Introduction
Primary malignant melanoma of the esophagus (PMME)
has only ~300 cases reported in the literature (1,2). It is a
rare malignant disease, accounting for 0.1–0.2% of esophageal
malignancies. Definitive diagnoses depends on pathology and
immunohistochemical examination with positive results for S-100,
human melanoma black (HMB)-45 and melanoma-specific antigen
(Melan-A) proteins. PMME has a 5-year survival rate of 2.2–4.2% and
the present standard treatment is surgical resection (3). In recent years, few cases of PMME
patients undergoing endoscopic mucosal resection (EMR) have been
reported. At present, endoscopic submucosal dissection (ESD) is
used to treat tumors originating from the mucosal or submucosal
layer due to the possibility of en bloc resection of lesions
regardless of their size and shape (4). Theoretically, due to the similarity of
the site of origin of PMME compared with other operable tumors, ESD
may be a viable option for its treatment. However, to the best of
our knowledge, no case of PMME treated with ESD has been reported,
and the follow-up requirements and complications of PMME subsequent
to endoscopic treatment remain unexplored.
The present study reports the case of a 71-year-old
PMME patient who was treated by ESD at the Third Affiliated
Hospital of Soochow University (Changzhou, China) in 2011. In
addition, a follow-up period of >3 years was conducted.
Case report
A 71-year-old female patient was admitted to the
Third Affiliated Hospital of Soochow University with complaints of
progressive dysphagia in December 2011. The patient had been
diagnosed with breast carcinoma and had undergone radical surgery
>2 years earlier. Physical examination was normal and revealed
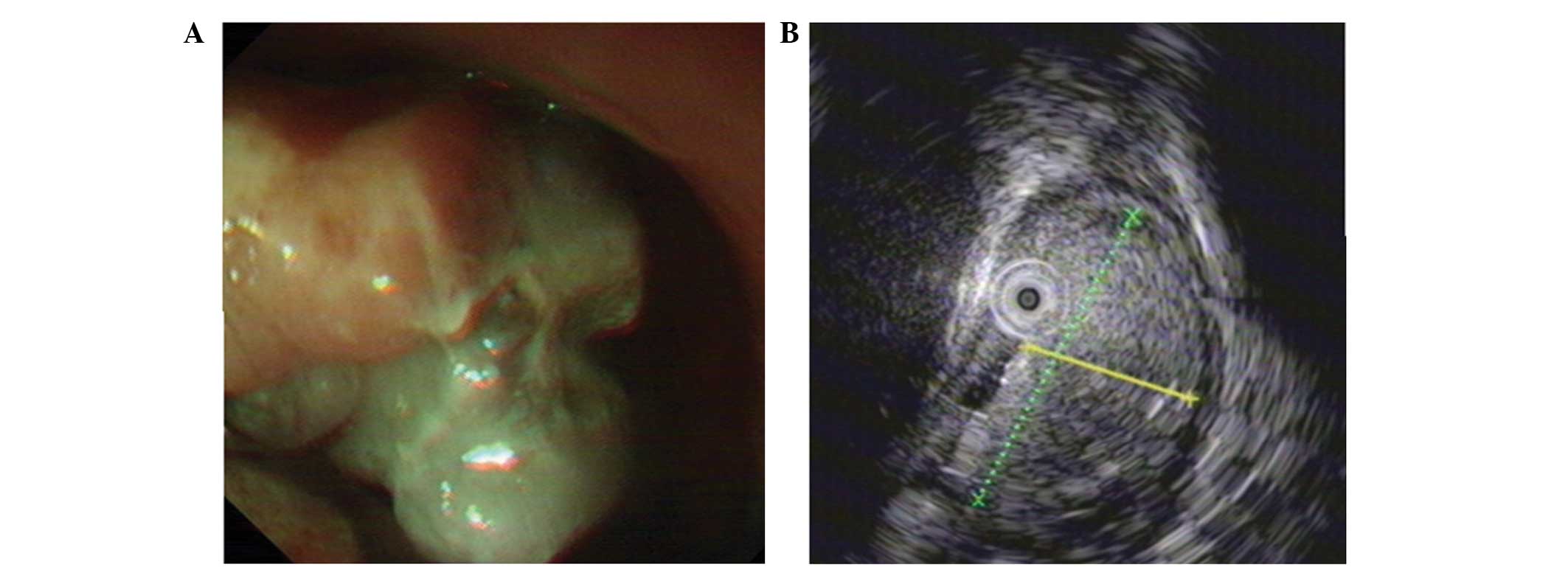
no skin lesions. Esophagogastroscopy indicated an irregularly
shaped pigmented polypoid mass located between 29 and 32 cm from
the incisors (Fig. 1A). Biopsy was
performed and the mass was found to be a primary malignant
melanoma. In addition, endoscopic ultrasound (EUS) was performed
and indicated a hyperechoic mass originating from the mucosal
layer, which was 0.8×2.8 cm in size with a pedicle diameter of ~0.8
cm (Fig. 1B). The mass originated
from the mucosal layer and displayed a tubiform structure in the
center with no echo, while the submucosal layer and muscularis
propria were intact. Chest and abdominal computerized tomography
(CT) indicated no metastasis to the lymph nodes, mediastinum or the
lungs. Further examinations also ruled out distant metastasis.
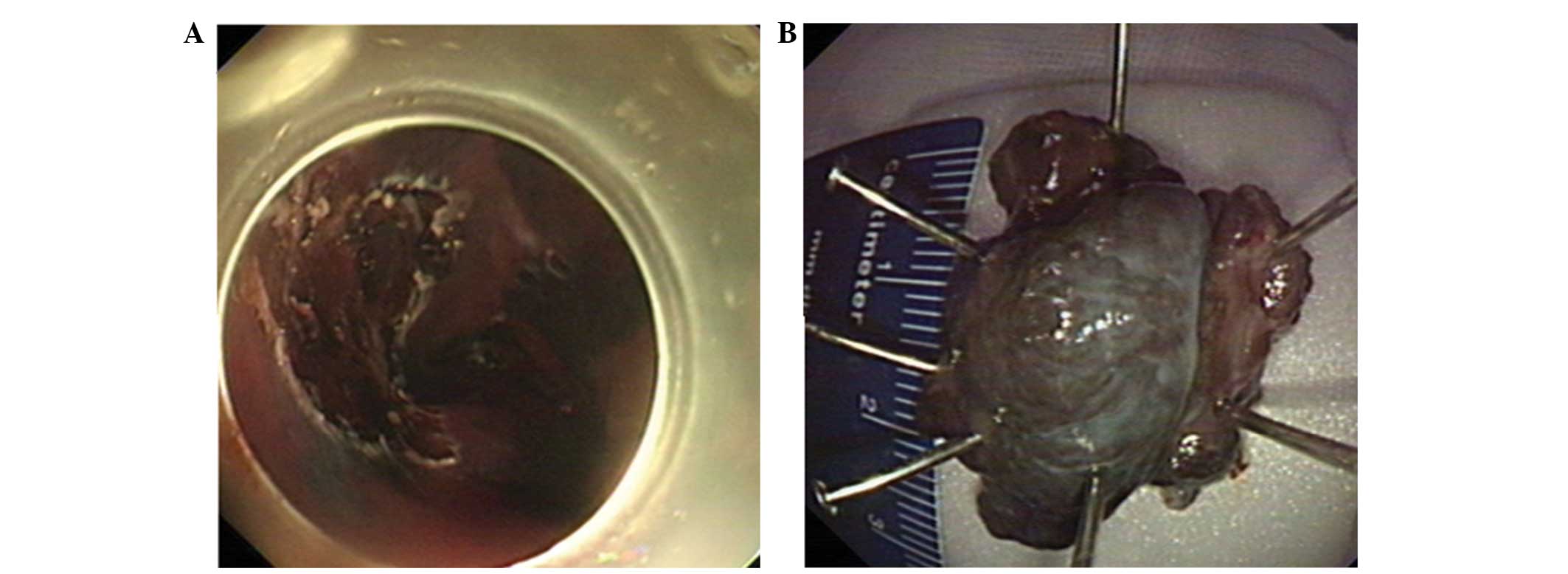
Additionally, the patient declined surgery for the PMME, and was
thus treated with ESD (Fig. 2),
subsequent to providing informed consent.
Prior to the procedure, the patient received
conscious sedation [using midazolam (Jiuxu Pharmaceutical Co.,
Ltd., Zhejiang, China) or propofol (Fresenius Kabi Deutschland
GmbH, Bad Homburg, Germany)] in the left lateral position.
Following the submucosal injection, the overlying mucosa was
dissected circumferentially. Then, the mass was resected. The
primary techniques included the use of a standard therapeutic
endoscope (GIF-Q260; Olympus Corporation, Tokyo, Japan),
insulated-tip knife (KD-640L; Olympus Corporation) and a hook-knife
(KD-620UR; Olympus Corporation). Subsequent to fasting for 3 days
after surgery, the patient was allowed to eat. The post-operative
white blood cell count was normal and the patient did not complain
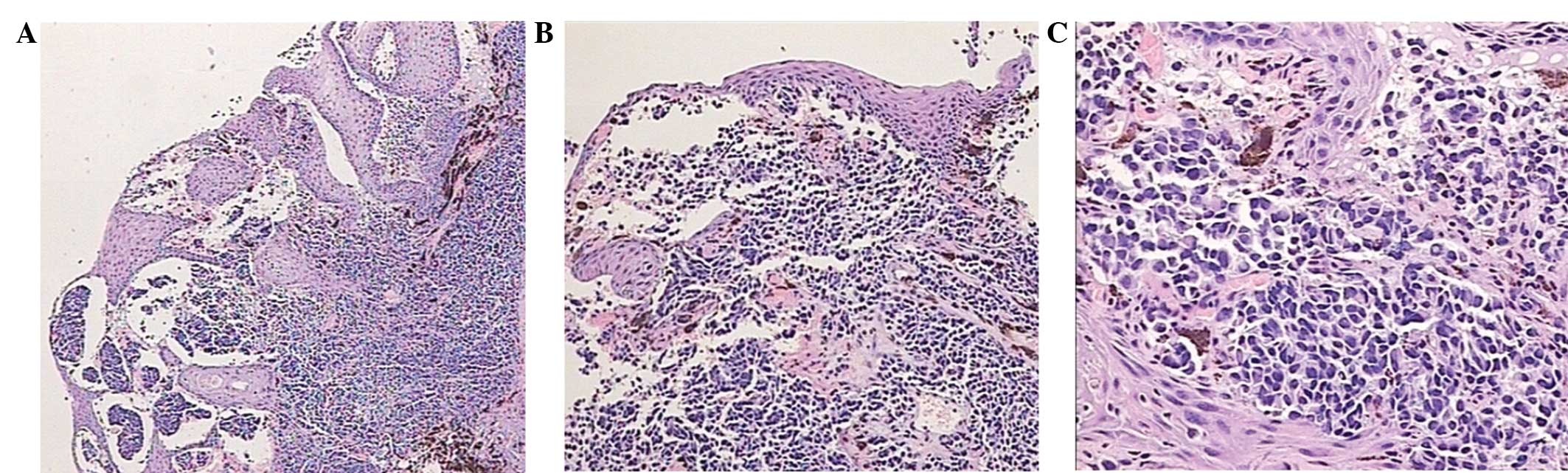
of discomfort. Pigmented cellular tumor was stained with
hematoxylin and eosin, and positive HMB-45 (A-AH10203; Abgent, San
Diego, CA, USA) in immunohistochemical examination confirmed the
diagnosis of a PMME (Fig. 3).
Following ESD, the patient received three cycles of immunotherapy.
Ipilimumab was used as the immunotherapy for 3 cycles, which is a
monoclonal antibody that blocks the checkpoint of cytotoxic
T-lymphocyte-associated protein 4 (180 mg per 3 weeks). Follow-up
esophagogastroscopy examinations and CT scans showed no signs of
disease recurrence for >3 years. The latest clinical follow-up
was in January 2016, and the esophagogastroscopy examinations and
CT scans showed no signs of disease recurrence. The patient has had
a normal life for >4.5 years.
Discussion
PMME is an aggressive and fatal disease associated
with poor prognosis, and the incidence of PMME accounts for
0.1–0.2% of esophageal malignancies (5). It typically occurs within the lower
two-thirds of the esophagus due to the presence of a high
concentration of melanin pigment. The etiology and natural course
of the disease remains unknown; however, several studies have
hypothesized that esophageal melanocytosis may be a precursor to
PMME (6,7). This melanoma tends to metastasize
readily via the hematogenous and lymphogenous routes. The most
common sites of metastases are the liver, mediastinum, mediastinal
lymph nodes, the lungs and the brain (8). The symptoms of PMME are not unique
compared with other types of esophageal malignancies. The most
common complaint include dysphagia and retrosternal pain. HMB-45,
AE1/AE3 and S-100 cytoplasmic proteins are the monoclonal
antibodies most frequently used to identify PMME (2,3).
Recently, EUS has been employed to identify the
depth of invasion and the node status in patients diagnosed with
PMME (9). On EUS, PMME appears as a
hypoechoic mass or with mixed echogenicity compared with the
surrounding healthy tissue (9). It
has previously been reported that endoscopic ultrasound guided
fine-needle aspiration may be used to diagnose PMME (10). The proposed criteria for the
diagnosis of PMME are the following: i) The mass possesses the
characteristic structure of a melanoma, containing melanin; ii) the
adjacent epithelium contains melanocytes; iii) the mass arises from
the area of junctional changes in the squamous epithelium; and iv)
metastasis of malignant melanoma from other parts of the body is
excluded (11).
There is currently no gold standard for the
treatment of PMME. Furthermore, it has been reported that 30–40% of
patients with PMME present nodal or distant metastases at
diagnosis, and ~85% will succumb as a result of distant metastasis
regardless of the treatment (2). It
is agreed that treatment should be adjusted individually according
to the tumor size, location, presence of metastases, patient age
and comorbidities observed. However, surgical resection should be
considered as the first line treatment for all patients with PMME,
since radical surgical extirpation increases the chances of
long-term survival (12).
For patients that decline or are unable to undergo
radical surgery, palliative treatment is an alternative method and
includes radiotherapy, chemotherapy and immunotherapy. However, the
aforementioned therapies are not successful in the treatment of
PMME, and the mean survival time has been reported to be 13.4
months (10). In recent years, novel
therapeutic agents have been approved for the treatment of
melanoma; however, few randomized clinical trial studies have
supported their efficacy (13).
Thus, the resection of the tumor remains the only viable treatment
with the potential to cure patients with PMME.
In the present study, the patient had a history of
breast carcinoma and had previously undergone surgery 2 years prior
to presenting with dysphagia. Subsequent to excluding breast
metastasis, the tumor was diagnosed as PMME. As the patient
declined radical surgery, ESD was performed, followed by three
cycles of immunotherapy. Follow-up examinations with endoscopy and
CT revealed that ESD had successfully removed the tumor the entire
tumor.
To the best of our knowledge, only a limited number
of reports have described the use of endoscopic treatment for PMME
(14–16). Kastl et al previously
described the implantation of a metal stent to alleviate PMME
symptoms, which improved the quality of life in the patient
(17). Local endoscopic laser
ablation may also serve a role in the palliation of locally
advanced tumors that are unresectable (18,19).
Furthermore, Miyatani et al reported a case involving a slow
growing flat-type PMME that was successfully treated by
cap-assisted EMR, with no evidence of recurrence during the
20-month follow-up (15). Compared
with EMR, the ESD technique enables the en bloc resection of
lesions regardless of their size and shape. In 2013, Eleftheriadis
et al reported a unique case of PMME undergoing successful
treatment with ESD and described an uneventful 8-month follow-up
period (20).
In the case presented in the current study, the
neoplasm originated from the mucosal layer. The patient refused
radical surgery, and thus ESD was performed given the large size of
the mass. The treatment successfully removed the PMME mass.
Notably, this is the first study to describe a case of PMME
undergoing treatment with ESD followed by a long-term follow-up
period.
In conclusion, to the best of our knowledge, this
study is the first to describe the long-term efficacy of ESD for
the treatment of PMME. The result of the present study revealed
that ESD may be an alternative option to EMR for the treatment PMME
in patients that do not present distant metastasis.
References
|
1
|
Garfinkle JM and Cahan WG: Primary
melanocarcinoma of the esophagus. First histologically proved case.
Cancer. 5:921–926. 1952. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang W, Zou Z and Liu B: Primary
malignant melanoma of the esophagus: A case report and review of
the literature. Oncol Lett. 9:2036–2040. 2015.PubMed/NCBI
|
|
3
|
Hamdy FC, Smith JH, Kennedy A and Thorpe
JA: Long survival after excision of a primary malignant melanoma of
the oesophagus. Thorax. 46:397–398. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y, Wang X, Xiong G, Qian Y, Wang H,
Liu L, Miao L and Fan Z: Complete defect closure of gastric
submucosal tumors with purse-string sutures. Surg Endosc.
28:1844–1851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanamori N and Igaki H: A case of primary
malignant melanoma of the esophagus. Jpn J Clin Oncol. 38:2332008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eigentler TK, Caroli UM, Radny P and Garbe
C: Palliative therapy of disseminated malignant melanoma: a
systematic review of 41 randomised clinicaltrials. LancetOnco.
14:748–759. 2003.
|
|
7
|
Wallis G, Sehgal V, Haider A, Bridgewater
J, Novelli M, Dawas K and Haidry R: Primary malignant melanoma of
the esophagus. Endoscopy. 47:E81–E82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sabanathan S, Eng J and Pradhan GN:
Primary malignant melanoma of the esophagus. Am J Gastroenterol.
84:1475–1481. 1989.PubMed/NCBI
|
|
9
|
Yu H, Huang XY, Li Y, Xie X, Zhou JL,
Zhang LJ, Fu JH and Wang X: Primary malignant melanoma of the
esophagus: A study of clinical features, pathology, management and
prognosis. Dis Esophagus. 24:109–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dennis KL, Thomas PA, Fan F and Olyaee M:
Primary malignant melanoma of the esophagus: A rare entity
diagnosed by endoscopic ultrasound guided fine-needle aspiration.
Diagn Cytopathol. 40:462–465. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levy MJ, Clain JE, Clayton A, Halling KC,
Kipp BR, Rajan E, Roberts LR, Root RM, Sebo TJ, Topazian MD, et al:
Preliminary experience comparing routine cytology results with the
composite results of digital image analysis and fluorescence in
situ hybridization in patients undergoing EUS-guided FNA.
Gastrointest Endosc. 66:483–490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Volpin E, Sauvanet A, Couvelard A and
Belghiti J: Primary malignant melanoma of the esophagus: A case
report and review of literature. Dis Esophagus. 15:244–249. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chapman PB, Hauschild A, Robert C, Haanen
JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et
al: BRIM-3 Study Group: Improved survival with vemurafenib in
melanoma with BRAF V600E mutation. N Engl J Med. 364:2507–216.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herman J, Duda M, Lovecek M and Svach I:
Primary malignant melanoma of the esophagus treated by endoscopic
ablation and interferon therapy. Dis Esophagus. 14:239–240. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyatani H, Yoshida Y, Ushimaru S,
Sagihara N and Yamada S: Slow growing flat-type primary malignant
melanoma of the esophagus treated with cap-assisted EMR. Dig
Endosc. 21:255–257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tipirneni E, Gunaratnam NT, Tworek JA and
Kodali S: Primary malignant melanoma of esophagus treated with
endoscopic mucosal resection and esophagectomy. J Gastrointest
Cancer. 42:266–268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kastl S, Wutke R, Czeczatka P, Hohenberger
W and Horbach T: Palliation of a primary malignant melanoma of the
distal esophagus by stent implantation. Report of a case. Surg
Endosc. 15:1042–1043. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lohmann CM, Hwu WJ, Iversen K, Jungbluth
AA and Busam KJ: Primary malignant melanoma of the oesophagus: A
clinical and pathological study with emphasis on the
immunophenotype of the tumours for melanocyte differentiation
markers and cancer/testis antigens. Melanoma Res. 13:595–601. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wayman J, Irving M, Russell N, Nicoll J
and Raimes SA: Intraluminal radiotherapy and Nd:YAG laser
photoablation for primary malignant melanoma of the esophagus.
Gastrointest Endosc. 59:927–929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eleftheriadis N, Inoue H, Ikeda H, Onimaru
M, Yoshida A, Hosoya T, Maselli R, Hamatani S and Kudo SE:
Endoscopic submucosal dissection for primary malignant esophageal
melanoma (with video). Gastrointest Endosc. 78:359–360. 2013.
View Article : Google Scholar : PubMed/NCBI
|