initiated
by two pathways, the mitochondria-mediated intrinsic pathway and
the death-receptor-induced extrinsic pathway (47). Mitochondria have a key role in
apoptosis and have been recognized as a central executioner,
releasing apoptotic factors including Cyt c and
apoptosis-inducing factors (48). In
cases of mitochondrial dysfunction, the mitochondrial permeability
transition pores open and Cyt c is released from the
mitochondria to the cytosol (49).
The release of Cyt c from the mitochondrion has a crucial
role in the apoptotic pathway, as Cyt c may stimulate the
formation of apoptotic bodies and subsequently activate caspase-9
which activates caspase-3. Caspase-3 activation results in the
destruction of target cells (50)
and it has been demonstrated that caspase-3 also participates in
the process of MC-induced apoptosis (51). Zhang et al(46) reported that MC-LR stimulated
hepatocytes to release Cyt c, which subsequently increased
the protein expression levels of Bax, caspase-3 and caspase-9 and
inhibited Bcl-2 expression over time via the mitochondrial pathway.
Previous studies have demonstrated that caspase-3 is closely
associated with apoptosis due to its ability to induce
morphological changes in several types of cells (52–54). The
results of the present study demonstrated that the expression
levels of caspase-3, caspase-9 and Cyt c increased after
exposure to MC-LR for 24 h. Following exposure to MC-LR for 48 h,
similar proteins expression trends were observed. In addition, at
specific concentrations of MC-LR, the expression of caspase-3,
caspase-9 and Cyt c increased over time. The aforementioned
findings indicated that MC-LR is capable of increasing the
expression levels of caspase-3, caspase-9 and Cyt c in a
time- and concentration-dependent manner. Furthermore, the present
study also demonstrated the apoptotic rate of HBE cells and the
expression levels of caspase-3 and caspase-9 were inhibited
following MC-LR treatment when cells underwent pretreatment with
Z-VAD-FMK.
In conclusion, the present study investigated the
effects of MC-LR on HBE cells and explored the potential mechanism
underlying MC-LR-induced apoptosis. The results suggested that
MC-LR inhibits proliferation, increases ROS generation, reduces
membrane potential and induces apoptosis of HBE cells in a dose-
and time-dependent manner. In addition, it was demonstrated that
the MC-LR-induced apoptosis of HBE cells may be associated with the
mitochondria-dependent apoptotic pathway. Notably, the present
study suggested that pretreatment with Z-VAD-FMK may attenuate the
adverse effects of MC-LR in HBE cells, although further studies are
required in order to fully elucidate the underlying mechanism. A
further understanding of the effects of Cyt c and Bcl-2/Bax
in caspase activation pathways is required in order to fully
elucidate the mechanism underlying respiratory toxicity induced by
MC-LR.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472948) and the
Scientific and Technological Project of Henan Province (grant no.
142102310344) and the Program of Science and Technology Development
of Henan province (grant no. 122102310208).
Glossary
Abbreviations
Abbreviations:
|
HBE
|
human bronchial epithelial cell
|
|
MTT
|
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide
|
|
DCFH
|
2′,7′-dichlorofluorescein; mcs,
microcystins
|
References
|
1
|
Wood R: Acute animal and human poisonings
from cyanotoxin exposure - a review of the literature. Environ Int.
91:276–282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen DN, Zeng J, Wang F, Zheng W, Tu WW,
Zhao JS and Xu J: Hyperphosphorylation of intermediate filament
proteins is involved in microcystin-LR-induced toxicity in HL7702
cells. Toxicol Lett. 214:192–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Y, Chen Y, Yuan M, Xiang Z and Han X:
In vivo study on the effects of microcystin-LR on the
apoptosis, proliferation and differentiation of rat testicular
spermatogenic cells of male rats injected i.p. with toxins. J
Toxicol Sci. 38:661–670. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Christen V, Meili N and Fent K:
Microcystin-LR induces endoplasmatic reticulum stress and leads to
induction of NFκB, interferon-alpha, and tumor necrosis
factor-alpha. Environ Sci Technol. 47:3378–3385. 2013.PubMed/NCBI
|
|
5
|
Carmichael WW, Azevedo SM, An JS, Molica
RJ, Jochimsen EM, Lau S, Rinehart KL, Shaw GR and Eaglesham GK:
Human fatalities from cyanobacteria: Chemical and biological
evidence for cyanotoxins. Environ Health Perspect. 109:663–668.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oliveira VR, Mancin VG, Pinto EF, Soares
RM, Azevedo SM, Macchione M, Carvalho AR and Zin WA: Repeated
intranasal exposure to microcystin-LR affects lungs but not nasal
epithelium in mice. Toxicon. 104:14–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duy TN, Lam PK, Shaw GR and Connell DW:
Toxicology and risk assessment of freshwater cyanobacterial
(blue-green algal) toxins in water. Rev Environ Contam Toxicol.
163:113–185. 2000.PubMed/NCBI
|
|
8
|
Giannuzzi L, Sedan D, Echenique R and
Andrinolo D: An acute case of intoxication with cyanobacteria and
cyanotoxins in recreational water in Salto Grande Dam, Argentina.
Mar Drugs. 9:2164–2175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Turner PC, Gammie AJ, Hollinrake K and
Codd GA: Pneumonia associated with contact with cyanobacteria. BMJ.
300:1440–1441. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Backer LC, McNeel SV, Barber T,
Kirkpatrick B, Williams C, Irvin M, Zhou Y, Johnson TB, Nierenberg
K, Aubel M, et al: Recreational exposure to microcystins during
algal blooms in two California lakes. Toxicon. 55:909–921. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pilotto LS, Douglas RM, Burch MD, Cameron
S, Beers M, Rouch GJ, Robinson P, Kirk M, Cowie CT, Hardiman S, et
al: Health effects of exposure to cyanobacteria (blue-green algae)
during recreational water-related activities. Aust N Z J Public
Health. 21:562–566. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stewart I, Webb PM, Schluter PJ, Fleming
LE, Burns JW Jr, Gantar M, Backer LC and Shaw GR: Epidemiology of
recreational exposure to freshwater cyanobacteria - an
international prospective cohort study. BMC Public Health.
6:932006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Backer LC, Carmichael W, Kirkpatrick B,
Williams C, Irvin M, Zhou Y, Johnson TB, Nierenberg K, Hill VR,
Kieszak SM and Cheng YS: Recreational exposure to low
concentrations of microcystins during an algal bloom in a small
lake. Mar Drugs. 6:389–406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmeck B, Gross R, N'Guessan PD, Hocke
AC, Hammerschmidt S, Mitchell TJ, Rosseau S, Suttorp N and
Hippenstiel S: Streptococcus pneumoniae-induced caspase 6-dependent
apoptosis in lung epithelium. Infect Immun. 72:4940–4947. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walsh GM, Sexton DW and Blaylock MG:
Corticosteroids, eosinophils and bronchial epithelial cells: New
insights into the resolution of inflammation in asthma. J
Endocrinol. 178:37–43. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Qiu P, Chen B, Lu Y, Wu K, Thakur C,
Chang Q, Sun J and Chen F: Reactive oxygen species contribute to
arsenic-induced EZH2 phosphorylation in human bronchial epithelial
cells and lung cancer cells. Toxicol Appl Pharmacol. 276:165–170.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Myerburg MM, Latoche JD, McKenna EE,
Stabile LP, Siegfried JS, Feghali-Bostwick CA and Pilewski JM:
Hepatocyte growth factor and other fibroblast secretions modulate
the phenotype of human bronchial epithelial cells. Am J Physiol
Lung Cell Mol Physiol. 292:L1352–L1360. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao W, Li L, Wang Y, Zhang S, Adcock IM,
Barnes PJ, Huang M and Yao X: Bronchial epithelial cells: The key
effector cells in the pathogenesis of chronic obstructive pulmonary
disease? Respirology. 20:722–729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen DJ, Xu YM, Du JY, Huang DY and Lau
AT: Cadmium induces cytotoxicity in human bronchial epithelial
cells through upregulation of eIF5A1 and NF-kappaB. Biochem Biophys
Res Commun. 445:95–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoon DH, Lim MH, Lee YR, Sung GH, Lee TH,
Jeon BH, Cho JY, Song WO, Park H, Choi S and Kim TW: A novel
synthetic analog of Militarin, MA-1 induces mitochondrial dependent
apoptosis by ROS generation in human lung cancer cells. Toxicol
Appl Pharmacol. 273:659–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alverca E, Andrade M, Dias E, Sam Bento F,
Batoréu MC, Jordan P, Silva MJ and Pereira P: Morphological and
ultrastructural effects of microcystin-LR from Microcystis
aeruginosa extract on a kidney cell line. Toxicon. 54:283–294.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang X, Chen L, Liu W, Qiao Q, Wu K, Wen
J, Huang C, Tang R and Zhang X: Involvement of oxidative stress and
cytoskeletal disruption in microcystin-induced apoptosis in CIK
cells. Aquat Toxicol. 165:41–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Xie P and Guo L: Antioxidant
response in liver of the phytoplanktivorous bighead carp
(Aristichthys nobilis) intraperitoneally-injected with extracted
microcystins. Fish Physiol Biochem. 36:165–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Turrens JF: Mitochondrial formation of
reactive oxygen species. J Physiol. 552:335–344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding WX and Nam Ong C: Role of oxidative
stress and mitochondrial changes in cyanobacteria-induced apoptosis
and hepatotoxicity. FEMS Microbiol Lett. 220:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Y, Xie P, Tang R, Zhang X, Li L and
Li D: In vivo studies on the toxic effects of microcystins
on mitochondrial electron transport chain and ion regulation in
liver and heart of rabbit. Comp Biochem Physiol C Toxicol
Pharmacol. 148:204–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang HZ, Zhang FQ, Li CF, Yi D, Fu XL and
Cui LX: A cyanobacterial toxin, microcystin-LR, induces apoptosis
of sertoli cells by changing the expression levels of
apoptosis-related proteins. Tohoku J Exp Med. 224:235–242. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shah P, Djisam R, Damulira H, Aganze A and
Danquah M: Embelin inhibits proliferation, induces apoptosis and
alters gene expression profiles in breast cancer cells. Pharmacol
Rep:. 68:638–644. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Afri M, Frimer AA and Cohen Y: Active
oxygen chemistry within the liposomal bilayer. Part IV: Locating
2′,7′-dichlorofluorescein (DCF), 2′,7′-dichlorodihydrofluorescein
(DCFH) and 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) in
the lipid bilayer. Chem Phys Lipids. 131:123–133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Helm A, Lee R, Durante M and Ritter S: The
Influence of C-Ions and X-rays on human umbilical vein endothelial
cells. Front Oncol. 6:52016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
MacKintosh C, Beattie KA, Klumpp S, Cohen
P and Codd GA: Cyanobacterial microcystin-LR is a potent and
specific inhibitor of protein phosphatases 1 and 2A from both
mammals and higher plants. FEBS Lett. 264:187–192. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawamoto M, Fujiwara A and Yasumasu I:
Changes in the activities of protein phosphatase type 1 and type 2A
in sea urchin embryos during early development. Dev Growth Differ.
42:395–405. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xue L, Li J, Li Y, Chu C, Xie G, Qin J,
Yang M, Zhuang D, Cui L, Zhang H and Fu X: N-acetylcysteine
protects Chinese Hamster ovary cells from oxidative injury and
apoptosis induced by microcystin-LR. Int J Clin Exp Med.
8:4911–4921. 2015.PubMed/NCBI
|
|
34
|
Rymuszka A: Microcystin-LR induces
cytotoxicity and affects carp immune cells by impairment of their
phagocytosis and the organization of the cytoskeleton. J Appl
Toxicol. 33:1294–1302. 2013.PubMed/NCBI
|
|
35
|
Sun Y, Meng GM, Guo ZL and Xu LH:
Regulation of heat shock protein 27 phosphorylation during
microcystin-LR-induced cytoskeletal reorganization in a human liver
cell line. Toxicol Lett. 207:270–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vyssokikh MY, Antonenko YN, Lyamzaev KG,
Rokitskaya TI and Skulachev VP: Methodology for use of
mitochondria-targeted cations in the field of oxidative
stress-related research. Methods Mol Biol. 1265:149–159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen L, Zhang X, Zhou W, Qiao Q, Liang H,
Li G, Wang J and Cai F: The interactive effects of cytoskeleton
disruption and mitochondria dysfunction lead to reproductive
toxicity induced by microcystin-LR. PloS One. 8:e539492013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oh SH and Lim SC: A rapid and transient
ROS generation by cadmium triggers apoptosis via caspase-dependent
pathway in HepG2 cells and this is inhibited through
N-acetylcysteine-mediated catalase upregulation. Toxicol Appl
Pharmacol. 212:212–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ding WX, Shen HM and Ong CN: Critical role
of reactive oxygen species and mitochondrial permeability
transition in microcystin-induced rapid apoptosis in rat
hepatocytes. Hepatology. 32:547–555. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao M, Zhang Y, Wang C, Fu Z, Liu W and
Gan J: Induction of macrophage apoptosis by an organochlorine
insecticide acetofenate. Chem Res Toxicol. 22:504–510. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li G, Bush JA and Ho VC: p53-dependent
apoptosis in melanoma cells after treatment with camptothecin. J
Invest Dermatol. 114:514–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ji YB, Ji CF and Yue L: Study on human
promyelocytic leukemia HL-60 cells apoptosis induced by fucosterol.
Biomed Mater Eng. 24:845–851. 2014.PubMed/NCBI
|
|
43
|
Vander Heiden MG, Chandel NS, Williamson
EK, Schumacker PT and Thompson CB: Bcl-xL regulates the membrane
potential and volume homeostasis of mitochondria. Cell. 91:627–637.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim HG, Song H, Yoon DH, Song BW, Park SM,
Sung GH, Cho JY, Park HI, Choi S, Song WO, et al: Cordyceps
pruinosa extracts induce apoptosis of HeLa cells by a caspase
dependent pathway. J Ethnopharmacol. 128:342–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wiebe JP, Beausoleil M, Zhang G and
Cialacu V: Opposing actions of the progesterone metabolites,
5alpha-dihydroprogesterone (5alphaP) and 3alpha-dihydroprogesterone
(3alphaHP) on mitosis, apoptosis, and expression of Bcl-2, Bax and
p21 in human breast cell lines. J Steroid Biochem Mol Biol.
118:125–132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang H, Cai C, Fang W, Wang J, Zhang Y,
Liu J and Jia X: Oxidative damage and apoptosis induced by
microcystin-LR in the liver of Rana nigromaculata in vivo.
Aquat Toxicol. 140–141:11–18. 2013. View Article : Google Scholar
|
|
47
|
Spencer SL and Sorger PK: Measuring and
modeling apoptosis in single cells. Cell. 144:926–939. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ferri KF and Kroemer G: Mitochondria––the
suicide organelles. BioEssays. 23:111–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang S, Zhang Y, Zhuang Y, Wang J, Ye J,
Zhang S, Wu J, Yu K and Han Y: Matrine induces apoptosis in human
acute myeloid leukemia cells via the mitochondrial pathway and Akt
inactivation. PLoS One. 7:e468532012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xiong Q, Xie P, Li H, Hao L, Li G, Qiu T
and Liu Y: Involvement of Fas/FasL system in apoptotic signaling in
testicular germ cells of male Wistar rats injected i.v. with
microcystins. Toxicon. 54:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fladmark KE, Brustugun OT, Hovland R, Boe
R, Gjertsen BT, Zhivotovsky B and Døskeland SO: Ultrarapid
caspase-3 dependent apoptosis induction by serine/threonine
phosphatase inhibitors. Cell Death Differ. 6:1099–1108. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hirata H, Takahashi A, Kobayashi S,
Yonehara S, Sawai H, Okazaki T, Yamamoto K and Sasada M: Caspases
are activated in a branched protease cascade and control distinct
downstream processes in Fas-induced apoptosis. J Exp Med.
187:587–600. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jänicke RU, Sprengart ML, Wati MR and
Porter AG: Caspase-3 is required for DNA fragmentation and
morphological changes associated with apoptosis. J Biol Chem.
273:9357–9360. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Woo M, Hakem R, Soengas MS, Duncan GS,
Shahinian A, Kägi D, Hakem A, McCurrach M, Khoo W, Kaufman SA, et
al: Essential contribution of caspase 3/CPP32 to apoptosis and its
associated nuclear changes. Genes Dev. 12:806–819. 1998. View Article : Google Scholar : PubMed/NCBI
|