Introduction
Fibroblasts (Fbs) are present in the dermal layer of
the skin and are the primary dermal cells to produce collagen and
other extracellular matrix (ECM) components, and their normal
proliferation and differentiation maintain the structure and
physiological function of the skin (1,2).
Depressed scars are characterized by a change in the structure of
the dermal skin layer, as well as a reduced number of Fbs (3,4).
Surgery, trauma, infections or other causes can make defects in the
dermis layer of the skin and subcutaneous tissues, and to deplete
the ECM forming a permanent depressed scar (5–7). These
scars distress patients and push towards applying medical
cosmetics. ECM is significant in the process of skin repair. It
bridges intercellular signal transduction, connection, and
physiological processes, of which Type I and III collagens (in
particular type I collagen) is predominantly associated with the
repair of depressed scars (8,9). Fbs are
significant repair cells for wound healing that generate a large
number of tissue healing factors as the skin is damaged (10–12).
Their abilities to produce collagens may lead to accelerated wound
healing and improved scar repair (1,2,9). In the present study, autologous skin
Fbs (asFbs) were cultured in vitro using a depressed trauma
rat model. The function of asFbs in the repair of depressed scars
was examined at the whole-animal and cellular levels, in order to
provide a reliable scientific basis for the use of asFbs in medical
cosmetology.
Materials and methods
Reagents
RPMI-1640 medium and type IV collagenase were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA); rabbit anti-rat type I collagen polyclonal antibody (BA0325)
and rabbit anti-rat type III collagen polyclonal antibody (BA0326)
and mouse anti-rat vimentin monoclanal antibody (BM0135),
biotinylated rabbit anti-goat secondary antibody IgG (BA1003) and
biotinylated goat anti-mouse secondary antibody IgG (BA1001) were
purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China); the hydroxyproline (HYP) kit, S-ABC immunohistochemistry
kit and DAB color kit were purchased from Fuzhou Maixin Biotech
Co., Ltd. (Fuzhou, China).
Animals and construction of a
depressed trauma model
A total of 20 male Wistar rats weighing 250–300 g
were provided by the Animal Department of the Bethune Medicine
Division of Jilin University (Changchun, China). They were fed in
25°C, 12:12/light:dark cycle, and allowed free access to food and
water. As previously described by Ren et al (2), a surgical method was used to establish
a rat model of depressed trauma. The surgical procedure was
conducted as follows: The rats were fixed in a dorsal position
prior to hair removal. The rats were under anesthesia with 10 wt.%
chloral hydrate (3 ml/kg; Tianjin Fuchen Chemical Reagent Factory,
Tianjin, China). Conventional surgical sterilization was performed
with 20% iodine and 75% ethanol. A skin and deep muscular layer of
~3×3 cm was removed on either side of the spine. The surface of the
wounds were then sutured to cause depressed scars. Following
creation of the wound, each rat was fed separately and the wound
was carefully monitored in order to prevent infection. The rats
were observed daily to determine their systemic reactions and wound
healing. The excised skin was used for the in vitro culture
of asFbs. Animal experiments in the present study was approved by
the Animal Ethics Committee of Jilin University ad the experiments
were performed in the College of Pharmacy at the University.
Isolation and culture of asFbs
Following removal from the rats, the skin was soaked
in 75% ethanol for 1 min, placed in a sterile petridish, rinsed
with phosphate-buffered saline (PBS), and cut into 5×5 mm sections
using a pair of sterile scissors. The tissue sections were then
digested with 0.25% trypsin and incubated overnight at 4°C. The
following day, sterile ophthalmic scissors and tweezers were used
to gently remove the epidermis, subcutaneous muscle and adipose
tissues, retaining the dermis. The skin tissue samples were
sectioned and digested with 0.25% type IV collagenase at 37°C for 4
h. Following filtering with 200-mesh sieves and centrifugation at
1,000 rpm at room temperature for 15 min, the supernatant was
removed. RPMI-1640 medium supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.,) was added to form a cell
suspension. The cells were counted and inoculated into 25 ml
culture flasks at a density of 1×104 cells/ml, then
placed in CO2 incubator with 37°C, 5% CO2 and
90% humidity. After 3 days of static culture, the cells were
observed under an inverted microscope, and then the medium was
refreshed every other day.
Reinjection of asFbs
When the cultured cells had proliferated to the
third generation and reached 90% confluence (~21 days following
found creation), the cells were digested with 0.25% trypsin and
suspended in 1 ml PBS to form a density of 1×107
cells/ml. The cells were then injected into the scar dermis of the
left side of the rats (cell injection side). The right dermal scar
served as a control (scar control side). The changes in the scar
following cell injection were observed by eye at 7 and 30 days.
Finally, the rats were sacrificed and the skin from the
experimental sites of the rats was removed for histological
examination.
Morphological observation and
identification
Cells were digested with 0.25% trypsin and prepared
for cell suspension, adjusting the cell density to
~1×104/ml. The cells were then seeded onto 24-well
plates, containing pre-placed sterilized coverslips at the bottom
to allow cells to adhere. When the cells grew to a monolayer, they
were washed three times with PBS and fixed with 95% ethanol for 30
min. Conventional hematoxylin and eosin (H&E) staining was
performed in order to observe cell morphology under an optical
microscope.
The cells were identified with an
immunohistochemical S-ABC staining method using mouse anti-rat
vimentin monoclonal antibodies (dilution, 1:100) incubated at room
temperature overnight and biotinylated rabbit anti-mouse secondary
antibody IgG (dilution, 1:500) incubated at 37°C for 2 h (13,14). DAB
substrate liquid was used for staining at room temperature for ~5
min. An optical microscope was used at ×100 to count the number of
positively-stained cells, and the percentage of positive cells was
calculated.
AsFb viability and growth curve
The 3–10 passaged cells were digested 0.25% trypsin
to form a single cell suspension with a density of
1×106/ml. Trypan blue staining was used to test cell
viability (15). The cells were then
counted and inoculated in 96-well plates at a final concentration
of 1×103/ml. The growth curves were measured by MTT
assay (16).
Cell morphology at the cell injection
sites
H&E staining was routinely conducted as follows:
The cells were fixed with formalin, embedded in paraffin, cut into
4 μm sections, dewaxed in xylene, dehydrated with gradient ethanol
and stained with H&E (17).
Immunohistochemistry staining of type
I and type III collagens at the cell injection site
The S-ABC method was used for immunohistochemical
staining according to the manufacturer's instructions in the S-ABC
immunohistochemistry kit (Fuzhou Maixin Biotech Co., Ltd.).
Briefly, goat anti-rat type I collagen and type III collagen
polyclonal antibodies were used at a 1:100 dilution at 37°C for 4
h. Biotinylated rabbit anti-goat secondary IgG was used at a 1:500
dilution at 37°C for 2 h. S-ABC reagents were used following the
manufacturer's instructions (Wuhan Boster Biological Technology,
Ltd.). Freshly prepared DAB substrate solution was used at room
temperature for ~3–5 min for staining. The collagen-positive area
was examined by observing 5 randomly-selected dermal layer
specimens from each rat under 5 randomly-selected fields using a
microscope (magnification, ×400; Eclipse TE-2000-U, Nikon, Tokyo,
Japan) equipped with an attached SXM1200F digital camera from the
dermal layer of skin for each specimen. Morphological image
analysis software Image-Pro® Plus version 6.0. (Media
Cybernetics, Inc., Rockville, MD, USA) was used to discriminate
between areas of collagen and non-collagen by grayscale and to
calculate the percentage of collagen-positive areas.
Statistical analysis
Data were analyzed using the SPSS 11.5 software
package (SPSS, Inc., Chicago, IL, USA). The growth curve of the
asFbs, the results of the HYP content measurement and the collagen
data were presented as means ± standard deviation. Comparison
between groups was performed using one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Morphological observation and
identification
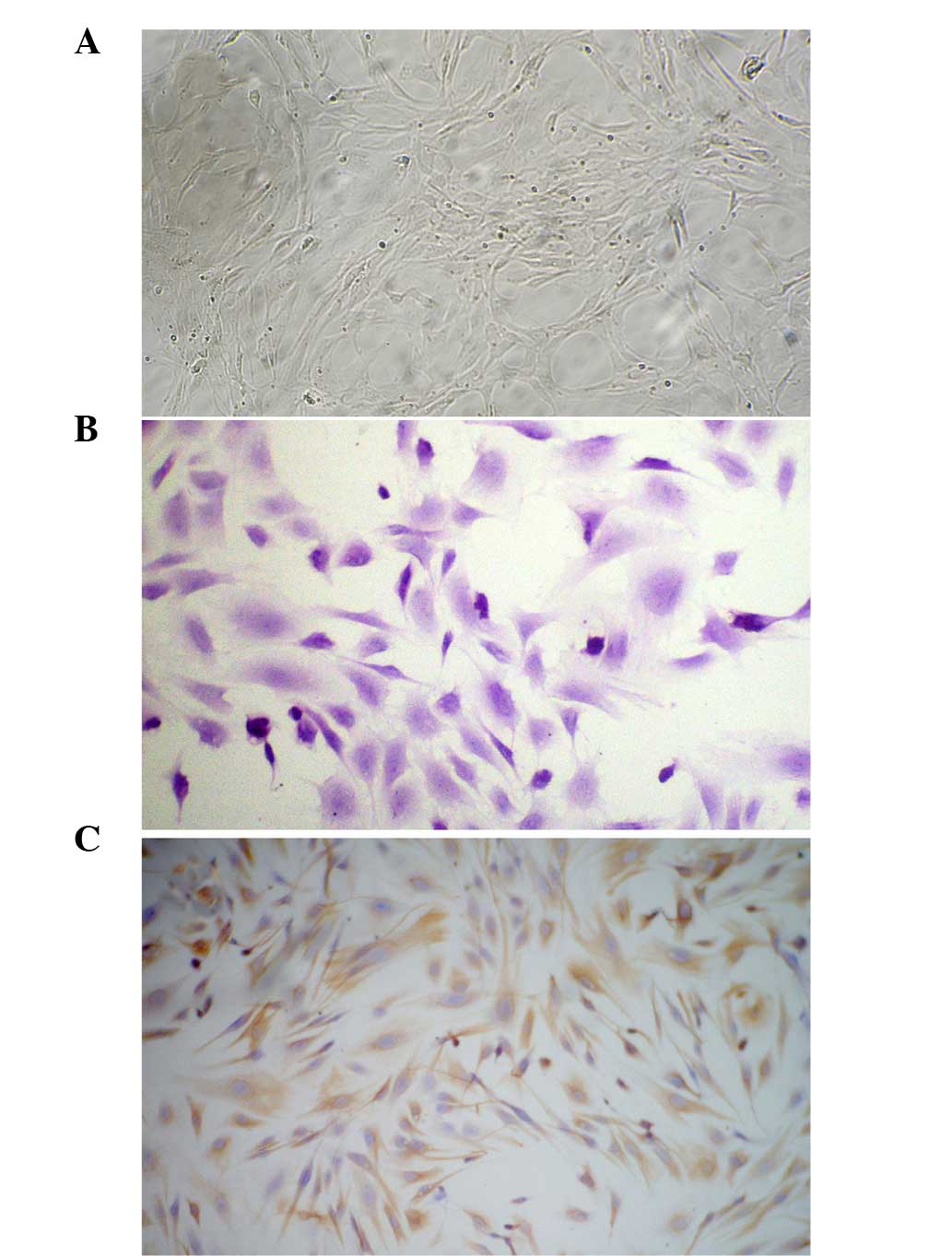
A total of 4 h after inoculation, rat asFbs became
adherent, long and filamentous, and grew rapidly. The proliferated
cells were confluent after 2–3 days, then the cells were closely
packed with a whirlpool, radial or palisade arrangement (Fig. 1A).
The third generation of cultured asFb cells was
stained using H&E and observed under light microscopy. The
cells were polygonal or long and filamentous with large nuclei, the
cell cytoplasm was stained pink, and the nuclei were stained blue
and purple as shown in Fig. 1B. The
S-ABC method was used to stain the vimentin of cultured skin asFbs.
The results showed a brown granular cytoplasmic reaction with
uncolored nuclei, and >98% of the cells were positively stained.
The cells expressing vimentin were asFbs, as shown in Fig. 1C.
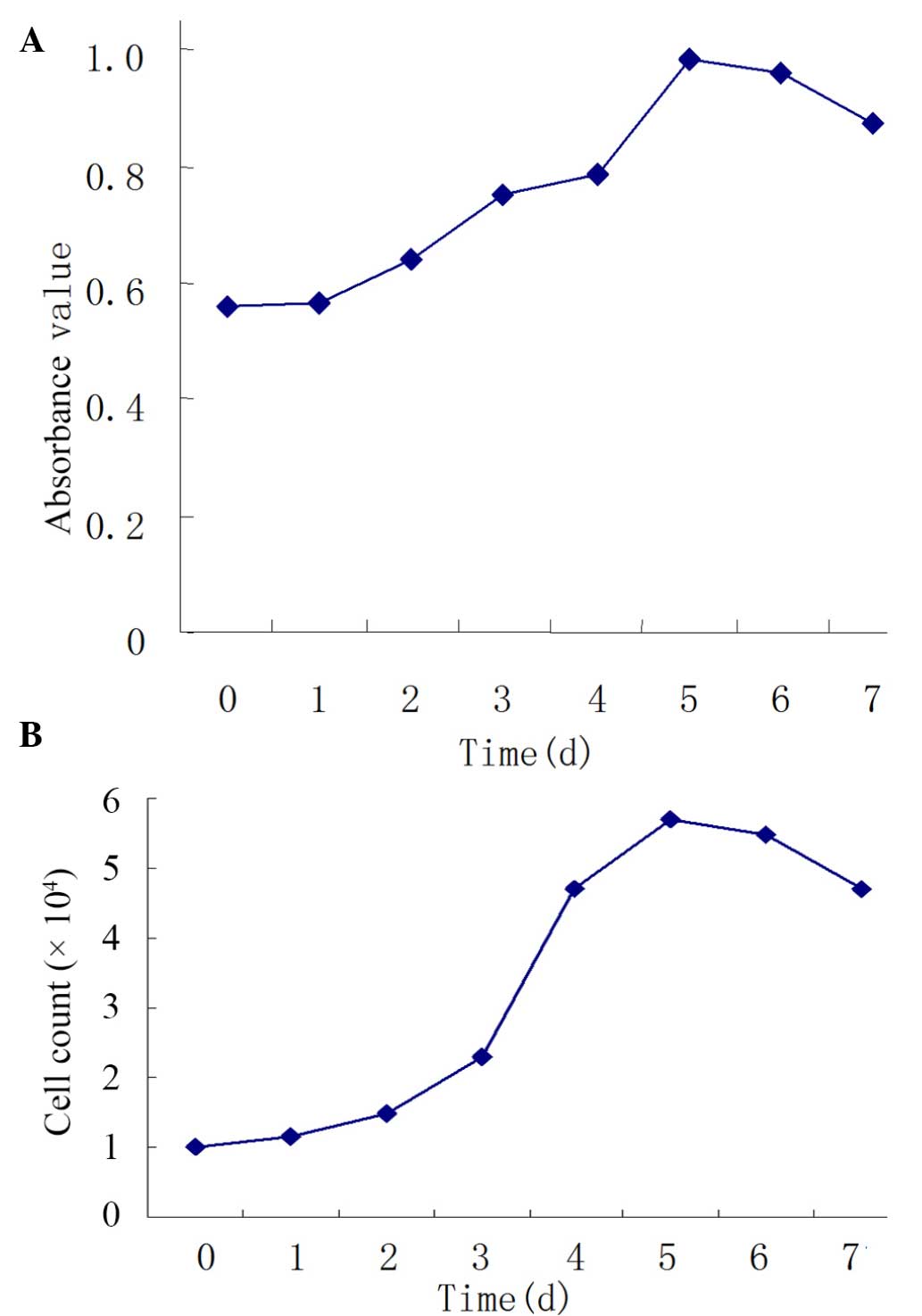
Skin asFb vitality and growth
curve
In the present study, trypan blue exclusion was
performed 3 times prior to observation under an inverted
microscope. The results demonstrated that few cells were stained
blue and the majority of cells resisted staining. After the asFb
cells were seeded into 96-well plates at 1×103
cells/well, we found that cells increased in the amount within 5
days and grew well. Cell proliferation at days 3–7 was
significantly increased compared with day 0 (P<0.01). The
incubation period, logarithmic growth phase, and plateau phase were
on days 0–1, days 1–5 and days 5–7, respectively (Fig. 2A).
Skin asFb recovery following
cryopreservation
Following asFb recovery, energy detection was
conducted. All cells recovered in good condition with a survival
rate of ~85%. The growth curves prior to and following recovery
were similar, suggesting the biological characteristics of asFbs
were stable (Fig. 2B). These results
suggest that cells can be successfully cryopreserved and
recovered.
Determination of the HYP content in
the culture medium of skin asFbs
The HYP kit demonstrated that HYP levels increased
with the proliferation of cells in the culture medium at 3–5 days,
and the HYP levels at days 3–6 significantly increased compared
with day 1 (P<0.01; Table I).
 | Table I.Levels of hydroxyproline in the
culture supernatants of skin fibroblasts. |
Table I.
Levels of hydroxyproline in the
culture supernatants of skin fibroblasts.
| Time (days) | HYP (mg/l) |
|---|
| 1 | 3.85±0.16 |
| 2 | 5.13±0.08 |
| 3 |
9.40±0.09b |
| 4 |
13.99±0.30b |
| 5 |
18.00±0.23b |
| 6 |
7.38±0.47a |
| 7 |
3.63±1.72a |
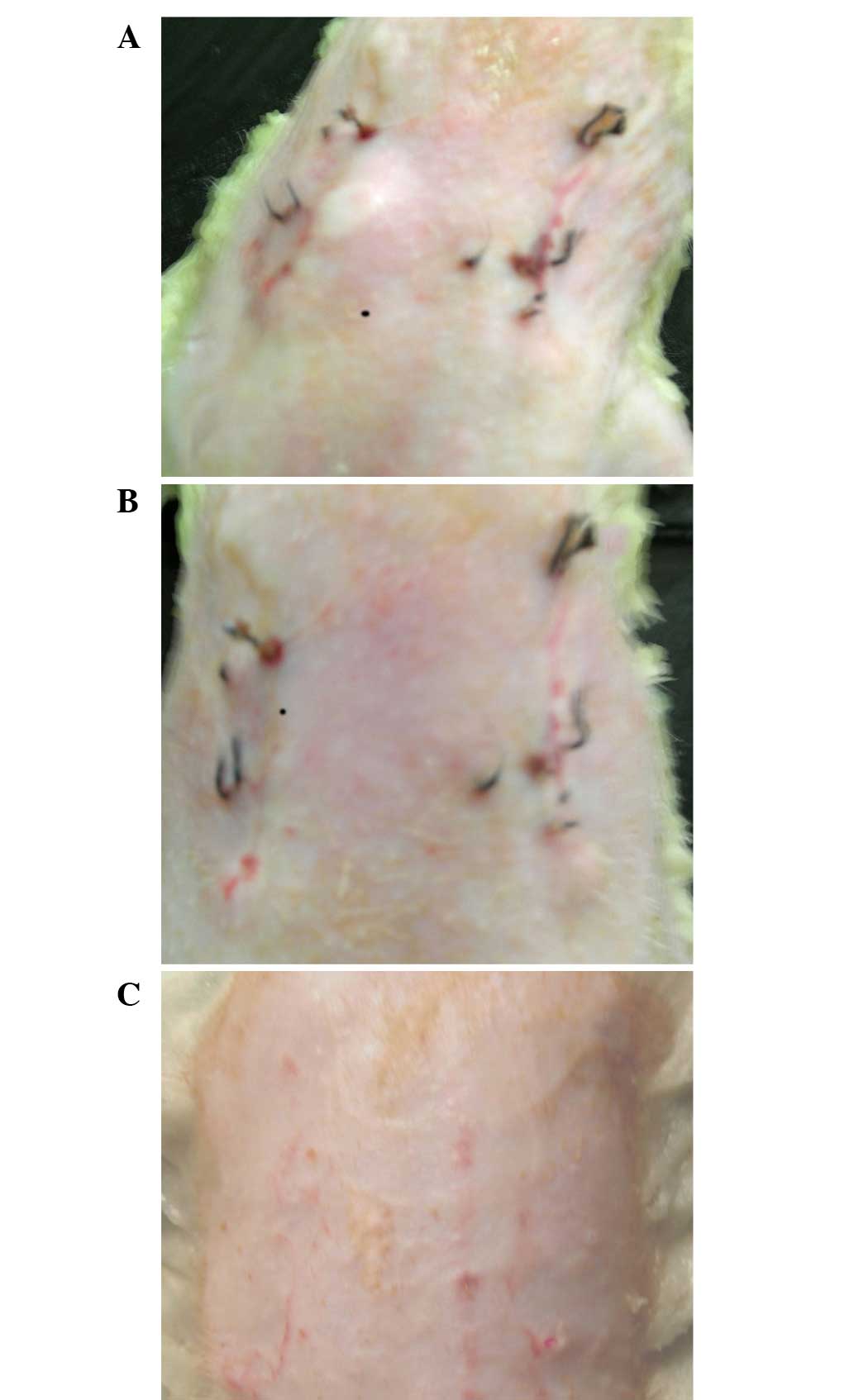
Observation of the repair of the
depressed scars
After 3 h of modeling, the bleeding at the wounds of
the rats stopped. At 3 days, the wounds were dry and had formed a
scab; the sunken scars on the backs of the rats had formed after 10
days, manifesting as linear scars, with lower than normal skin.
Prior to reinjection, the scars exhibited no significant difference
on either side. A total of 7 days after reinjection, the scar sites
injected with asFBs were significantly lighter compared with the
control side; the scar color had faded and the sunken appearance of
the scars were improved compared with the control skin. A total of
30 days after reinjection, the scars sites further improved and the
scars disappeared at the injected sites, with a similar color and
height as the normal control skin (Fig.
3).
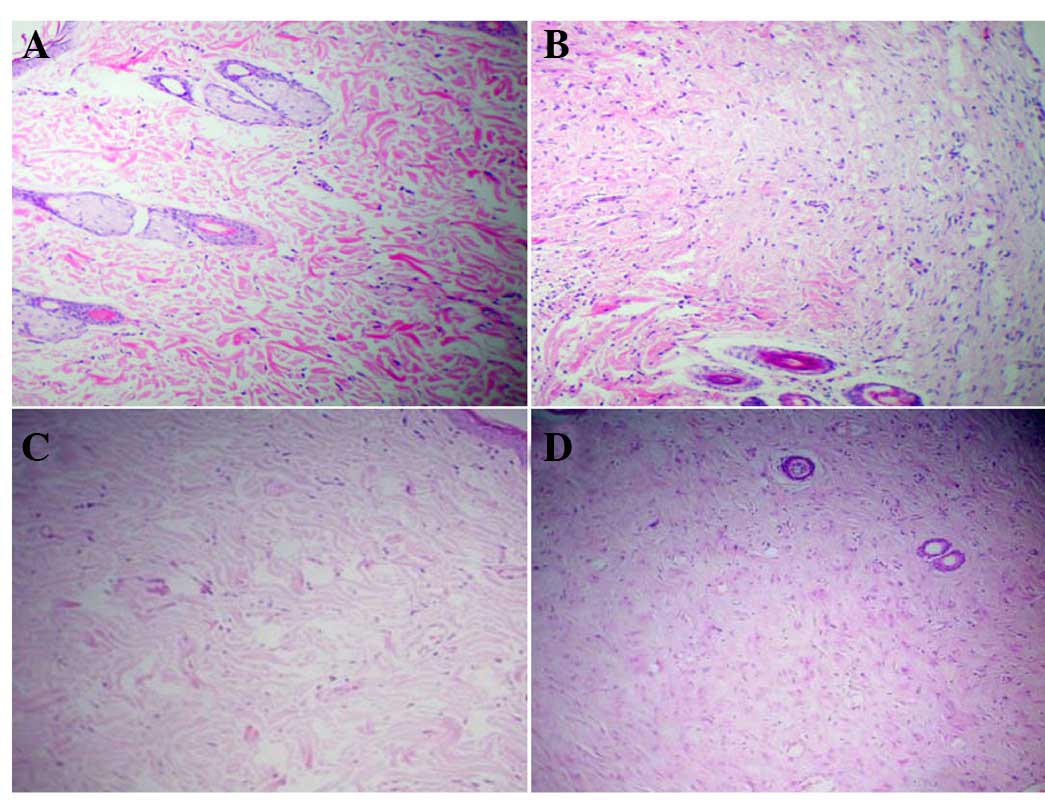
Morphological observation
H&E staining demonstrated that in the dermal
layer of the scar skin on the control side, the cells appeared in a
spindle shape with pale blue nuclei and pink cytoplasm. A total of
7 days after cell injection, the number of cells in the dermal
layer of the skin on the cell injection side increased
significantly compared with the control side. A total of 30 days
following cell injection, the number of cells in the dermal skin
layer on both the cell injection and control side increased
compared with the number of cells counted on day 7 (Fig. 4).
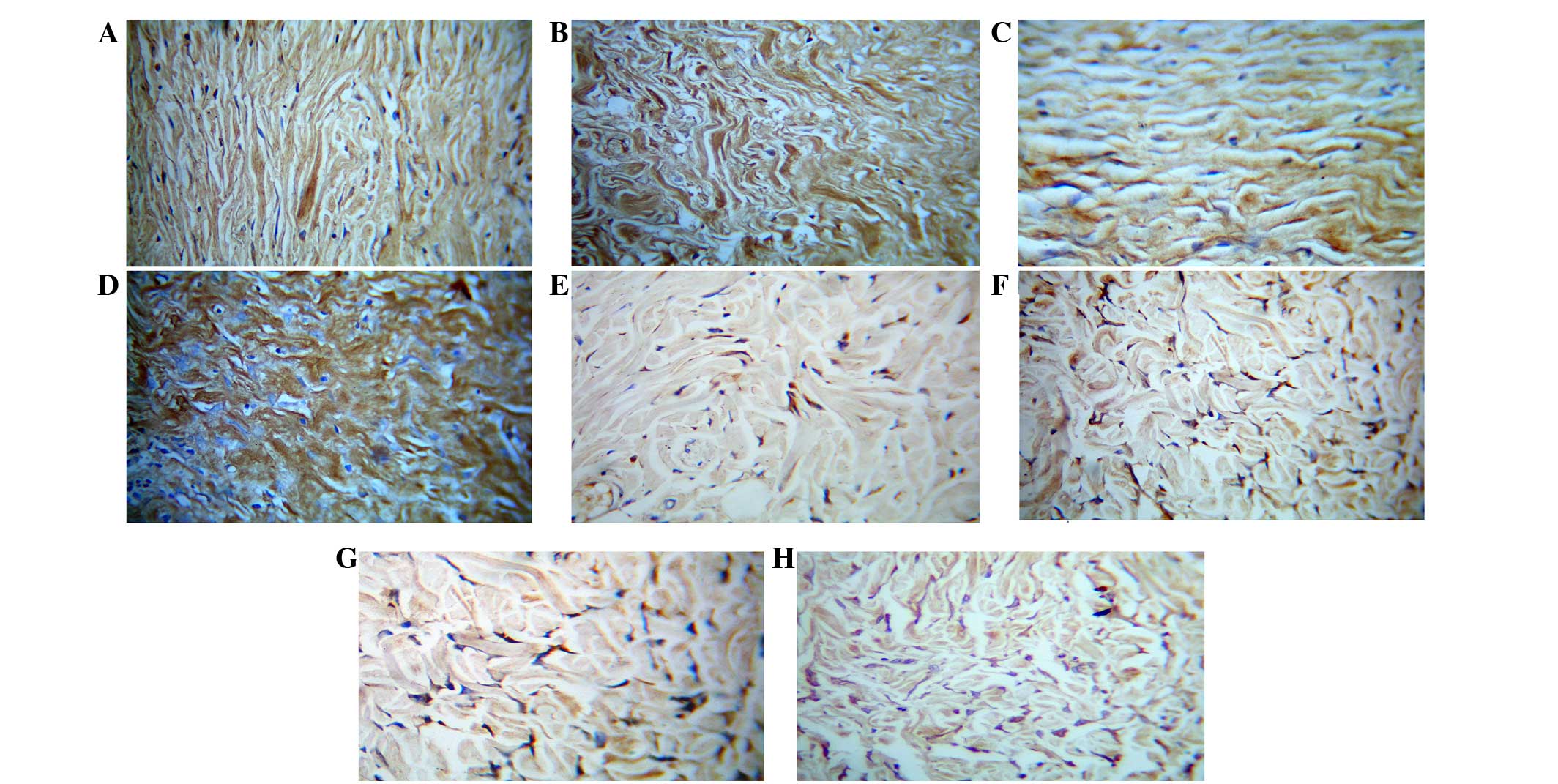
Immunohistochemistry of type I and
type III collagens
For type I and type III collagen staining, the
collagen fibers were positive and showed a brown color, the asFbs
in the dermis layer were also positive to be cytoplasmic brown
substance around the nucleus, but the nucleus was not colored.
Immunohistochemical observation of type I collagen staining
demonstrated that the percentage of collagen type I-positive areas
in all the groups were statistically significant (P<0.01;
Table II). A total of 7 days after
cell injection, the positive staining of collagen on the scar
control side was lower compared with the cell injection side. A
total of 30 days after injection, the number of asFbs and the
collagen staining in both the cell-injected and the control scar
were higher compared with those at 7 days. The immunohistochemical
staining of Type III collagen and type I collagen demonstrated
similar results, namely that 30 days after injection, the collagen
staining and the number of cells in the dermal layer were increased
compared with those at 7 days, and their collagen area fractions
were statistically significant (P<0.01; Table II). Although after cell injection
the number of asFbs, type I collagen levels and type III collagen
levels in the dermal layer were all increased compared with the
control side, immunohistochemical staining demonstrated that the
amount of type I collagen increased significantly more compared
with type III collagen. These results are shown in Table II and Fig. 5.
 | Table II.Type I and type III collagen-positive
areas at various time points (%). |
Table II.
Type I and type III collagen-positive
areas at various time points (%).
| Group | Cell injection time
(days) | Number of
animals | Area of type I
collagen (%) | Area of type III
collagen (%) |
|---|
| Cell injection | 7 | 5 |
36.33±0.41a |
10.06±0.67a |
| Scar control | 7 | 5 |
23.20±0.34a |
1.61±0.28a |
| Cell injection | 30 | 5 |
25.79±0.20a |
15.84±0.74a |
| Scar control | 30 | 5 |
9.80±0.18a |
5.41±0.47a |
Discussion
With the development of modern society and the
improvement of living standards, an increased emphasis is placed on
quality of life. As an important part of medical cosmetics, trauma
repair using beauty treatments has become increasingly popular
(18–21). Depressed scars, resulting from
surgery, trauma, infections or other causes, lead to defects in the
dermis layer of the skin and subcutaneous tissues, which are
depleted of collagen and elastin in the subsequent healing process,
forming a permanent depressed scar (5–7). These
scars can be distressing for patients.
In the present study, the autologous culture of
asFbs and a rat trauma healing model of depressed scars was used to
investigate the role of asFbs in depressed scars both at the
whole-animal level and cellular level. Depressed scars are
characterized by a change in the structure of the dermis layer of
the skin, as well as a reduced number of Fbs (22,23).
Therefore, in vitro cultivation of asFbs is an indispensable
procedure to study the repair of depressed scars. Combined
digestion methods using trypsin and collagenase for the in
vitro culture of asFbs are effective laboratory methods, and
asFbs cell cultures are highly proliferative, easily adaptable and
stable (24–27). The successful culture of asFbs has
provided a reliable source of cells for the repair of human
depressed scars (22).
Under normal circumstances, skin asFbs are in a
relatively quiescent state. When the skin is damaged, cells are
activated and enter into the proliferative and metabolic state, and
generate a large number of tissue healing factors (10–12).
asFbs are important repair cells for wound healing, and therefore
the study of the biological behavior of asFbs may lead to the
acceleration of wound healing and improve the recovery of the scar
tissue.
AsFbs are able to synthesize and secrete collagen,
elastin, fibronectin and laminin (1,2,8,9,22,23).
These ECM proteins not only support and connect cells, but also act
as intercellular bridges during signal transduction, thereby
participating in the physiological and pathological processes of
cells, and collagen is dominant in the ECM (8,9). The
present study examined the main indicators of depressed scars and
demonstrated that upon re-injection of in vitro proliferated
autologous asFbs into the wound dermis of rat depressed scars, the
synthesis and secretion of type I and type III collagens increased,
with type I collagen being dominant, markedly promoting the repair
of depressed scars.
In conclusion, the cell culture technique may
produce a large number of asFbs with the ability to synthesize and
secrete skin collagens. When a certain amount of asFbs are
re-injected subcutaneously, they can survive, synthesize and
secrete large amounts of collagens, thereby achieving the
therapeutic effect of filling depressed scars. As they are
autologous tissue cells, the disadvantages of immune and allergic
reactions are eliminated. The use of asFBs in cosmetic medicine
would be a repair method with simple, safe, minimally-invasive and
long-lasting advantages, improving the quality of life of patients
who elect to remove their depressed scars.
Acknowledgements
The present study was funded by The First Youth
Talent Project and the Key Disciplines Projects of the Hebei
Province.
References
|
1
|
Zeng W, Wei ZR, Liu D, Chai M and Zhao YM:
Preliminary clinical observations on autologous cultured skin
fibroblasts transplantation to treat the facial soft tissue
deficiencies. Zhonghua Zheng Xing Wai Ke Za Zhi. 29:29–33. 2013.(In
Chinese). PubMed/NCBI
|
|
2
|
Ren HT, Hu H, Li Y, Jiang HF, Hu XL and
Han CM: Endostatin inhibits hypertrophic scarring in a rabbit ear
model. J Zhejiang Univ Sci B. 14:224–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung DH, Medikeri GS, Chang GU and Hyun
SM: Surgical techniques for the correction of postrhinoplasty
depressed scars on the nasal tip. JAMA Facial Plast Surg.
17:405–412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seok J, Choi SY, Park KY, Jang JH, Bae JH,
Kim BJ, Kim MN and Hong CK: Depressed scar after filler injection
successfully treated with pneumatic needleless injector and
radiofrequency device. Dermatol Ther. 29:45–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Skigen AL, Bedrock RD and Stopperich PS:
Correction of the depressed, retracted, post-tracheostomy scar.
Plast Reconstr Surg. 103:1703–1705. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inchingolo F, Tatullo M, Abenavoli FM,
Marrelli M, Inchingolo AD, Corelli R, Inchingolo AM and Dipalma G:
Surgical treatment of depressed scar: A simple technique. Int J Med
Sci. 8:377–379. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan F, Richards K and Rashid RM:
Hyaluronic acid filler for a depressed scar. Dermatol Online J.
18:152012.PubMed/NCBI
|
|
8
|
Kubo K and Kuroyanagi Y: A study of
cytokines released from fibroblasts in cultured dermal substitute.
Artif Organs. 29:845–849. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burd A and Chiu T: Allogeneic skin in the
treatment of burns. Clin Dermatol. 23:376–387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang H, Chi JT, Dudoit S, Bondre C, van
de Rijn M, Botstein D and Brown PO: Diversity, topographic
differentiation, and positional memory in human fibroblasts. Proc
Natl Acad Sci USA. 99:12877–12882. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clark DP, Hanke CW and Swanson NA: Dermal
implants: Safety of products injected for soft tissue augmentation.
J Am Aced Dermatol. 21:992–998. 1989. View Article : Google Scholar
|
|
12
|
Viyoch J, Buranajaree S, Grandmottet F,
Robin S, Binda D, Viennet C, Waranuch N and Humbert P: Evaluation
of the effect of Thai breadfruit's heartwood extract on the
biological functions of fibroblasts from wrinkles. J Cosmet Sci.
61:311–324. 2010.PubMed/NCBI
|
|
13
|
Kotaska K, Petrak B, Kukacka J, Kraus J
and Prusa R: Anti-vimentin antibodies and neuron-specific enolase
in children with neurofibromatosis type-1. Neuro Endocrinol Lett.
28:761–764. 2007.PubMed/NCBI
|
|
14
|
Chen X, Xu R, Jiang YN, Zhu WN and Wang
YH: Simultaneous separation of primary cardiomyocytes and cardiac
fibroblasts from neonatal rats with density gradient
centrifugation. Sheng Li Xue Bao. 67:423–430. 2015.(In Chinese).
PubMed/NCBI
|
|
15
|
Avelar-Freitas BA, Almeida VG, Pinto MC,
Mourão FA, Massensini AR, Martins-Filho OA, Rocha-Vieira E and
Brito-Melo GE: Trypan blue exclusion assay by flow cytometry. Braz
J Med Biol Res. 47:307–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suto A, Kubota T, Shimoyama Y, Ishibiki K
and Abe O: MTT assay with reference to the clinical effect of
chemotherapy. J Surg Oncol. 42:28–32. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Werely WA: Automated hematoxylin and eosin
staining for large volumes of tissue. Am J Med Technol. 42:285–287.
1976.PubMed/NCBI
|
|
18
|
Cooper JS and Lee BT: Treatment of facial
scarring: lasers, filler, and nonoperative techniques. Facial Plast
Surg. 25:311–315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han HH, Kim SY, Lee YJ, Moon SH and Oh DY:
Donor-site closure using absorbable dermal staple for deep inferior
epigastric artery perforator flaps: Its efficacy and cosmetic
outcomes. Springerplus. 23:3632016. View Article : Google Scholar
|
|
20
|
Byun SH, Ahn KM, Kim SM and Lee JH:
Functional and cosmetic outcome after closure of radial forearm
free flap donor defect with porcine collagen membrane. J
Craniomaxillofac Surg. 44:527–532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanaydin V, Conings J, Malyar M, van der
Hulst R and van der Lei B: The role of topical vitamin E in scar
management: A systematic review. Aesthet Surg J. Mar 14–2016.(Epub
ahead of print) pii: sjw046. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Munavalli GS, Smith S, Maslowski JM and
Weiss RA: Successful treatment of depressed, distensible acne scars
using autologous fibroblasts: A multi-site, prospective, double
blind, placebo-controlled clinical trial. Dermatol Surg.
39:1226–1236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McAnulty RJ: Fibroblasts and
myofibroblasts: Their source, function and role in disease. Int J
Biochem Cell Biol. 39:666–671. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan S, Chen W, Liu X, Xiao J, Wang Y, Liu
J, Du Y, Wang Y and Zhang Y: Application of a novel population of
multipotent stem cells derived from skin fibroblasts as donor cells
in bovine SCNT. PLoS One. 10:e01144232015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Doucet YS and Owens DM: Isolation and
functional assessment of cutaneous stem cells. Methods Mol Biol.
1235:147–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tondato F, Zeng H, Goodchild T, Ng FS,
Chronos N and Peters NS: Autologous dermal fibroblast injections
slow atrioventricular conduction and ventricular rate in atrial
fibrillation in swine. Circ Arrhythm Electrophysiol. 8:439–446.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Walmsley GG, Maan ZN, Hu MS, Atashroo DA,
Whittam AJ, Duscher D, TEvlin R, Marecic O, Lorenz HP, Gurtner GC
and Longaker MT: Murine dermal fibroblast isolation by FACS. J Vis
Exp. 107:e534302016.
|