Introduction
The platelet membrane glycoprotein IIb/IIIa receptor
antagonist (GPRA) tirofiban is widely used for the treatment of
acute coronary syndrome (ACS). However, tirofiban may induce
thrombocytopenia in certain patients (1). Prior studies have shown that the
incidence of GPRA-induced thrombocytopenia (GIT) is 1–5% (2), and 0.2–0.5% for severe GIT (3). Tirofiban is currently the only
available GPRA in China, and the incidence of GIT caused by
tirofiban in the Chinese population has not been reported, to the
best of our knowledge. Due to a lack of associated guidelines or
expert consensus, treatment of GIT is inconsistent. Thrombopoietin
(TPO) is one of the most effective thrombocytopenic medication
(4). While there are some reports on
TPO in the treatment of heparin-induced thrombocytopenia (HIT)
(5,6), it is not clear whether TPO can be
effectively used for the treatment of GIT (4).
In the present study, a case of tirofiban-induced
severe thrombocytopenia, or GIT, with secondary in-stent thrombosis
and cerebral infarction following TPO treatment is reported. This
example of the diagnosis and treatment of a case of ACS highlights
the need for caution and careful monitoring of the clinical use of
tirofiban and TPO in patients with ACS and recognition of the risk
of tirofiban-induced thrombocytopenia.
Case report
A 68-year-old man presented in the Department of
Cardiology on July 4th, 2012, Chinese PLA General Hospital
(Beijing, China), with paroxysmal chest depression of 1-month
duration. The patient had a past medical history of hypertension,
and was not allergic to any medication. After admission, the
patient began to receive dual antiplatelet therapy with aspirin
(100 mg, Bayer AG, Leverkusen, Germany) and clopidogrel (75 mg,
Sanofi Pharmaceutical Co., Ltd., Hangzhou, China) without heparin
(SPH No. 1 Biochemical & Pharmaceutical Co., Ltd., Shanghai).
Patient informed consent was obtained for the present study.
Five days after admission, coronary angiography (GE
Medical Systems Ltd., Chalfont St Giles, UK) detected a 50%
stenosis at the proximal left anterior descending artery, 30%
stenosis at the proximal circumflex branch, and 90% stenosis at the
mid-portion of the right coronary artery. A sirolimus-eluting stent
(3.5×21 mm; Cypher™; Cordis Corporation, Bridgewater Township, NJ,
USA) was implanted at the right coronary artery and the patient
received a total of 10,500 units of heparin (SPH No. 1 Biochemical
& Pharmaceutical Co., Ltd.,). During the surgical procedure,
the patient displayed coronary slow flow, and tirofiban (8 ml/h,
total 0.4 mg; Great Medical China Co., Ltd., Wuhan, Hubei, China)
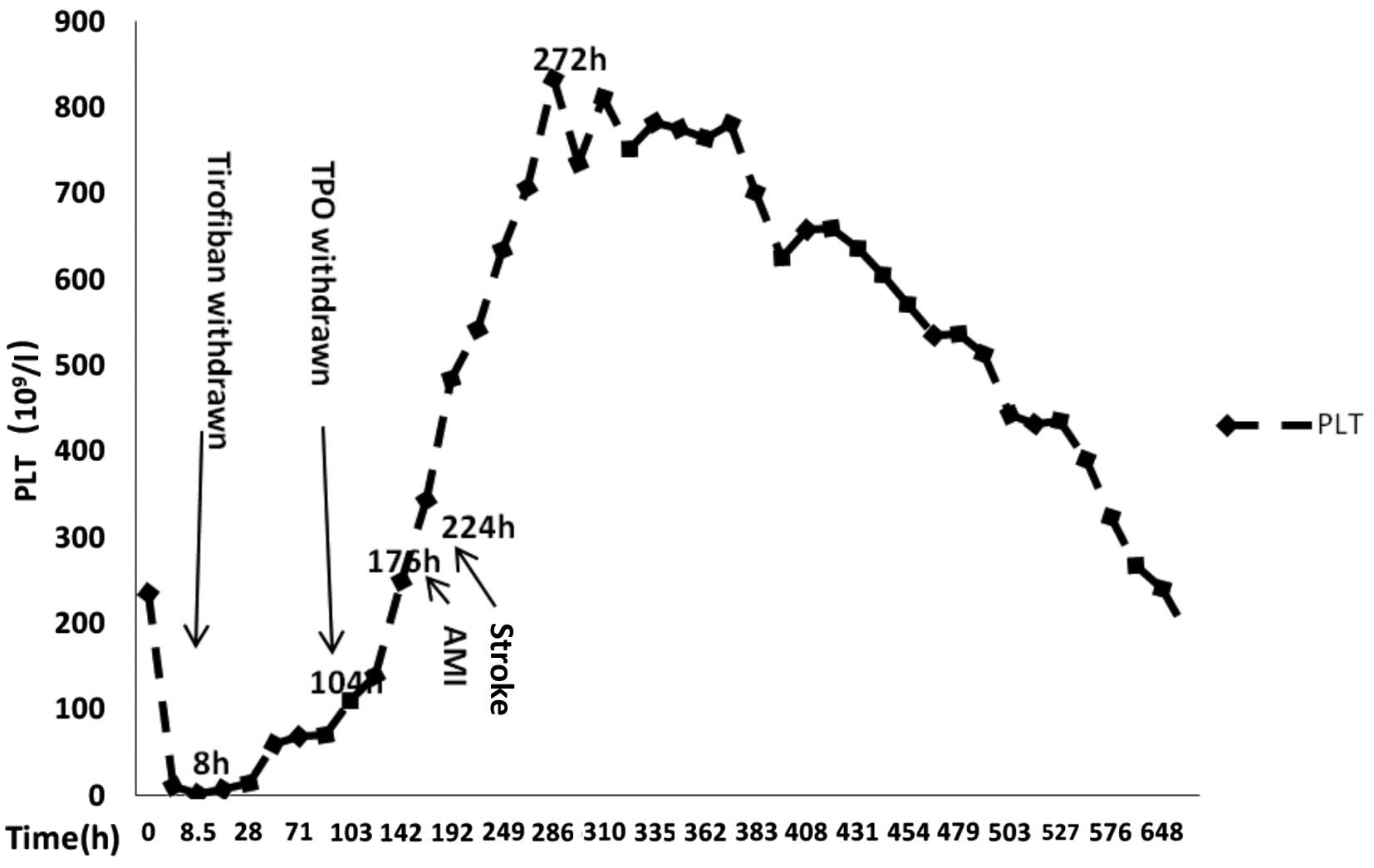
was administered intravenously. At 8 h after the procedure, the
platelet count of the patient decreased from 234×109/l
to 10×109/l, while hemoglobin levels, clotting function,
and hepatic and renal functions were normal. When the platelet
count was repeated, manually, a further reduction to
3×109/l was noted. All antiplatelet and antithrombotic
agents were discontinued immediately, and following consultation
with a hematologist, 40 mg methylprednisolone (Pfizer Inc., New
York, NY, USA) and 1 unit platelets were administered
intravenously, and 15,000 units TPO (3S Bio Inc., Hong Kong, China)
were injected subcutaneously once daily. The platelet count rose to
34×109/l after 48 h and to 71×109/l after 4
days of treatment. TPO was discontinued (total of 60,000 IU), and
aspirin, clopidogrel and a low-molecular-weight heparin (60 mg,
twice a day, Sanofi Pharmaceutical Co., Ltd.) therapy were
administered.
On the seventh morning of GIT and 3 days after the
discontinuation of TPO, the patient complained of acute chest pain.
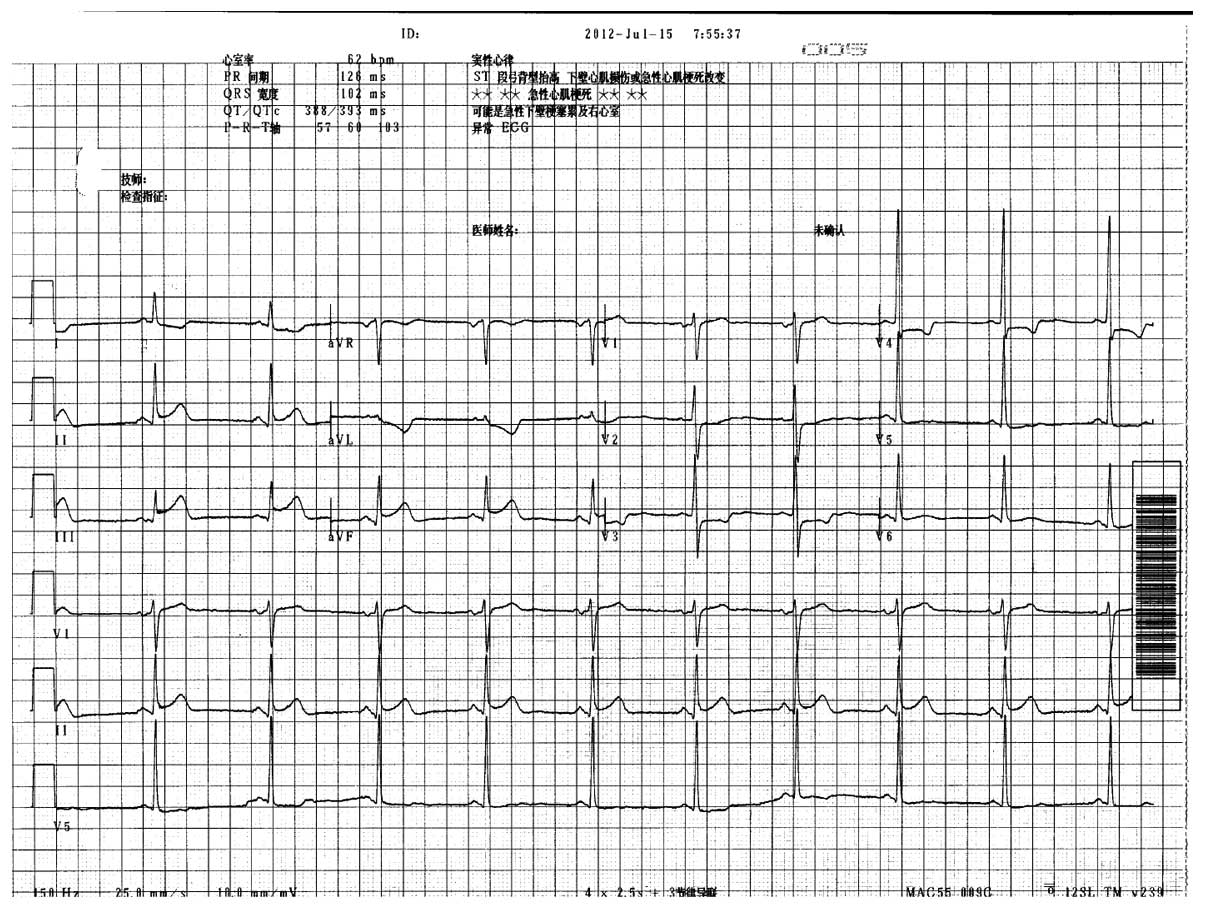
Electrocardiography (MAC 5000 ECG Analysis System, GE Medical
Systems Ltd.) suggested acute inferior wall myocardial infarction
(Fig. 1). The platelet count was
179×109/l (normal range: 100–300×109/l), and
the platelet aggregation test showed a value of 62% (normal range:
20–60%, calculated using Turbidimetric platelet aggregometry,
aggregation remote analyzer module, Helena Laboratories, Beaumont,
TX, USA). In brief, the procedure is as follows: Blood specimen is
collected and handled. The aggregation reagents are reconstituted
according to the directions of the manual. ADP, epinephrine and
collagen reagents should be used undiluted and will have the final
concentrations given below when mixed with the PRP during testing.
The aggregometer was prepared for use and a 450 µl pipette of
platelet poor plasma (PPP) was added to a cuvette. This blank was
used to set the 100% aggregation value. The cuvette was incubated
at 37°C between one and three minutes. Next, the PPP cuvette was
inserted into the appropriate channel and the instrument was set to
100% aggregation, and the cuvette set into the appropriate channel.
Next, 50 µl of the aggregating reagent dilutions were added to the
PRP cuvette and the percentage of aggregation was recorded
(instrument sets 0% when the aggregation agent is added and the
channel activated).
Following intensified antiplatelet treatment with
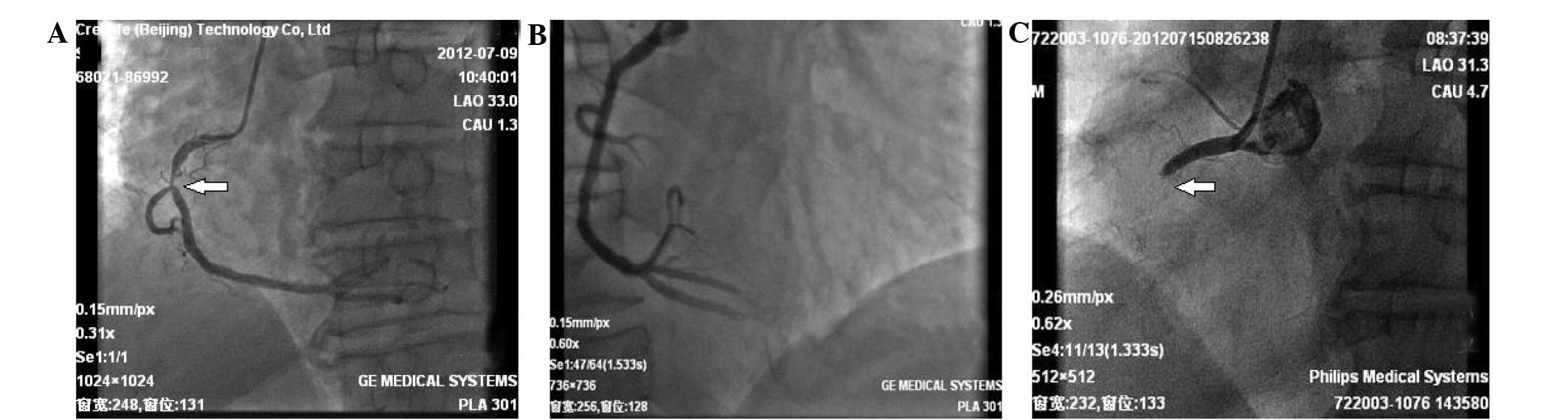
300 mg clopidogrel and 100 mg aspirin, a repeat coronary
angiography (Philips Medical Systems, Eindhoven, Holland) was
performed, which showed in-stent thrombosis and total occlusion of
the right coronary artery (Fig. 2).
After thrombus aspiration, another sirolimus-eluting stent (3.5×33
mm; Firebird; MicroPort Medical Group, Co., Ltd., Shanghai, China)
was implanted, and a Thrombolysis In Myocardial Infarction blood
flow at level 3 was restored. Dual anti-platelet therapy with 100
mg of aspirin and 150 mg of clopidogrel was applied. Heparin was
injected intravenously for anticoagulation.
On day 9 after GIT and 5 days after the
discontinuation of TPO, the platelet count rose to
314×109/l, and the platelet aggregation rate was 54%.
The patient had dysarthrosis, flatness of the right nasolabial
groove, and a deteriorated pain sensation; the muscle force of his
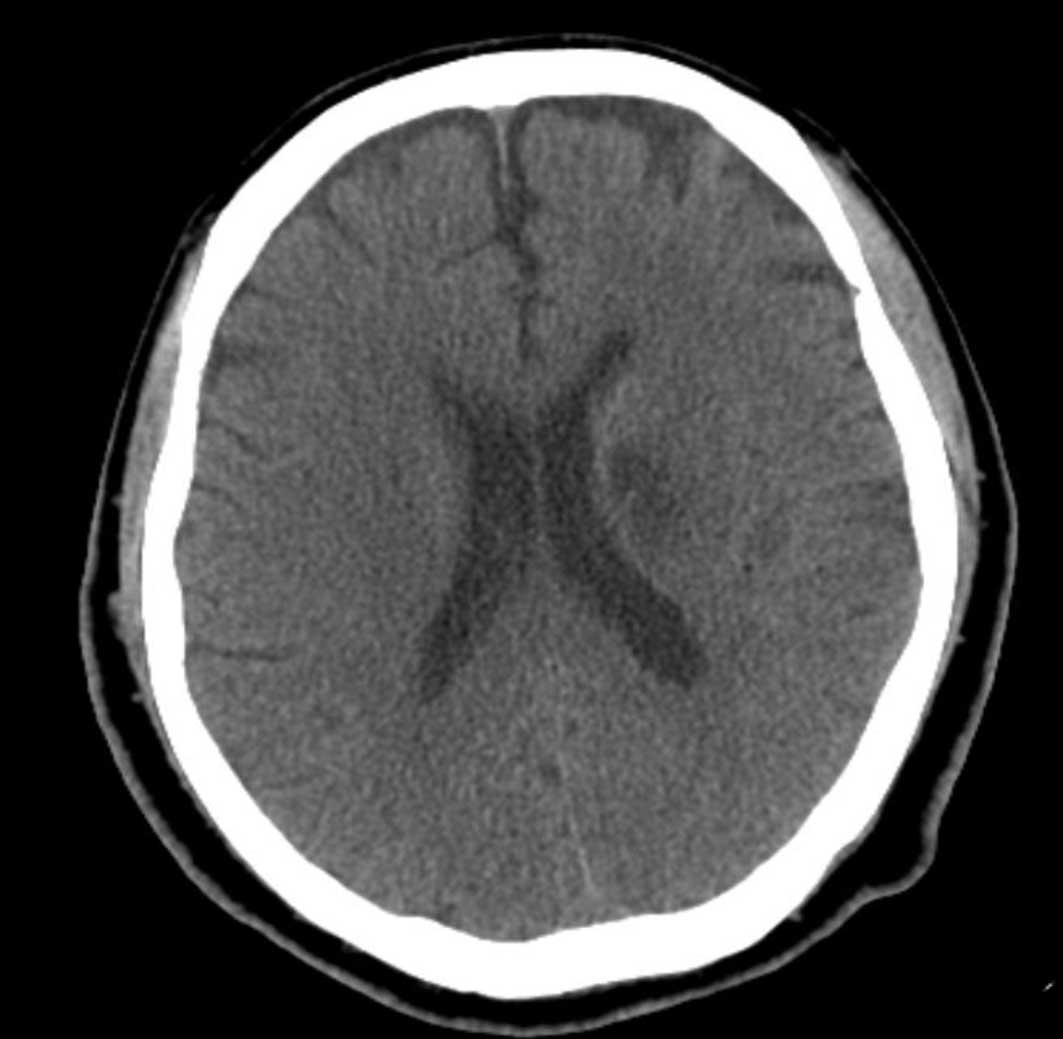
right-sided extremities became <5°. An emergency cranial
computed tomography (CT; Siemens dual-source computer tomography,
Munich, Germany) scan ruled out cerebral hemorrhage, and no
definite ischemic lesion was found. As these symptoms appeared on
the third day after coronary intervention, cerebral embolism caused
by plaque shedding during the procedure could be ruled out. Acute
cerebral infarction was confirmed by cranial CT 1 week later
(Fig. 3). The platelet count
increased progressively, and 11 days after the initiation of GIT
treatment (7 days after discontinuing TPO), it leveled out at
834×109/l, while the platelet aggregation rate was 24%.
Hydroxycarbamide (1 g, twice daily; (Shandong Qilu, Xinghua, China)
was applied to reduce the platelet count. After administering
hydroxycarbamide for 7 days, the platelet count was gradually
reduced to 636×109/l, and hydroxycarbamide was
discontinued. Antiplatelet treatment with aspirin (100 mg) and
clopidogrel (150 mg) daily and anticoagulant treatment was
continued with fondaparinux sodium (2.5 mg; GlaxoSmithKline, Cedex,
France).
At 10 days after the withdrawal of hydroxycarbamide,
when the platelet count was reduced to 186×109/l
(Fig. 4), the patient was
discharged. At the 28-month follow-up examination, the patient's
medical condition was stable.
Discussion
The mechanism of GIT remains unclear, but may be
associated with an autoimmune reaction (5). According to this theory, a GRPA can
alter the configuration of the platelet glycoprotein receptor,
thereby creating a new antigenic determinant. This antigen is
recognized by serum antibodies, leading to the removal of
thrombocytes from the blood circulation. The prognosis for patients
with GIT is poor (7). Evaluation of
abciximab (c7E3 Fab), a monoclonal antibody that binds to the
platelet glycoprotein IIb/IIIa receptor and inhibits platelet
aggregation, in the Prevention of Ischemic Complications (EPIC)
study (8) showed that patients with
GIT have a high risk of mortality, myocardial infarction and
revascularization. In the present case, the patient received
multiple antithrombotic agents, such as aspirin, clopidogrel,
unfractionated heparin and tirofiban prior to thrombocytopenia.
After analyzing the case, and considering that the clinical
diagnostic criteria of GIT are primarily based on the relationship
between the timing of medical administration and thrombocytopenia,
a clear conclusion could be made that the patient's
thrombocytopenia was induced by tirofiban. Since heparin was
administered during the stent implantation procedure,
heparin-induced thrombocytopenia could be ruled out. The patient
had no past history of heparin contact, and thrombocytopenia
developed fairly early and severely, which was not compliant with
the typical characteristics of type I or type II HIT (9). In addition, following the
discontinuation of tirofiban, the platelet count reverted to the
normal range. During the procedure of revascularization of the
right coronary artery, the large dosage of heparin did not trigger
thrombocytopenia. Therefore, it is likely that in the present case
thrombocytopenia was induced by tirofiban.
TPO is an effective thrombocytopenic medication that
may act directly on bone marrow hematopoietic stem cells to
specifically raise platelet counts (10). However, the effect of TPO starts
slowly; the platelet count starts to rise at 4–9 days, and reaches
a peak at 12–16 days after subcutaneous injection (8). Since the patient with GIT in the
present study had normal bone marrow function, when the effect of
TPO started, the patient theoretically might have already recovered
from GIT, and therefore TPO could have promoted a further rise in
platelet numbers, thus increasing the possibility of thrombosis.
However, when the thrombotic events occurred, the platelet count
was relatively low within the normal range. It is difficult to
determine whether the thrombotic events were drug-related, but the
use of TPO may have been an important contributing factor.
In summary, from this case, the conclusion can be
drawn that administration of tirofiban should be conducted under
careful clinical monitoring in order to detect GIT as early as
possible. GIT usually develops within 24 h after tirofiban
administration, and the platelet count resumes to a normal range
1–6 days after discontinuation of tirofiban (11). In the present case, it was critically
important to discontinue tirofiban once GIT was detected. Moreover,
in view of the possible thrombotic risk of TPO application, it
should be cautiously used in the treatment of GIT.
References
|
1
|
Dasgupta H, Blankenship JC, Wood GC, Frey
CM, Demko SL and Menapace FJ: Thrombocytopenia complicating
treatment with intravenous glycoprotein IIb/IIIa receptor
inhibitors: A pooled analysis. Am Heart J. 140:206–211. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeung J and Holinstat M: Newer agents in
antiplatelet therapy: A review. J Blood Med. 3:33–42.
2012.PubMed/NCBI
|
|
3
|
Labinaz M, Ho C, Banerjee S, Martin J,
Chen S and Mensinkai S: Meta-analysis of clinical efficacy and
bleeding risk with intravenous glycoprotein IIb/IIIa antagonists
for percutaneous coronary intervention. Can J Cardiol. 23:963–970.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li B, Ji Y, Shao Q, Zhu Z, Ji D, Li F and
Chen G: Comparative efficacy and cost effectiveness of splenectomy
and thrombopoietin prior to peginterferon and ribavirin therapy
with compensatory cirrhosis associated with hepatitis C and
thrombocytopenia. Exp Ther Med. 10:2180–2186. 2015.PubMed/NCBI
|
|
5
|
Gudbrandsdottir S, Frederiksen H, Birgens
HS, Nielsen CH, Nielsen OJ, Stentoft J and Hasselbalch HC: New
treatment options for primary immune thrombocytopenia. Ugeskr
Laeger. 173:271–274. 2011.(In Danish). PubMed/NCBI
|
|
6
|
Yuan SM: Report: Heparin-induced
thrombocytopenia associated with cardiopulmonary bypass:
Preliminary attempt with recombinant human thrombopoietin therapy.
Pak J Pharm Sci. 28:1793–1796. 2015.PubMed/NCBI
|
|
7
|
Tuhta AG, Yeşildağ O and Köprülü D:
Tirofiban-associated acute thrombocytopenia. Acta Cardiol.
61:577–579. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuter DJ: The biology of thrombopoietin
and thrombopoietin receptor agonists. Int J Hematol. 98:10–23.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huxtable LM, Tafreshi MJ and Rakkar AN:
Frequency and management of thrombocytopenia with the glycoprotein
IIb/IIIa receptor antagonists. Am J Cardiol. 97:426–429. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuter DJ: Thrombopoietin: Biology and
clinical applications. Oncologist. 1:98–106. 1996.PubMed/NCBI
|
|
11
|
Aster RH: Immune thrombocytopenia caused
by glycoprotein IIb/IIIa inhibitors. Chest. 127(2 Suppl): 53S–59S.
2005. View Article : Google Scholar : PubMed/NCBI
|