Introduction
Lung cancer is the most common cause of
cancer-associated mortality cause in the world (1). In particular non-small cell lung cancer
(NSCLC) accounts for ~80% of all lung cancer cases (1). NSCLC is commonly diagnosed at the
advanced stages of the disease, and platinum-based chemotherapy is
the standard treatment for advanced NSCLC, with response rates to
currently-used regimens of 30–40% (2). However, patients eventually develop
resistance to chemotherapy, resulting in a median survival of only
8–10 months (3).
Targeted therapies are another option in patients
who harbor mutations on key genes, such as in the epidermal growth
factor receptor (EGFR) and anaplastic lymphoma kinase genes
(4–9). EGFR-tyrosine kinase inhibitors
(EGFR-TKIs) are used in the treatment of advanced NSCLC,
particularly in patients with activating mutations in the EGFR gene
(4–8). Two types of mutation have been reported
to comprise up to 90% of all activating EGFR mutations, including
the deletion in exon 19, clustered around the amino-acid residues
747–750, and a specific point mutation in exon 21 (L858R) (10–12). The
progression-free survival (PFS) of the first-line EGFR-TKI drugs
ranges between 8.4 and 13.1 months (4–8), and the
overall survival reached 34.8 months (13). The common side effects of EGFR-TKIs
include rash, diarrhea, fatigue and loss of appetite (14).
Erlotinib is an oral EGFR-TKI used in the treatment
of advanced NSCLC, particularly for patients with activating
mutations in the EGFR gene. EGFR is expressed in basal and
suprabasal layers of the epidermis, in sebaceous glands and in the
outer root sheath of the hair follicles (15), which may explain the cutaneous side
effects of erlotinib, including acneform rash. Along with the wide
use of erlotinib, there is an increasing number of studies
reporting uncommon side effects, such as cardiac adverse effects
(16), interstitial lung disease
(17) and ocular side effects
(18). The present study described
the cases of 6 pulmonary adenocarcinoma patients receiving
erlotinib, who presented rare side effects associated with
epidermis, such as trichomegaly and alterations of scalp hair
(Table I). In the present study, all
patients provided written informed consent.
 | Table I.Characteristics of the six pulmonary
adenocarcinoma patients administered erlotinib. |
Table I.
Characteristics of the six pulmonary
adenocarcinoma patients administered erlotinib.
| Patient No. | Age (Years) | Gender | EGFR mutation | PFS (Months) | Disease progress | Trichomegaly | Scalp hair
alterations | Time appears
(Month) |
|---|
| 1 | 29 | F | c.2235_2249del15 | 6 | Y | Elongated, curly and
irregular | Curly, brittle and
fine | 1 |
| 2 | 85 | M | c.2543C>T
(p.P848L) | 20 | Y | Elongated, brittle
and curly | N | 3 |
| 3 | 68 | M | c.2573T>G
(p.L858R) | 45 | N | Curly and
brittle | Curly, grew in a
slower rate and less in volume | 3 |
| 4 | 75 | F | c.2307_2308ins9
(GCCAGCGTG) (p.V769_D770insASV) | 36 | N | N | Curly and rigid | 6 |
| 5 | 77 | M | c.2573T>G
(p.L858R) | 24 | Y | Curly and longer and
brittle | Curly and
brittle | 3 |
| 6 | 60 | M | c.2573T>G
(p.L858R) | 25 | N | Elongated, curly and
irregular | Less in volume,
straight | 6 |
Case report
Case 1
A 29-year-old female patient presented with a cough
and sputum in March 2013. She underwent chest computed tomography
(CT) scan which showed miliary nodules diffused at bilateral lobes
of lungs. Bronchoscopic biopsy (Olympus-BF-260; Olympus Corp.,
Tokyo, Japan).) was performed and a diagnosis of stage cT4N2M1b
lung adenocarcinoma primarily located at right upper lobe of lung,
metastasis in both lungs and mediastinal lymph nodes was made
following pathological (hematoxylin and eosin staining) and
immunohistochemical (thyroid transcription factor-1) examination.
First line treatment with 1,250 mg/m2 gemcitabine (days
1 and 8; Lilly France, Neuilly-sur-Seine, France) plus 75
mg/m2 cisplatin (day 1; Qilu Pharmaceutical Co., Ltd.,
Jinan, China) was administered for two cycles (21 days).
Simultaneously, EGFR mutation with a deletion at exon 19
(c.2235_2249del15) was detected in tumor tissue by direct Sanger
sequencing test. Image examination with CT and magnetic resonance
imaging (MRI) was performed after two cycles of chemotherapy. Chest
CT showed no significant change in the diffuse miliary nodes. Brain
MRI scanning showed a new focus at the right parietal lobe. The
tumor progression was diagnosed and the patient received erlotinib
(150 mg, daily; Roche, Welwyn Garden City, UK) treatment as the
second line therapy from May 11, 2013. After one month of erlotinib
treatment, the diffuse miliary nodes considerably reduced or
disappeared. The brain lesion had disappeared completely as well,
and the therapeutic evaluation was partial response (PR). The
patient's disease progressed again after six months of treatment.
Chemotherapy with 500 mg/m2 pemetrexed on day 1 (Lilly
France), and carboplatin with AUC of 5 mg/ml/min on day 1 (in
cycles of 21 days) was administered afterward as a third line
therapy. In December 2014, the patient remained alive and was
receiving follow-up. During the erlotinib treatment, she complained
of skin rashes on her face, chest and scalp, which were not
adequately controlled with topical therapy. In addition, she
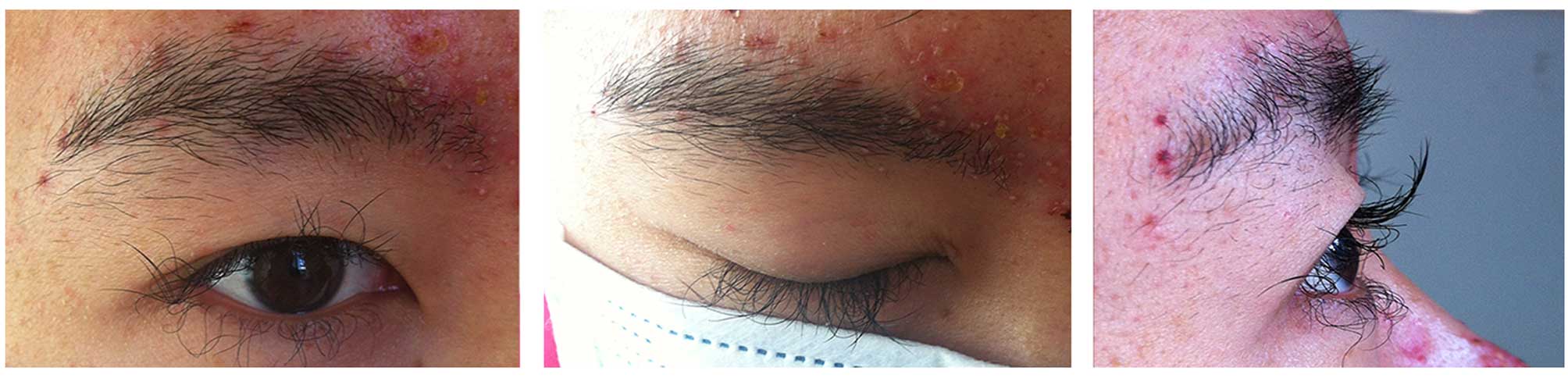
experienced excessively elongated, curly and irregular growth of
both eyelashes (Fig. 1) and a change
of hair texture from straight and thick to curly, brittle and fine
after one month of initiating erlotinib treatment. After one month
of withdrawal of erlotinib while the disease progressed, the rashes
on the patient's skin gradually disappeared and the hair and
eyelashes gradually became normal.
Case 2
An 85-year-old male patient with a diagnosis of
stage cT4N2M1b lung adenocarcinoma primarily located in the right
lower lobe of the lung, metastasis in bilateral lungs, mediastinal
lymph nodes, pleural membrane and bone had a EGFR mutation at exon
21 of EGFR [c.2543C>T (p.P848L)], detected in the tumor
tissue by direct Sanger sequencing test. PR was diagnosed after two
cycles of chemotherapy with single pemetrexed (500 mg/m2
on day 1, in cycles of 21 days). As maintenance therapy, the
patient received erlotinib (150 mg, daily) from April 21, 2011. All
lesions reduced or disappeared gradually. The patient's disease
slowly progressed after 20 months of treatment. He continued to
receive erlotinib although the disease progressed rapidly in June
2013. Chemotherapy (single pemetrexed) has been concomitantly
performed for two cycles as second line therapy. Therapeutic
evaluation was ‘stable disease’. The patient refused further
chemotherapy and succumbed to their illness in November 13, 2013.
During the single erlotinib treatment, the patient experienced
severe skin rashes and/or acne on his face, chest and extremities,
which improved with topical therapy (vitamin E emulsion). In
addition, his eyelashes became excessively elongated, brittle and
curly after 3 months initiation of erlotinib. However, while the
patient received erlotinib together with pemetrexed, the skin
reactions notably reduced and he temporarily stopped erlotinib
treatment during the second chemotherapy. In total, the patient was
administered erlotinib for 27 months. Although his disease
progressed, he continued to exhibit trichomegaly and skin
reactions. Following the withdrawal of erlotinib, his skin and
eyelashes gradually became normal.
Case 3
A 68-year-old male patient with a diagnosis of stage
cT1bN2M1b lung adenocarcinoma primarily located in the right upper
lobe of the lung, metastasis to mediastinal lymph nodes, pleural
membrane and bone had EGFR mutations at exon 21 of EGFR
[c.2573T>G (p.L858R)] detected by direct Sanger sequencing test.
As second line therapy, the patient received erlotinib (150 mg,
daily) from March 22, 2011. All lesions reduced or disappeared
gradually. His disease remained stable as of December 22, 2014 (45
months of erlotinib). During the treatment, he experienced slight
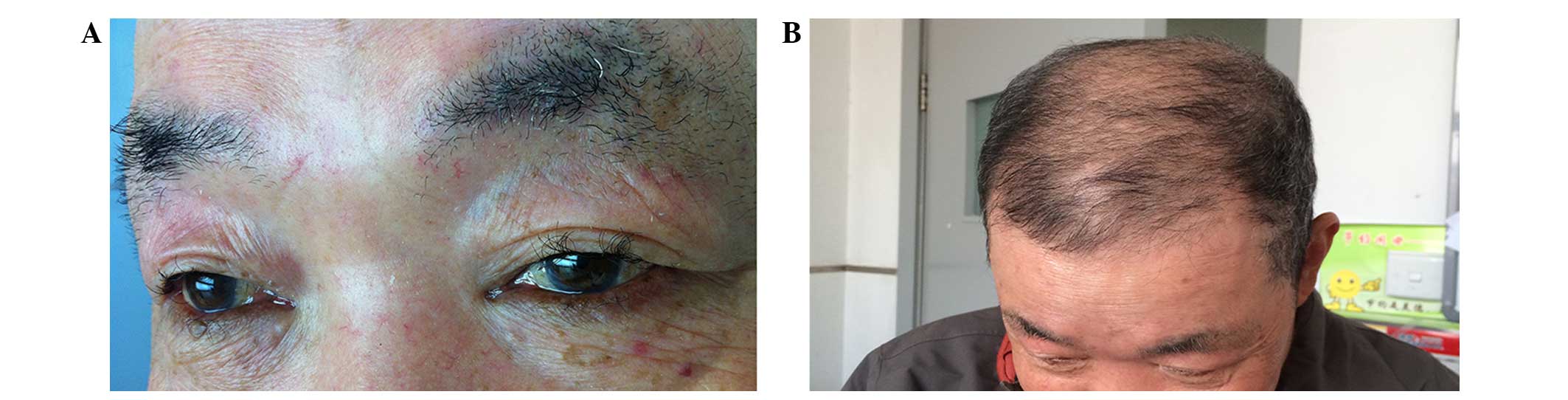
skin rashes on his face. His eyelashes and eyebrows became curly
and brittle after 3 months of treatment with erlotinib (Fig. 2). Additionally, his scalp hair became
curly, grew at a slower rate and reduced in volume (Fig. 2) due to hair loss. Since these side
effects did not influence the patient's life quality, no treatment
was performed.
Case 4
A 75-year-old female patient with a diagnosis of
stage cT1bN0M1a lung adenocarcinoma primarily located in the right
upper lobe of lung, metastasis in pleural membrane was administered
erlotinib (150 mg, daily), starting December 12, 2011 as third line
therapy. All lesions reduced or disappeared gradually. She
underwent follow-up examinations every two months. Her disease
remained stabled as of December 22, 2014 (36 months). Recently, the
EGFR mutations were checked by amplification refractory mutation
system test and the result showed that the patient has an insertion
mutation at exon 20 (c.2307_2308ins9 (GCC AGC GTG)
(p.V769_D770insASV). During the erlotinib treatment, the patient
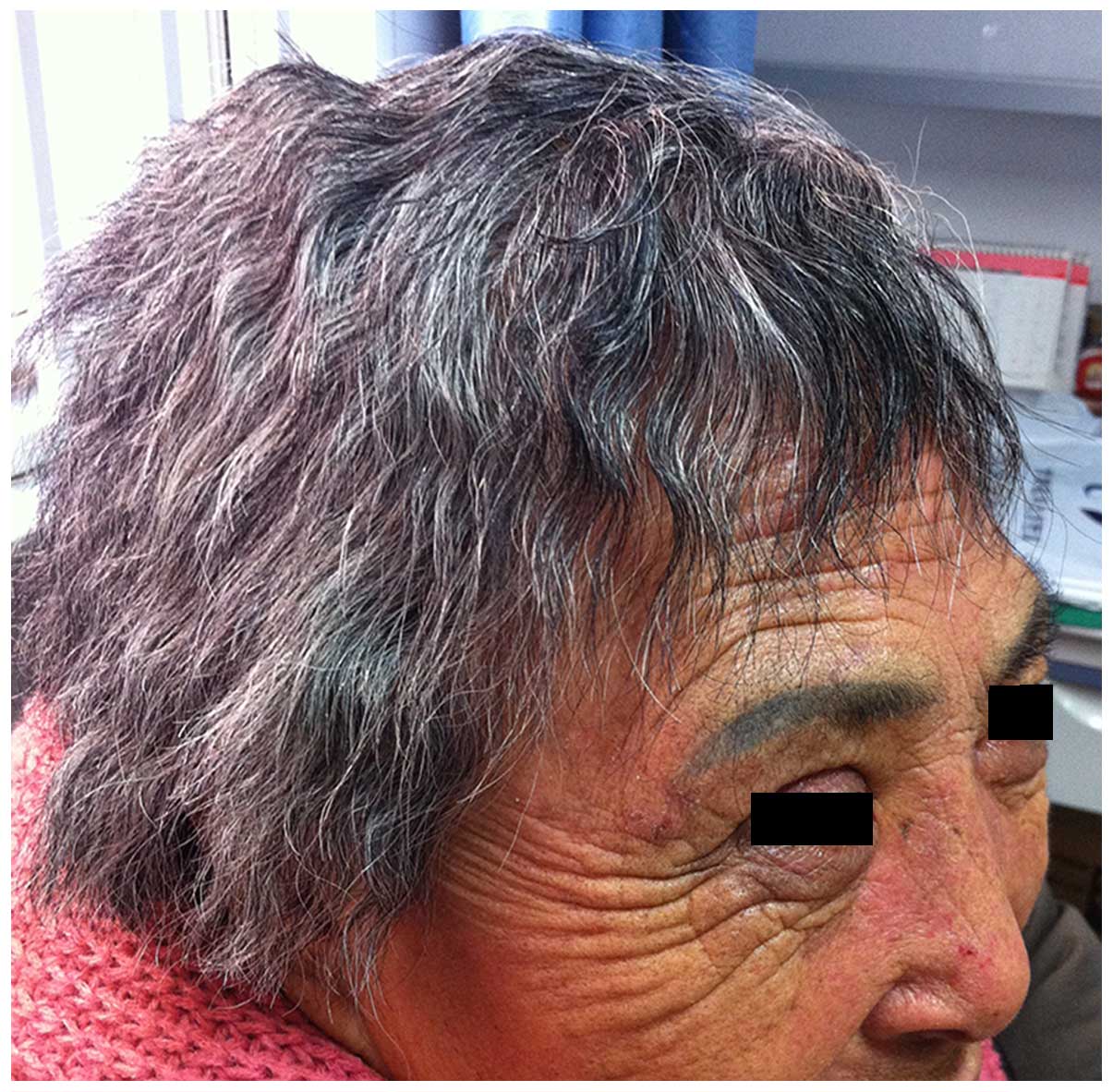
experienced slight skin rashes and diarrhea. There was no change at
eyelashes, but the patient's hair became curly and rigid after 6
months of erlotinib treatment (Fig.
3). No management was performed since the side effects did not
impact the patient's quality of life.
Case 5
A 77-year-old male patient had a confirmed diagnosis
of stage IIIa (pT3N1M0) lung adenocarcinoma primarily located in
the left upper lobe of the lung, metastasis in left hilar lymph
nodes was established through surgery. Adjuvant chemotherapy was
administered as a single dose of docetaxel (75 mg/m2, in
cycles of 21 days) for four cycles. The tumor had a missense
mutation at exon 21 of EGFR [c.2573T>G (p.L858R)] by
direct Sanger sequencing test. After his disease progressed,
erlotinib (150 mg, daily) was administered as first line therapy
from September 19, 2011. The patient's leg pain reduced
considerably and the node in the lung disappeared. His disease
progressed at September 2013. He refused further treatment and
succumbed to his illness on October 23, 2013. The PFS of erlotinib
is 24 months. During the erlotinib treatment, the patient
experienced slight skin rashes and diarrhea. His eyelashes became
curly, elongated and brittle, while his hair became curly and
brittle as well after receiving 3 months treatment with erlotinib.
No management was performed since these side effects did not affect
his quality of life.
Case 6
A 60-year-old male patient with a diagnosis of stage
cT4N2M1b lung adenocarcinoma primarily located in the left hilar
and metastasis to mediastinal lymph node and bone tissues had a
missense mutation at exon 21 of EGFR [c.2573T>G
(p.L858R)] by direct Sanger sequencing test. The patient received
maintenance therapy with erlotinib (150 mg, daily) from December 2,
2012 as second line therapy. His disease remained stable as of
December 22, 2014, and the PFS of erlotinib is 25 months. During
the erlotinib treatment, the patient complained of severe skin
rashes and acne on his face, neck, chest and scalp, which were not
controlled well with topical therapy. In addition, the patient
experienced slight diarrhea. The erlotinib administration was
reduced from daily to 5–6 times per week with unchanged per dose
(150 mg), and the side effects mentioned above improved. In
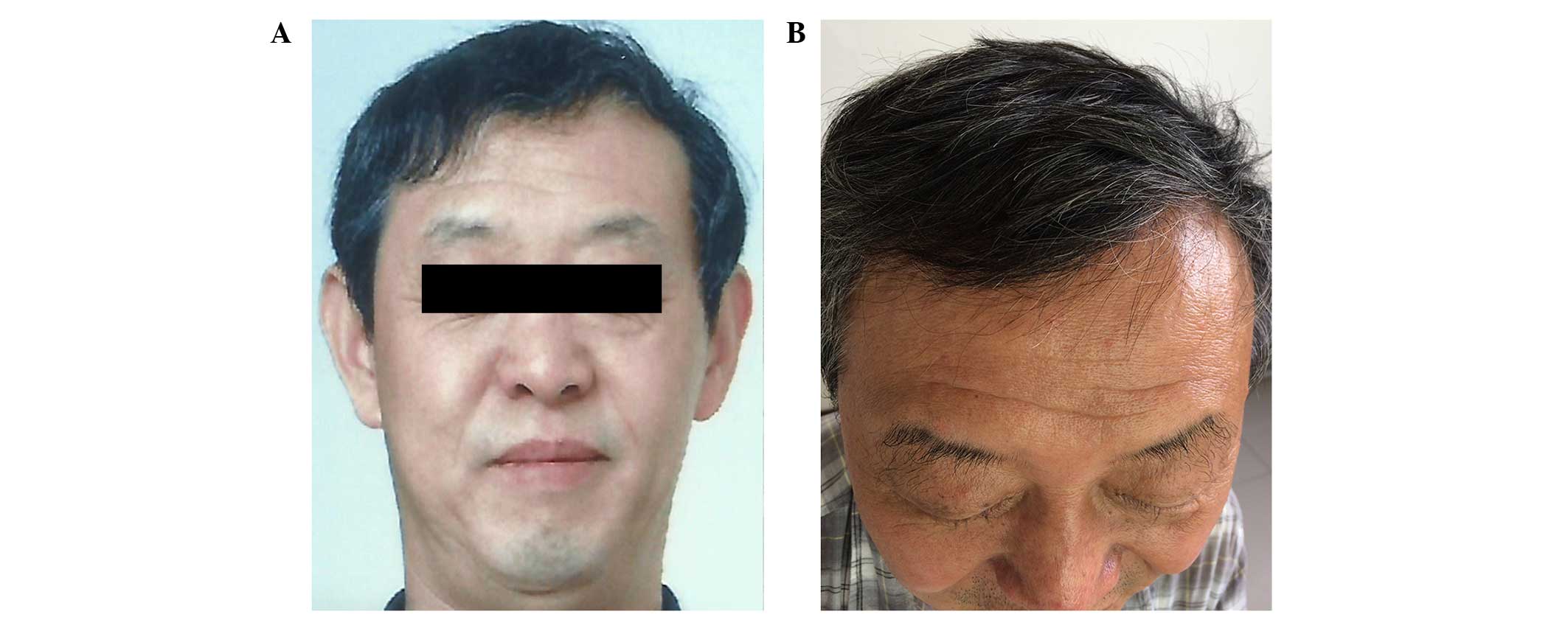
addition, the patient exhibited elongated, curly and irregular
growth of his eyelashes, and his scalp hair volume reduced at 6
months after initiating erlotinib treatment. Notably, the patients
hair became straight from curly after 6 months of treatment with
erlotinib (Fig. 4). No management
was performed since the side effects did not impact the patient's
quality of life.
Discussion
EGFR is a transmembrane glycoprotein that is found
on cells of epithelial origin and is expressed by solid tumors of
several organs, including the lungs (19). EGFR tyrosine kinase inhibitors
(EGFR-TKIs) have been developed and widely used in non-small-cell
lung cancer patients (4–8). Erlotinib is one of EGFR-TKIs and used
for the patients with activating mutations in the EGFR genes. The
common side effects of erlotinib include rash, diarrhea, fatigue
and loss of appetite (14). Herein,
we describe rare side effects, trichomegaly and alterations of
scalp hair in six pulmonary adenocarcinoma patients that received
erlotinib.
Trichomegaly was initially reported in congenital
diseases, such as Oliver-McFarlane syndrome (20). It was also described in acquired
hypertrichosis in patients infected with HIV type 1 (21) or secondary to certain drugs such as
zidovudine (22). In patients with
lung cancer, trichomegaly uncommonly occurs after treatment with
EGFR-TKIs, such as erlotinib (23–26) and
gefitinib (14,27) or the EGFR monoclonal antibody,
cetuximab (28). In the present
cases, their eyelashes became curly, brittle, irregular and
elongated after administration of erlotinib. The trichomegaly
occurred after 1–6 months of therapy and it was reversible after
cessation of the medication. EGFR mutations in the present cases
were as following: Case 1 featured deletion at exon 19; cases 2, 3,
5 and 6 harbored a missense mutation at exon 21; and case 4
possessed an insertion mutation at exon 20. Case 2 possessed a
p.L848R mutation but did not show similar scalp hair alterations as
those reported in cases 3, 5 and 6 with a p.L858R mutation. In
addition, case 4 did not present eyelash trichomegaly as reported
for other mutations in exons 19 and 21. In the majority of cases,
trichomegaly does not affect daily life and patients do not require
special treatment, with certain patients actually preferring their
appearance with trichomegaly. Trimming and epilation have been
found to be satisfactory, safe therapeutic options in cases whose
eyelashes grow excessively long.
Alteration of the scalp hair has been reported
(29,30) following the administration of EGFR
inhibitors. In the present cases, patients experienced hair loss
and a reduced growth rate, and commonly curly and brittle hair.
However, in case 4, the patient's hair became curly but rigid
(Fig. 3), which differed from the
previous three cases. However, she did not exhibit eyelash
trichomegaly, which was a different from the other five cases.
Notably, she has a rare mutation, which is an insertion mutation at
exon 20 (p.V769_D770insASV). The majority of exon 20 insertion
mutations reported to date are associated with resistance to TKIs
(31,32). Her disease remained stable as of
December 22, 2014, and the PFS is 36 months temporarily. The
mechanism underlying the differing manifestations of these
mutations is unclear. The hair became curly or straight, grew in
fast or slow speed, does not need special treatment. However, cases
of baldness which affects the appearance, the patient may need to
consult a doctor.
The side effects of EGFR-TKI were predominantly
cutaneous, including pruritis, xerosis, paronychia, skin fissures,
telangiectasias and most commonly, acne form rash. These symptoms
may be explained by the presence of EGFR in a variety of normal
tissues in addition to lung tissue, including the basal layer of
the epidermis and hair follicles and in sebaceous glands and
capillaries. In contrast to other drug-associated skin changes,
eyelash trichomegaly and alteration of scalp hair are not
frequently observed; in one study of 30 patients receiving EGFR
inhibitors, only 17% (five individuals) noted an overgrowth of
their eyelashes (33). These changes
typically occur at 2–5 months after the start of treatment and may
resolve in several weeks or months after discontinuation of the
treatment.
However, different EGFR-TKIs produced side effects
of varying severity. The discontinuation of erlotinib and gefitinib
due to of toxic side effects occurs in 5 and 2% of patients,
respectively (14,34). Icotinib showed reduced toxicity
compared with gefitinib (P=0.05), with 0% of discontinuation
(35). In previous clinical
observations, no patients exhibited trichomegaly and alteration of
scalp hair with icotinib treatment (35,36).
In conclusion, trichomegaly and alterations of scalp
hair are manifestations of erlotinib, which are uncommon, and
generally do not affect patient quality of life. A relatively
limited proportion of patients experience symptoms which influence
daily life and require treatment. The detail mechanisms underlying
these symptoms remain unknown and require further
investigation.
Acknowledgements
The authors would like to thank all participating
physicians and registered patients.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramalingam S and Belani C: Systemic
chemotherapy for advanced non-small cell lung cancer: Recent
advances and future directions. Oncologist. 13(Suppl 1): 5–13.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blackhall FH, Shepherd FA and Albain KS:
Improving survival and reducing toxicity with chemotherapy in
advanced non-small cell lung cancer: A realistic goal? Treat Respir
Med. 4:71–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han JY, Park K, Kim SW, Lee DH, Kim HY,
Kim HT, Ahn MJ, Yun T, Ahn JS, Suh C, et al: First-SIGNAL:
First-line single-agent iressa versus gemcitabine and cisplatin
trial in never-smokers with adenocarcinoma of the lung. J Clin
Oncol. 30:1122–1128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong
Z, Lu S, Cheng Y, Han B, Chen L, et al: First-line erlotinib versus
gemcitabine/cisplatin in patients with advanced EGFR
mutation-positive non-small-cell lung cancer: Analyses from the
phase III, randomized, open-label, ENSURE study. Ann Oncol.
26:1883–1889. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang JC, Wu YL, Schuler M, Sebastian M,
Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al:
Afatinib versus cisplatin-based chemotherapy for EGFR
mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6):
Analysis of overall survival data from two randomised, phase 3
trials. Lancet Oncol. 16:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Final overall
survival results from a randomised, phase III study of erlotinib
versus chemotherapy as first-line treatment of EGFR
mutation-positive advanced non-small-cell lung cancer (OPTIMAL,
CTONG-0802). Ann Oncol. 26:1877–1883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
PROFILE 1014 Investigators: First-line crizotinib versus
chemotherapy in ALK-positive lung cancer. N Engl J Med.
371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshioka H, Mitsudomi T, Morita S, Yatabe
Y, Negoro S, Okamoto I, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Final overall survival results of WJTOG 3405, a randomized
phase 3 trial comparing gefitinib (G) with cisplatin plus docetaxel
(CD) as the first-line treatment for patients with non-small cell
lung cancer (NSCLC) harboring mutations of the epidermal growth
factor receptor (EGFR). 2014 ASCO Annual Meeting Abstract Number:
8117. J Clin Oncol. 32(Suppl; abstr 8117): 5s2014.
|
|
14
|
Shah NT, Kris MG, Pao W, Tyson LB, Pizzo
BM, Heinemann MH, Ben-Porat L, Sachs DL, Heelan RT and Miller VA:
Practical management of patients with non-small-cell lung cancer
treated with gefitinib. J Clin Oncol. 23:165–174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alexandrescu DT, Kauffman CL and Dasanu
CA: The cutaneous epidermal growth factor network: Can it be
translated clinically to stimulate hair growth? Dermatol Online J.
15:12009.
|
|
16
|
Pinquié F, de Chabot G, Urban T and
Hureaux J: Maintenance Treatment by Erlotinib and Toxic
Cardiomyopathy: A Case Report. Oncology. 90:176–177. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Macerelli M, Mazzer M, Foltran L,
Cardellino GG and Aprile G: Erlotinib-associated interstitial lung
disease in advanced pancreatic carcinoma: A case report and
literature review. Tumori. 101:e122–e127. 2015.PubMed/NCBI
|
|
18
|
Celik T and Kosker M: Ocular side effects
and trichomegaly of eyelashes induced by erlotinib: A case report
and review of the literature. Cont Lens Anterior Eye. 38:59–60.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hirsch FR, Varella-Garcia M and Cappuzzo
F: Predictive value of EGFR and HER2 overexpression in advanced
non-small-cell lung cancer. Oncogene. 28(Suppl 1): S32–S37. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oliver GL and McFarlane DC: Congenital
trichomegaly: With associated pigmentary degeneration of the
retina, dwarfism, and mental retardation. Arch Ophthalmol.
74:169–171. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaplan MH, Sadick NS and Talmor M:
Acquired trichomegaly of the eyelashes: A cutaneous marker of
acquired immunodeficiency syndrome. J Am Acad Dermatol. 25:801–804.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klutman NE and Hinthorn DR: Excessive
growth of eyelashes in a patient with AIDS being treated with
zidovudine. N Engl J Med. 324:18961991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carser JE and Summers YJ: Trichomegaly of
the eyelashes after treatment with erlotinib in non-small cell lung
cancer. J Thorac Oncol. 1:1040–1041. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lane K and Goldstein SM:
Erlotinib-associated trichomegaly. Ophthal Plast Reconstr Surg.
23:65–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Braiteh F, Kurzrock R and Johnson FM:
Trichomegaly of the eyelashes after lung cancer treatment with the
epidermal growth factor receptor inhibitor erlotinib. J Clin Oncol.
26:3460–3462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang SB, Lei KJ, Liu JP and Jia YM:
Eyelash trichomegaly following treatment with erlotinib in a
non-small cell lung cancer patient: A case report and literature
review. Oncol Lett. 10:954–956. 2015.PubMed/NCBI
|
|
27
|
Pascual JC, Bañuls J, Belinchon I, Blanes
M and Massuti B: Trichomegaly following treatment with gefitinib
(ZD1839). Br J Dermatol. 151:1111–1112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bouché O, Brixi-Benmansour H, Bertin A,
Perceau G and Lagarde S: Trichomegaly of the eyelashes following
treatment with cetuximab. Ann Oncol. 16:1711–1712. 2005. View Article : Google Scholar
|
|
29
|
Price TJ and Nott L: Unusual hair changes
with prolonged erlotinib exposure. Intern Med J. 38:8072008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Becker A, van Wijk A, Smit EF and Postmus
PE: Side-effects of long-term administration of erlotinib in
patients with non-small cell lung cancer. J Thorac Oncol.
5:1477–1480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Naidoo J, Sima CS, Rodriguez K, Busby N,
Nafa K, Ladanyi M, Riely GJ, Kris MG, Arcila ME and Yu HA:
Epidermal growth factor receptor exon 20 insertions in advanced
lung adenocarcinomas: Clinical outcomes and response to erlotinib.
Cancer. 121:3212–3220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang M, Xu X, Cai J, Ning J, Wery JP and
Li QX: NSCLC harboring EGFR exon-20 insertions after the regulatory
C-helix of kinase domain responds poorly to known EGFR inhibitors.
Int J Cancer. 139:171–176, Epub ahead of print. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roé E, García Muret MP, Marcuello E,
Capdevila J, Pallarés C and Alomar A: Description and management of
cutaneous side effects during cetuximab or erlotinib treatments: A
prospective study of 30 patients. J Am Acad Dermatol. 55:429–437.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shepherd FA, Pereira Rodrigues J, Ciuleanu
T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S,
Smylie M, Martins R, et al: National Cancer Institute of Canada
Clinical Trials Group: Erlotinib in previously treated
non-small-cell lung cancer. N Engl J Med. 353:123–132. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi Y, Zhang L, Liu X, Zhou C, Zhang L,
Zhang S, Wang D, Li Q, Qin S, et al: Icotinib versus gefitinib in
previously treated advanced non-small-cell lung cancer (ICOGEN): A
randomised, double-blind phase 3 non-inferiority trial. Lancet
Oncol. 14:953–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng H, Wang Q, Shi H, Zhang H, Hu F and
Li B: Favorable response to icotinib in a lung cancer patient with
a special mutation at exon 19 of epidermal growth factor receptor.
Thorac Cancer. 5:358–361. 2014. View Article : Google Scholar : PubMed/NCBI
|