Introduction
The β-adrenergic receptor (β-AR) constitutes a
G-protein-coupled, catecholamine-mediated receptor that is
important in the regulation of cardiac function. The main subtypes
of β-AR in cardiac muscle tissue are, β1-AR and β2-AR. The
distribution, function and effect of the two receptor subtypes in
cardiac muscle tissue differ in normal and multiple pathological
conditions (1). β1-AR and β2-AR are
commonly expressed on the surface of cardiac muscle cell membranes
and mediate the systolic function of cardiac muscle, but they
generate different functions through different signaling pathways
(2,3). Under normal physiological conditions,
systolic function of the heart is mainly related to β1-AR, while
the signal system of β2-AR has a weak response to catecholamines.
During the development of chronic heart failure (CHF), β2-AR plays
a key role. For an aged heart in failure, the amount and function
of β1-AR decreases, while the amount of β2-AR is not altered, which
means that the physiological effects of β2-AR can be influenced and
improved significantly (4,5).
β-AR blockers have been widely used in China for the
treatment of congestive heart failure (CHF). Through clinical
observation, symptoms are improved, quality of life is increased
and the mortality rate decreases after long-term use (6). After treatment of β receptor blockers,
the increase of β receptor is regarded as the possible mechanism of
improvement for specific β receptor blocker medications such as
metoprolol (1). This increase occurs
much earlier than the improvement of clinical symptoms. For some β
receptor blocker medications, such as carvedilol and bucindolol,
there is no increase in β receptors following treatment but obvious
clinical benefits occur (3). In
addition, the increase of β receptors increases the sensitivity of
cardiac muscle cells to sympathetic nerve stimulation, which may
actually be detrimental. Currently, a selective β1-AR blocker has
been used in the treatment of patients with heart failure and
findings have shown that it also increases β2-AR (7). If this is the case the effects of
selective β1-AR blockers on cardiac muscle cells in heart failure
remain to be determined.
Previous findings showed that the amount of activity
of the calcium pump sarcoplasmic reticulum Ca2+-ATPase
(SERCA2a) in the sarcoplasmic reticulum during heart failure was
decreased (8–10). This decrease in activity affected the
contraction and relaxation of cardiac muscle. A large number of
experiments demonstrate that SERCA2a activity during heart failure
is 30% lower than normal (11).
Furthermore, contractility increases with enhanced SERCA2a activity
as in vitro experiments of cardiac muscle in heart failure
indicate (12,13). Previous studies examining the
mechanism of β receptor blockers and renin-angiotensin system
inhibitors on heart failure identified that the amount of activity
of SERCA2a increased with the improvement of heart failure symptoms
(14,15), indicating that SERCA2a is important
in heart failure. Therefore, β2-AR blockers potentially influence
the systolic function of cardiac muscle cells through the
regulation of SERCA2a.
The aim of the study was to determine the effects of
the highly selective β2-AR blocker ICI 118,551 on systolic function
and proteins of individual cardiac muscle cells in normal rats and
rats with heart failure. Additionally, the underlying molecular
mechanism of the β2-AR blocker on cells was examined. Influences of
systemic factors including nerve and body fluid were excluded.
Materials and methods
Experimental animals
In total, 250 male Sprague-Dawley rats weighing
180–220 g were provided by the Experimental Animal Center of Xuzhou
Medical College (Jiangsu, China).
The study was approved by the ethics committee of
Xuzhou Medical College.
Instruments and reagents
Collagenase II was purchased from Worthington
Biochemical Corp. (Freehold, NJ, USA). ICI 118,551, a β2 selective
blocker, was purchased from Sigma-Aldrich (St. Louis, MO, USA), and
required storage in the dark. The SDS-PAGE gel development kit was
purchased from Beyotime Institute of Biotechnology (Jiangsu,
China). Molecular weight marker, anti-mouse IgG and anti-rabbit IgG
were purchased from Sigma-Aldrich. Anti-β-actin was purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA); anti-β2-AR
(H-20): sc-569 was obtained from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The NBT/BCIP alkaline phosphatase color
development kit was purchased from Promega Corp. (Madison, WI, USA)
and the protease inhibitor cocktail set was purchased from Merck
Millipore (Darmstadt, Germany). Anti-SERCA2a monoclonal antibody
was purchased from Sigma-Aldrich, Langendorff cardiac muscle cell
perfusion apparatus and the dynamic boundary detection system of
individual cells were obtained from IonOptix (Westwood, MA, USA).
Gel electrophoresis system and semi-dry electrophoretic transfer
system were purchased from Bio-Rad Laboratories, Inc. (Hercules,
CA, USA). The discolored shaking table was obtained from Taicang.
Statistical analysis software used was ImageJ, SigmaStat and
SigmaPlot.
Establishment of heart failure model
for rats
Abdominal aortic constriction was performed to
prepare the model of a rat with heart failure. Briefly, male
Sprague-Dawley rats were weighed and anesthetized. After exposing
the internal structures, an in-house no. 7 silver clip was used as
banding along with aorta abdominalis over renal arteries with a
diameter of 0.7 mm. For the sham group, the aorta abdominalis was
separated without banding, and then closed. Twelve weeks after the
operation, multi-functional diagnostic ultrasound determined
intra-cardiac structure and function using a 10S probe at a
frequency of 11.0 MHz. The M-type ultrasound recorded contraction
and relaxation change curves of the left ventricle at the anterior
and posterior leaflet levels of the bicuspid valve to test LVEDD,
LVESD, FS and EF.
Separation, cultivation and
calculation of survival rate for cardiac muscle cells
A Sprague-Dawley rat was anesthetized and an
incision was made. The heart was removed and placed into cold 1
mM/l calcic KH solution for cardiac arrest. The heart was hung on a
Langendorff constant flow perfusion apparatus immediately after
arrest and then perfused with 1 mM/l calcic KH solution, low
calcium solution and enzyme solution, respectively. The heart was
removed while soft, sectioned into pieces, and the sections were
centrifuged at 400 × g for 1 min. The supernatant was then
discarded. The material was placed in 1 mM/l calcic KH solution,
and allowed to settle naturally after re-suspension. This was
repeated three times and then allowed to settle at a room
temperature of 32°C. After natural settlement, the solution was
changed. Visual counting was used to calculate cell density.
Cardiac muscle cell suspension liquid (1 ml) was added into one
well of a 24-well plate. Under a 10X objective lens five viewpoints
were selected and the total amount of rhabdocytes was calculated.
The total amount of cells and survival rate in these five
viewpoints were then calculated. Subsequently, the cells were
cultivated in serum-free medium for 48 h (at 37°C in a 5%
CO2 incubator). The survival rate was calculated by
random selection of five viewpoints from three groups of Petri
dishes. Images were captured under 10X objective lens and marked to
calculate the rod-shape rate following cultivation for 48 h. The
percentage of surviving cells was calculated as (amount of
rod-shaped cell/amount of total cells) ×100%.
Experimental group and administration
methods
The heart failure model comprised 75 rats with heart
failure and 70 rats in the Sham group. Cardiac muscle cells of
adult rats from primary culture were divided into the Sham, HF and
HF+ICI 50 nM groups. Indicators were observed after cultivation for
>48 h.
Testing of systolic function of
individual cardiac muscle cells
A dynamic boundary detection system was used for
individual cells (IonOptix) to record the length-time change curve
of cardiac muscle cells. Cardiac muscle cells in each group were
cultivated for >48 h and then washed with KH solution three
times. Cell suspension was placed into perfusion to test systolic
function. After standing for 5 min, the cell suspension was
perfused using 1 mM/l calcic KH solution with 5% CO2
mixed oxygen. Cardiac muscle cells were stimulated with electricity
(0.5 Hz). The liquid flow rate was 1.5 ml/min and the ISO
concentration response curve accumulated through semi-log
increments, ceasing when maximum contraction was reached or an
arrhythmia occurred. Rod-shaped cardiac muscle cells with clear
transverse striation, complete cell membrane and steady contraction
were selected to record the contraction curve. The data were
analyzed using IonWizard software (IonOptix Corp., Milton, MA, USA)
with the following results obtained: shortened rate of cardiac
muscle cell [(initial length of cell - length of cell after
contraction)/initial length of cell × 100%], time-to-peak (TTP) and
R50. When testing the systolic function of cardiac muscle cells,
newly prepared isoprenaline (10-7 mol/l, away from light) was added
into the circulated KH solution to observe the response of cells to
isoprenaline.
Western blot analysis and
immunoprecipitation
To prepare cell samples, cardiac muscle cells were
collected and cultivated for >48 h, washed twice, centrifuged
and the supernatant discarded. Homogenate with a protease inhibitor
cocktail was added and cells disintegrated through
ultrasonification. These cells were preserved at −80°C. The Lowry
method was used as a reference to test protein content with BSA as
the standard protein. To extract membrane protein, cardiac muscle
cell samples were preserved at −80°C, and placed in an ice box for
thawing, centrifuged for 5 min at 14,000 × g and the supernatant
was discarded. Cell lysis buffer 0.3% Triton X-100 × 100/PBS (mixed
with protease inhibitor) was added and blended with a micropipette
(Gilson, Villiers Le bel, France). The weight was 14,000 g.
Centrifugation followed for 15 min. The supernatant was the
cytoplasm layer, and the subnatant the cytomembrane layer. The
cytomembrane layer was drained and placed into 1.5 ml EP for
further experimentation.
For western blot analysis, all the following steps
occurred at 4°C. The samples were placed with the same protein
content into 4X Laemmli SDS-PAGE loading buffer of the same volume
and then in a boiling water bath for 5 min for degeneration
treatment. Denatured protein samples of equal amount (100 µg) were
removed, separated through SDS-PAGE and then transferred to NC
membrane through a semi-dry electrophoretic transfer method. The NC
membrane was placed into a confining liquid and incubated at room
temperature for 3 h. Primary mouse anti-serca2 ATPase monoclonal
antibody (Sigma, catalog no.: s1439) was added at a dilution of
1:1000 and incubated at room temperature for 4 h at 4°C overnight.
The membrane was washed with TBST (5 min × 3) and a secondary
antibody marked with AP was added. The membrane was incubated at
room temperature for 2 h, washed with TBST (5 min × 3) and then
rinsed with water. A NBT/BCIP kit was used for color development in
new AP coloring solution and the reaction was terminated using
running water. Image processing apparatus was used in the analysis
(to observe the expression and activation of protein).
Immunoprecipitation occurred at 4°C. Briefly, a
volume 5-fold that of the IP buffer solution was added to the
samples with the same protein content (400 µg). Then, 25 µl protein
A/G-agarose was used in pre-adsorption for 1 h, and centrifuged at
1,000 × g for 2 min. The supernatant was discarded and 1–2 µg
antibody was added, which was allowed to react on a rotating vortex
mixer for 4 h or overnight. Subsequently, 25 µl protein A/G-agarose
was added and allowed to react on a rotating vortex mixer for 2 h.
The agarose weighed 10.00 g. Following the reaction the agarose was
centrifuged at 1,000 × g for 2 min, and washed with IP buffer
solution three times. Subsequently, 2X Laemmli SDS-PAGE loading
buffer of the same volume was added, mixed and placed into a
boiling water bath for 5 min to elute protein from agarose, this
weighed 10.00 g. This was centrifuged at 1,000 × g for 2 min, and
the supernatant was absorbed for immunoprecipitation.
Western blot analysis
Ten percent separation gel and 4% spacer gel were
used to perform SDS-PAGE. After separation, the bands on the gel
were transferred to an NC membrane using a semi-dry electrophoretic
transfer method. The NC membrane was placed into confining liquid
and incubated at room temperature for 3 h. Primary antibody
(1:1,000) was added and incubated at room temperature for 4 h.
Then, secondary antibody (1:10,000) was added and incubated at room
temperature for 2 h. TBST was then used to wash the membrane (5 min
× 3). An NBT/BCIP kit was used for color development in a new AP
coloring solution and the reaction was terminated using running
water. Coloring bands on the membrane were scanned, processed and
analyzed through software such as ImageJ, SigmaStat and SigmaPlot.
Optical density in the bands was expressed by the multiple of the
normal group on the same membrane.
Statistical analysis
SPSS 16.0 software (Chicago, IL, USA) was used to
analyze data. Data were presented as mean ± SD. Comparison among
groups were analyzed through ANOVA and comparison between groups
tested by q. P<0.05 indicated statistically significant
results.
Results
Identification results of heart
failure model for rats
After 8 weeks of an established heart failure model,
symptoms such as decreased appetite, low spirits, no luster in fur,
fluffy fur and polypnea during a resting state occurred. No such
changes were observed in the Sham group during the same period.
Related parameters in ultrasonic cardiogram testing 12 weeks after
the operation in the heart failure model group indicated that the
inner diameters of the atrium and ventricle were increased,
myocardium was thinner and EF was significantly decreased (Table I). Other objective evidence for heart
failure was based on EF <64% in the ultrasonic cardiogram.
 | Table I.Selection of rats with heart failure
by cardiac function test through ultrasonic cardiogram. |
Table I.
Selection of rats with heart failure
by cardiac function test through ultrasonic cardiogram.
|
| Models of heart
failure rats |
|---|
|
|
|
|---|
| Test | Preoperative | Post-operative 12
weeks |
|---|
| LVDd (mm) |
5.02±0.84 |
5.46±0.64a |
| LVDs (mm) |
2.89±0.32 |
3.87±0.37a |
| FS (%) |
42.64±1.94 |
28.17±1.47a |
| EF (%) |
80.05±3.62 |
62.07±5.15a |
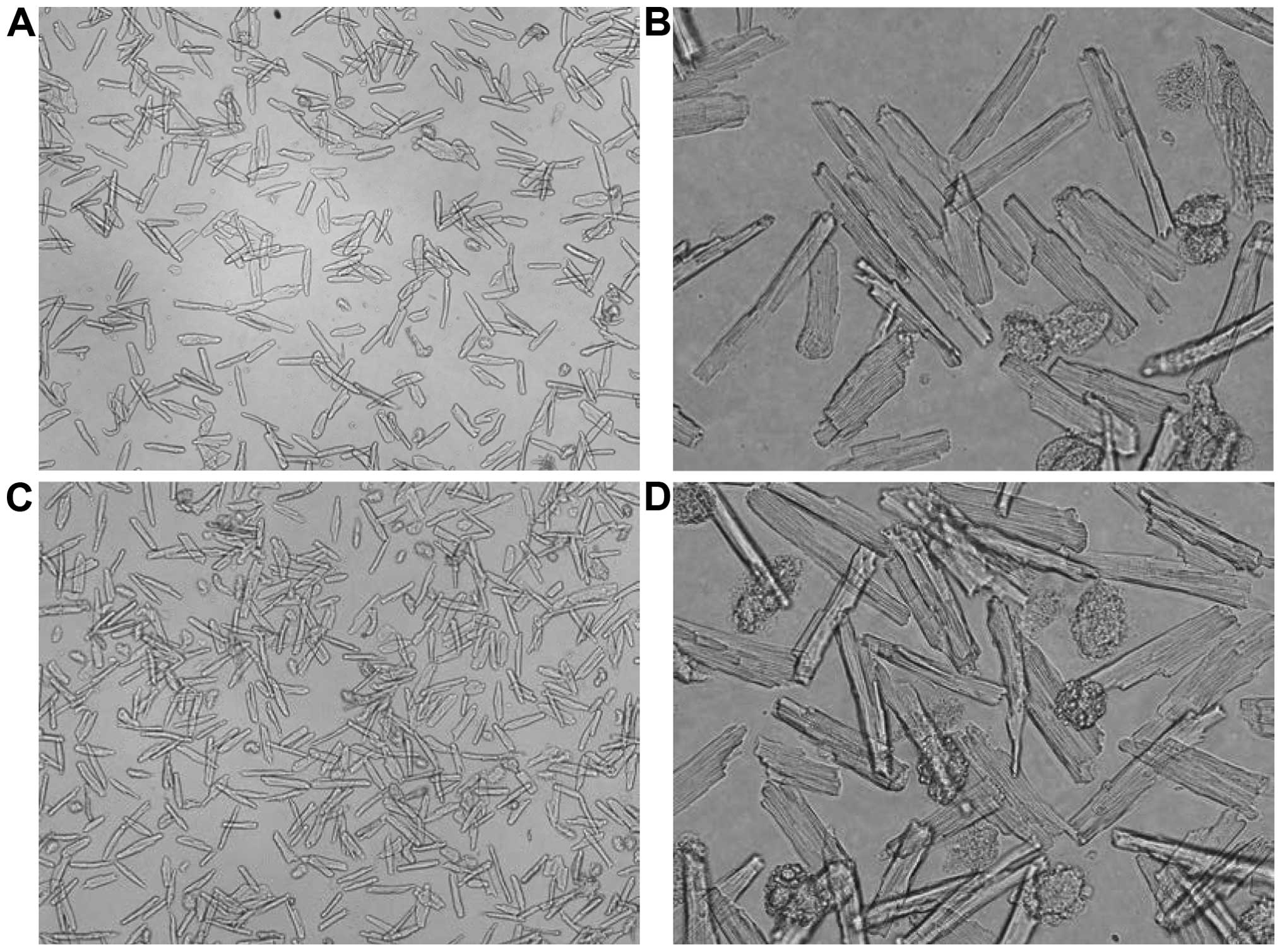
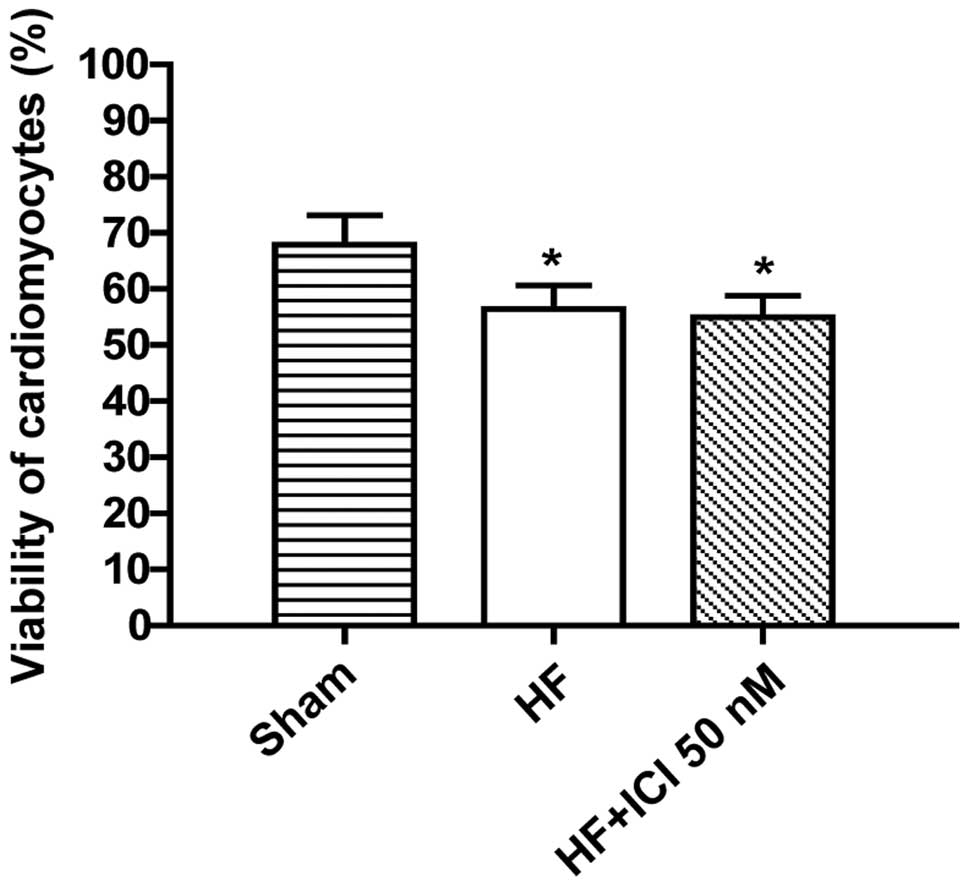
Survival rate of cardiac muscle cell
of rats with heart failure
Compared with the Sham group, the survival rates of
cardiac muscle cells in group HF and HF+ICI 50 nM were decreased
(P<0.05). No such changes were identified in terms of the
survival rate of cardiac muscle cells in group HF+ICI 50 nM
compared with group HF (P>0.05) (Figs. 1 and 2).
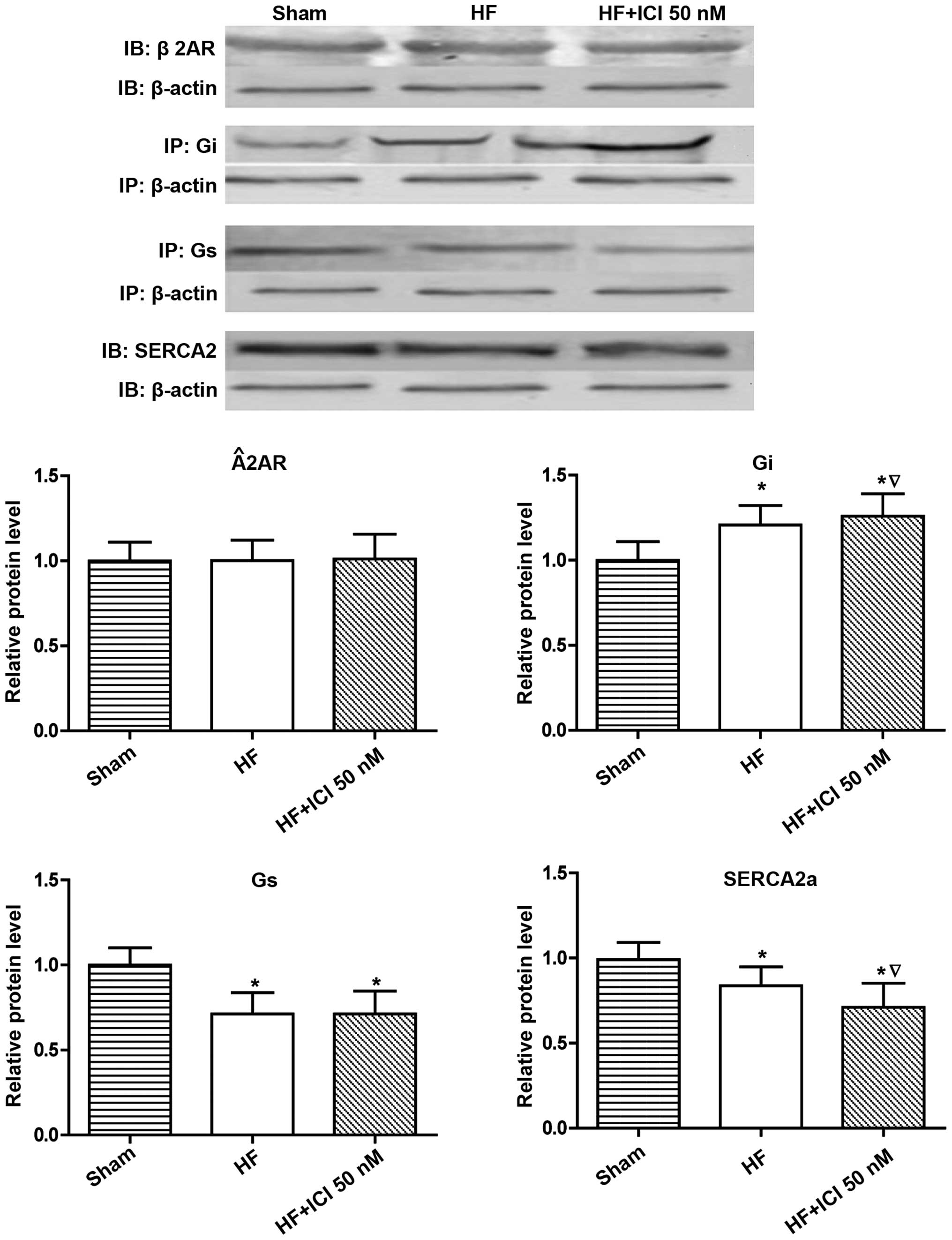
Molecular biology results for rats
with heart failure
Compared with the Sham group, Gi protein expression
levels in group HF and HF+ICI 50 nM increased (P<0.05), whereas
Gs protein expression (P<0.05) and SERCA2a protein expression
(P<0.05) decreased. Compared with group HF, there were no
obvious differences in terms of β2-AR protein and Gs protein
expression amounts for cardiac muscle cells in group HF+ICI 50 nM
(P>0.05). Gi protein expression increased (P<0.05) but the
SERCA2a protein expression amount was obviously decreased
(P<0.05) (Fig. 3).
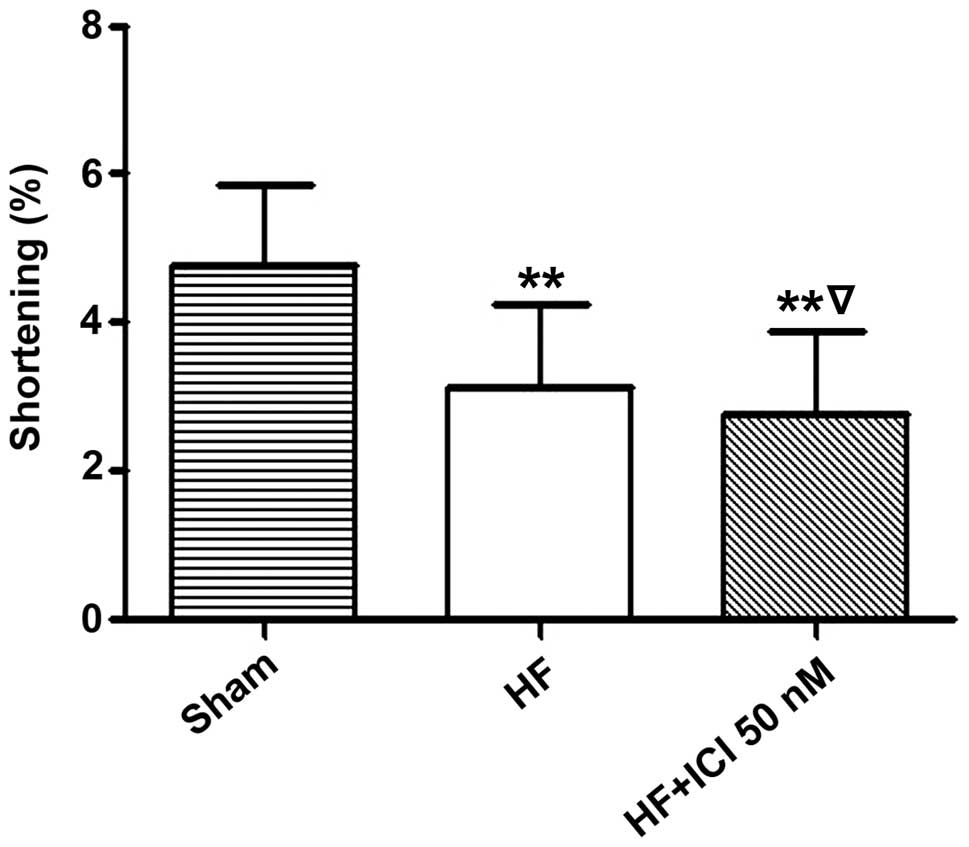
Systolic function test results of
cardiac muscle cells of rats with heart failure
Compared with the Sham group, the basic contraction
(1 mM Ca2+) amplitude percentage of cardiac muscle cells
in group HF significantly decreased (4.761±1.103 vs. 3.140±1.904%,
n=220, P<0.01). In addition, the basic contraction (1 mM
Ca2+) amplitude percentage of cardiac muscle cells in
group HF significantly decreased (4.761±1.103 vs. 2.761±1.110%,
n=220, P<0.01). Compared with group HF, the basic contraction (1
mM Ca2+) amplitude percentage of cardiac muscle cells in
group HF+ICI 50 nM decreased (3.140±1.094 vs. 2.761±1.110%,
P<0.05) (Fig. 4).
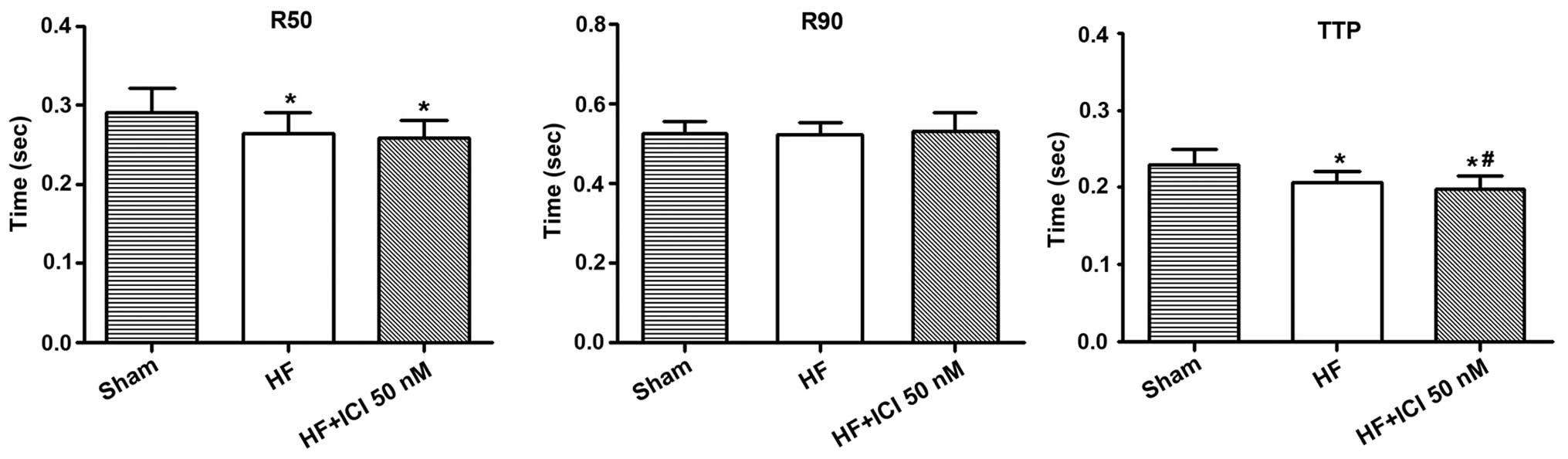
Compared with the Sham group, TTP contraction in
group HF was shortened (0.229±0.021 vs. 0.207±0.014 sec, n=60,
P<0.05) as was the TTP 50% relaxation (R50) of cardiac muscle
cells (0.291±0.031 vs. 0.264±0.027 sec, n=60, P<0.05).
Additionally, TTP contraction in group HF+ICI 50 nM was shortened
(0.229±0.021 vs. 0.198±0.018 sec, n=60, P<0.05) as was the TTP
50% relaxation (R50) of cardiac muscle cells (0.291±0.031 vs.
0.258±0.024 sec, n=60, P<0.05). There were no obvious
differences regarding R50 and R90 between heart failure cells
(P>0.05). Compared with group HF, TTP in group HF+ICI 50 nM
decreased (P<0.05) (Fig. 5).
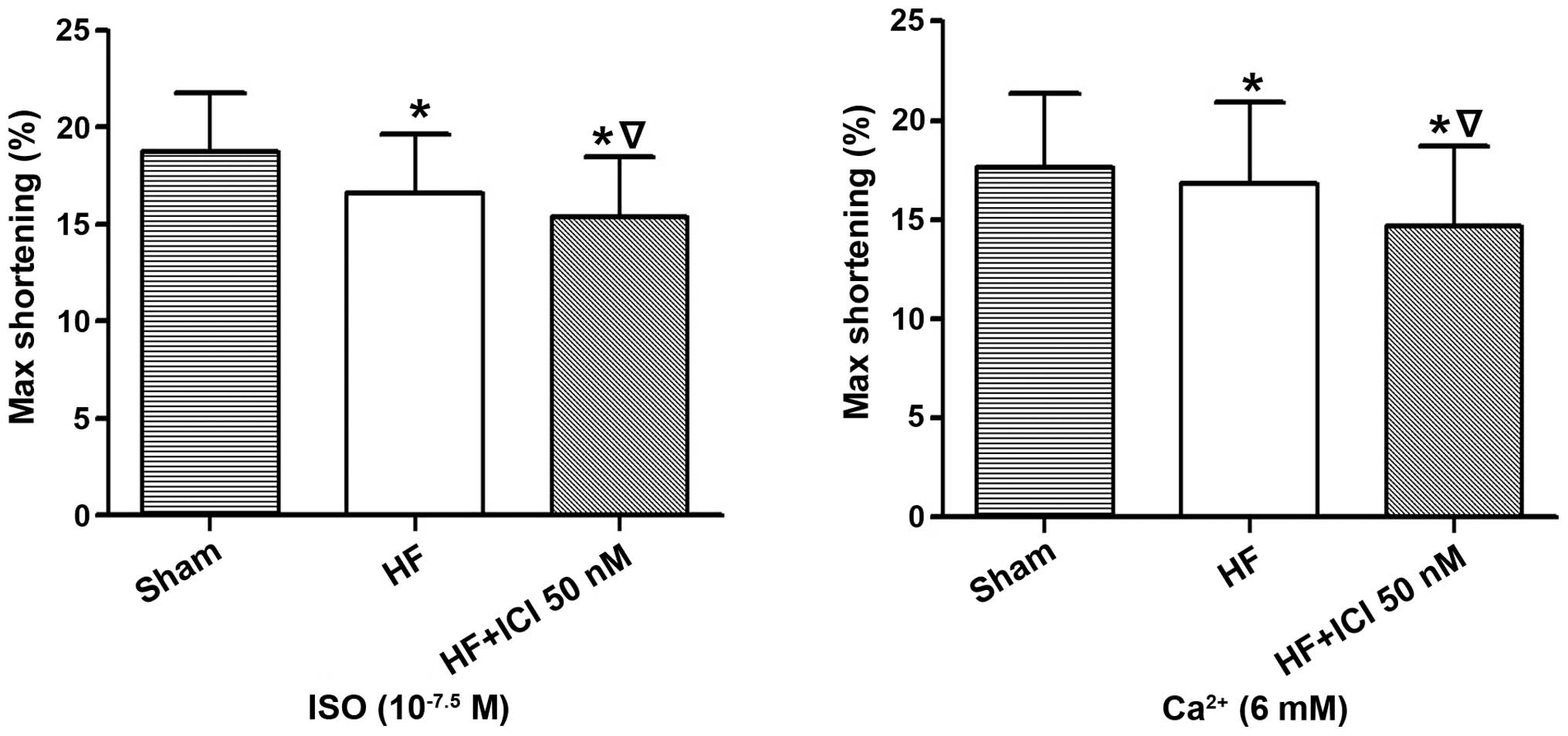
Following the stimulation of Ca2+, the
maximum contraction amplitude percentage of the cardiac muscle cell
in group HF decreased (17.664±3.683 vs. 16.821±4.104%, n=60,
P<0.05) when compared with the Sham group, as did that of group
HF+ICI 50 nM (17.664±3.683 vs. 14.670±4.021%, n=60, P<0.05). The
maximum contraction amplitude percentage of cardiac muscle cells in
group HF+ICI 50 nM was reduced compared with that in group HF
(16.821±4.104 vs. 14.670±4.021%, P<0.05) (Fig. 6).
Following the stimulation of ISO, the maximum
contraction amplitude percentage of cardiac muscle cells in group
HF decreased (18.757±3.051 vs. 16.587±3.075%, n=60, P<0.05) when
compared with the Sham group, as was the case for group HF+ICI 50
nM (18.757±3.051 vs. 15.384±3.112%, n=60, P<0.05). The maximum
contraction amplitude percentage of cardiac muscle cells in group
HF+ICI 50 nM was lower than that in group HF (16.587±3.075 vs.
15.384±3.112%, P<0.05) (Fig.
7).
Discussion
CHF is a common clinical syndrome that remains an
important lethal cardiovascular disease. The application of β-AR
blocker in the long-term treatment of heart failure has changed
previous therapeutic schedules. Although it may reduce the
contraction ability of cardiac muscle cells over a short period of
time, it does increase myocardial contractility or decrease the
mortality rate in the long term.
The surface of cardiac muscle cells mainly expresses
two ARs, β1 and β2. Activated β1-AR stimulates the classic
Gs-AC-cAMP-PKA signaling pathway (16) and causes a series of phosphorylation
of proteins associated with calcium treatment. However, activated
β2-AR, not only stimulates the abovementioned pathway and generates
positive contraction and relaxation effects, but also activates the
Gi-PI3K-AKt signaling pathway (17),
limiting and balancing out positive relaxation and contraction
effects generated by the Gs signaling pathway in terms of space and
function. It is considered that there are no changes regarding the
expression of β2-AR in heart failure (18). The results of the present study show
that compared with the Sham group, there were no changes in the
expression of β2-AR of cardiac muscle in group HF. By contrast, Gs
protein was decreased, Gi protein was increased and SERCA2a protein
expression was decreased. Function testing of cardiac muscle
indicates that basic contraction during heart failure decreases,
because of decreasing β1-AR. Therefore, the contraction effect
generated from coupling with Gs decreases and the function of
cardiac muscle is reduced.
The effects of systemic factors such as nerve and
body fluid were excluded from the present study, which identified
the effects of highly selective β2-AR blocker ICI 118,551 on
systolic function and the protein of individual cardiac muscle
cells of normal rats, as well as rats with heart failure based
directly on cell and receptor level. The results show that ICI
118,551 may decrease the systolic function of cardiac muscle in
isolated heart failure under basic contraction and the stimulation
of Ca2+ and ISO.
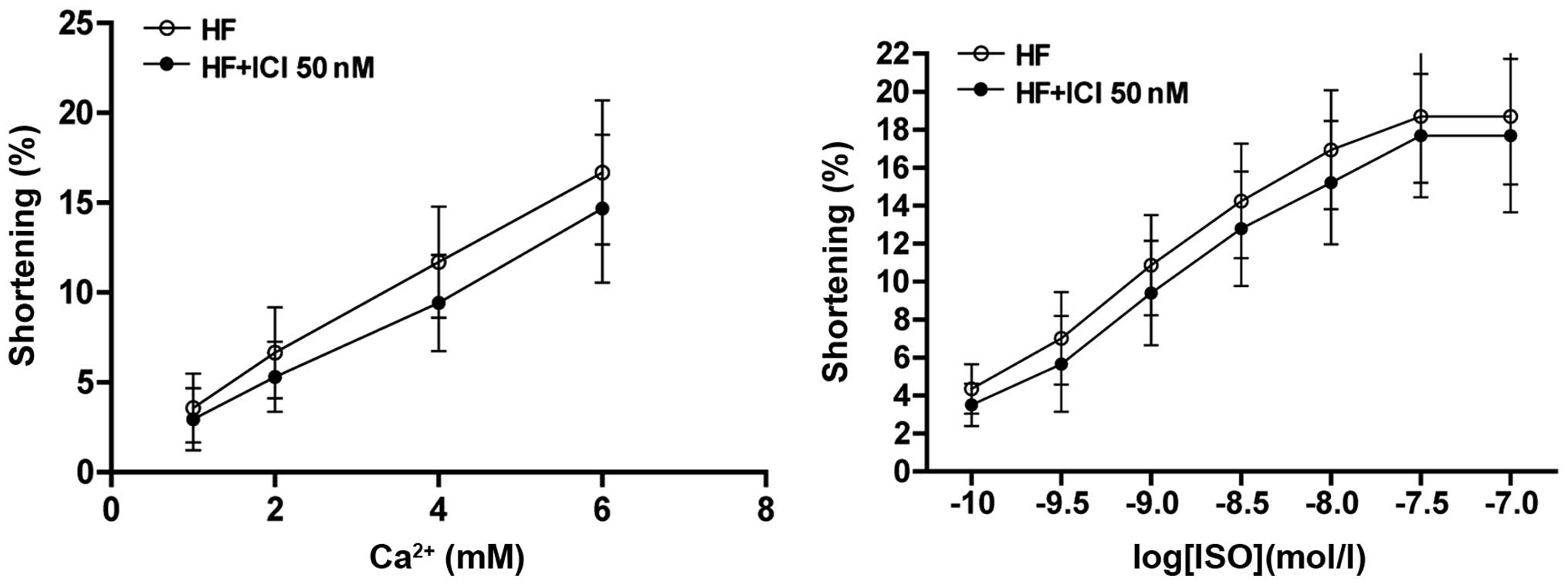
The present findings have shown that when the
concentration of Ca2+ is >6 mM, cardiac muscle in
heart failure begins to spasm, decreasing the function of
individual cardiac muscle cells. When stimulating cardiac muscle
with ISO of different concentrations, the contraction amplitude of
cardiac muscle in heart failure may increase with the increased
concentration of ISO and be lower than the contraction amplitude of
normal cardiac muscle cell under the same concentration. This
finding shows that compared with the normal cardiac muscle, the
reactiveness to catecholamines of cardiac muscle in heart failure
is lowered. According to literature (19), cardiac muscle tissues exposed to an
epinephrine agonist over a long period of time die easily. This may
be associated with the decreasing concentration of Ca ATPase
protein in the sarcoplasmic reticulum with myocardial hypertrophy
and heart failure (20). Following
the development of myocardial hypertrophy, the reduction becomes
more obvious and results in the dysfunction of Ca2+
intake. In addition, due to long-term sympathetic nerve
stimulation, cardiac muscle becomes hypertrophic and oxygen
consumption of myocardium increases, and the resulting insufficient
energy supply affects the systolic function of cardiac muscle
(21,22).
Compared with the HF control group, systolic
function in the ICI 118,551 (50 nM) HF group decreased, Gi protein
expression increased and SERCA2a protein level decreased. Thus, a
negative inotropic effect occurs for ICI 118,551 through the
Gi-PI3K-AKt signaling pathway. This increases Gi protein on the one
hand, whereas, the systolic function of cardiac muscle cells may be
reduced by decreasing SERCA2a pump function on intake, storage and
release of Ca2+. The survival rate of cardiac muscle in
heart failure is lower than that of normal hearts (19). ICI 118,551 has no effect on the
survival rate of cardiac muscle cells in heart failure, which
ensures that there are no differences in terms of cell amount
between groups. A large number of data have shown that loss of
cardiac muscle cells may be a key factor for the development of
heart failure (23). Cardiac muscle
cell apoptosis is the main reason for the continuous loss of
myocardial contraction units during the development of CHF. This is
because of the progressive decrease of cardiac function involved in
the physiopathologic changes occurring during CHF.
Acknowledgements
The study was partly financed by the Jiangsu
Provincial Special Program of Medical Science (BL2012019).
References
|
1
|
Dzimiri N: Regulation of β-adrenoceptor
signaling in cardiac function and disease. Pharmacol Rev.
51:465–501. 1999.PubMed/NCBI
|
|
2
|
Xiao RP and Lakatta EG: Beta
1-adrenoceptor stimulation and beta 2-adrenoceptor stimulation
differ in their effects on contraction, cytosolic Ca2+,
and Ca2+ current in single rat ventricular cells. Circ
Res. 73:286–300. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng M, Han QD and Xiao RP: Distinct
β-adrenergic receptor subtype signaling in the heart and their
pathophysiological relevance. Sheng Li Xue Bao. 56:1–15.
2004.PubMed/NCBI
|
|
4
|
Bristow MR, Ginsburg R, Umans V, Fowler M,
Minobe W, Rasmussen R, Zera P, Menlove R, Shah P and Jamieson S:
Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing
and failing human ventricular myocardium: coupling of both receptor
subtypes to muscle contraction and selective beta 1-receptor
down-regulation in heart failure. Circ Res. 59:297–309. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kiuchi K, Shannon RP, Komamura K, Cohen
DJ, Bianchi C, Homcy CJ, Vatner SF and Vatner DE: Myocardial
beta-adrenergic receptor function during the development of
pacing-induced heart failure. J Clin Invest. 91:907–914. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lv Z and Huang R: Common issues of
neuroendocrine antagonist in treatment of chronic cardiac failure.
Chin J Cardiol. 33:293–296. 2005.(In Chinese).
|
|
7
|
Aoyagi T, Yonekura K, Eto Y, Matsumoto A,
Yokoyama I, Sugiura S, Momomura S, Hirata Y, Baker DL and Periasamy
M: The sarcoplasmic reticulum Ca2+-ATPase (SERCA2) gene
promoter activity is decreased in response to severe left
ventricular pressure-overload hypertrophy in rat hearts. J Mol Cell
Cardiol. 31:919–926. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kögler H, Hartmann O, Leineweber K, van
Nguyen P, Schott P, Brodde OE and Hasenfuss G: Mechanical
load-dependent regulation of gene expression in
monocrotaline-induced right ventricular hypertrophy in the rat.
Circ Res. 93:230–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dash R, Frank KF, Carr AN, Moravec CS and
Kranias EG: Gender influences on sarcoplasmic reticulum
Ca2+-handling in failing human myocardium. J Mol Cell
Cardiol. 33:1345–1353. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spragg DD, Leclercq C, Loghmani M, Faris
OP, Tunin RS, DiSilvestre D, McVeigh ER, Tomaselli GF and Kass DA:
Regional alterations in protein expression in the dyssynchronous
failing heart. Circulation. 108:929–932. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei J, Liu HC, Lee FY, Lee MS, Huang CY,
Pan HP and Lin CI: Role of the sarcoplasmic reticulum in altered
action potential and contraction of myopathic human and hamster
ventricle. Clin Exp Pharmacol Physiol. 30:232–241. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van de Caetsbeek I, Raeymaekers L, Wuytack
F and Vangheluwe P: Factors controlling the activity of the SERCA2a
pump in the normal and failing heart. Biofactors. 35:484–499. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakata Y, Yamamoto K, Mano T, Nishikawa N,
Yoshida J, Nakayama H, Otsu K, Suzuki K, Tada M, Hori M, et al:
Angiotensin II type 1 receptor blockade prevents diastolic heart
failure through modulation of Ca(2+) regulatory proteins and
extracellular matrix. J Hypertens. 21:1737–1745. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sabbah HN, Sharov VG, Gupta RC, Mishra S,
Rastogi S, Undrovinas AI, Chaudhry PA, Todor A, Mishima T, Tanhehco
EJ, et al: Reversal of chronic molecular and cellular abnormalities
due to heart failure by passive mechanical ventricular containment.
Circ Res. 93:1095–1101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chinese Society of Cardiology of Chinese
Medical Association, Editorial Board of Chinese Journal of
Cardiology: Guidelines for the diagnosis and management of chronic
heart failure. Chin J Cardiol. 35:1076–1095. 2007.
|
|
16
|
Brixius K, Frank KF, Bölck B, Hoyer F and
Schwinger RH: Reverse remodeling of the intracellular
Ca(2+)-homeostasis: new concepts of pathophysiology and therapy of
heart failure. Wien Med Wochenschr. 156:209–215. 2006.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gong H, Adamson DL, Ranu HK, Koch WJ,
Heubach JF, Ravens U, Zolk O and Harding SE: The effect of
Gi-protein inactivation on basal, and beta(1)- and
beta(2)AR-stimulated contraction of myocytes from transgenic mice
overexpressing the beta(2)-adrenoceptor. Br J Pharmacol.
131:594–600. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hamdani N, de Waard M, Messer AE, Boontje
NM, Kooij V, van Dijk S, Versteilen A, Lamberts R, Merkus D, Dos
Remedios C, et al: Myofilament dysfunction in cardiac disease from
mice to men. J Muscle Res Cell Motil. 29:189–201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lohse MJ, Engelhardt S and Eschenhagen T:
What is the role of beta-adrenergic signaling in heart failure?
Circ Res. 93:896–906. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee DI, Vahebi S, Tocchetti CG, Barouch
LA, Solaro RJ, Takimoto E and Kass DA: PDE5A suppression of acute
beta-adrenergic activation requires modulation of myocyte beta-3
signaling coupled to PKG-mediated troponin I phosphorylation. Basic
Res Cardiol. 105:337–347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tóth A, Kiss L, Varró A and Nánási PP:
Potential therapeutic effects of Na+/Ca2+
exchanger inhibition in cardiac diseases. Curr Med Chem.
16:3294–3321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vinge LE, Raake PW and Koch WJ: Gene
therapy in heart failure. Circ Res. 102:1458–1470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun H, Zhou F, Wang Y, Zhang Y, Chang A
and Chen Q: Effects of beta-adrenoceptors overexpression on cell
survival are mediated by Bax/Bcl-2 pathway in rat cardiac myocytes.
Pharmacology. 78:98–104. 2006. View Article : Google Scholar : PubMed/NCBI
|