Introduction
Caesarean scar pregnancy (CSP) is an uncommon but
serious complication of a previous caesarean. The first case of CSP
was reported in the medical literature in 1978 by Larsen and
Solomon (1) and the reported
incidence of CSP was approximately 0.15% of ectopic pregnancies
(2). Since 2000, the incidence of
CSP has increased significantly to 6.1% of all ectopic pregnancies
in those with previous caesarean sections, which may reflect the
increasing number of caesarean sections being performed and the
improved accuracy of diagnosis of ectopic pregnancies using colour
Doppler transvaginal ultrasonography (3). In addition, the rates of CSP may have
increased as a result of the use of assisted reproduction
technology (4). Typically the
diagnosis was made at between 6 and 9 weeks gestation.
CSP was confirmed by a thorough medical history
evaluation, including obstetric, reproductive and surgical history,
a physical examination and increased levels of progesterone and
total serum human chorionic gonadotropin (HCG) between 6 and 9
weeks of gestation. The diagnosis of CSP was mainly accomplished by
combining transvaginal sonography (TVS) with Doppler flow imaging
(5).
There is no standard treatment for the management of
CSP and only a few cases have been reported in the literature.
Although CSP is rare, without a high index of suspicion and early
diagnosis, it may result in serious maternal morbidity, including
uterine rupture and haemorrhage, or mortality (6). Termination of pregnancy in the first
trimester is strongly recommended to prevent life-threatening
complications and to maintain the possibility of future pregnancies
(7). In the past, the preferred
treatment option was to perform a laparotomy with the possible need
for hysterectomy to avoid potential lethal haemorrhage (8). The current methods for the management
of CSP include systemic chemotherapy with methotrexate (MTX), local
injection of embryocidic agents, uterine curettage, hysteroscopic
evacuation, laparoscopic management, excision of the involved lower
segment of the uterus, uterine artery embolization (UAE) and
expectant management (9,10). In the present study, we reported the
outcome of 30 cases of CSP managed by using uterine curettage as a
primary approach or combined with prophylactic UAE and MTX prior to
uterine curettage. In the 25 cases managed with prophylactic UAE
and MTX, we compared CSP removal using ultrasound-guided uterine
curettage and laparoscopy-guided curettage.
Patients and methods
Patients
Thirty patients with CSP were treated with uterine
curettage with or without prophylactic UAE and MTX. These cases
were identified from the Beijing Obstetrics and Gynaecology
Hospital database and were analysed retrospectively. The
characteristics of the 30 patients are shown in Table I. Clinical data were analyzed with
respect to age, gravidity and parity, history of caesarean
delivery, interval from the most recent caesarean delivery to
diagnosis, clinical presentation, results of laboratory
examination, process of diagnosis and treatment sequence, and
outcome, which were collected from the original hospital charts,
operation notes and outpatient medical records via telephone
questionnaires.
 | Table I.Characteristics of the clinical
cases. |
Table I.
Characteristics of the clinical
cases.
| Values | Age (years) | No. of previous
caesarean sections | No. of
pregnancies | Interval time from
recent caesarean section (years) | Gestation
(weeks) |
|---|
| Mean | 32.20±4.83 | 1.20±0.61 | 3.60±1.55 | 4.45±1.34 | 8.34±3.70 |
| Range (min -
max) | 23–43 | 1–4 | 2–7 | 6 months-12
years | 5–12 |
At Beijing Obstetrics and Gynaecology Hospital,
transabdominal ultrasound with full bladder was performed initially
to assess the pelvis and uterus with careful inspection of the
interface between the anterior lower uterine segment and bladder.
This was followed by a transvaginal ultrasound to allow for the
fine-detail evaluation of the gestational sac in relation to the
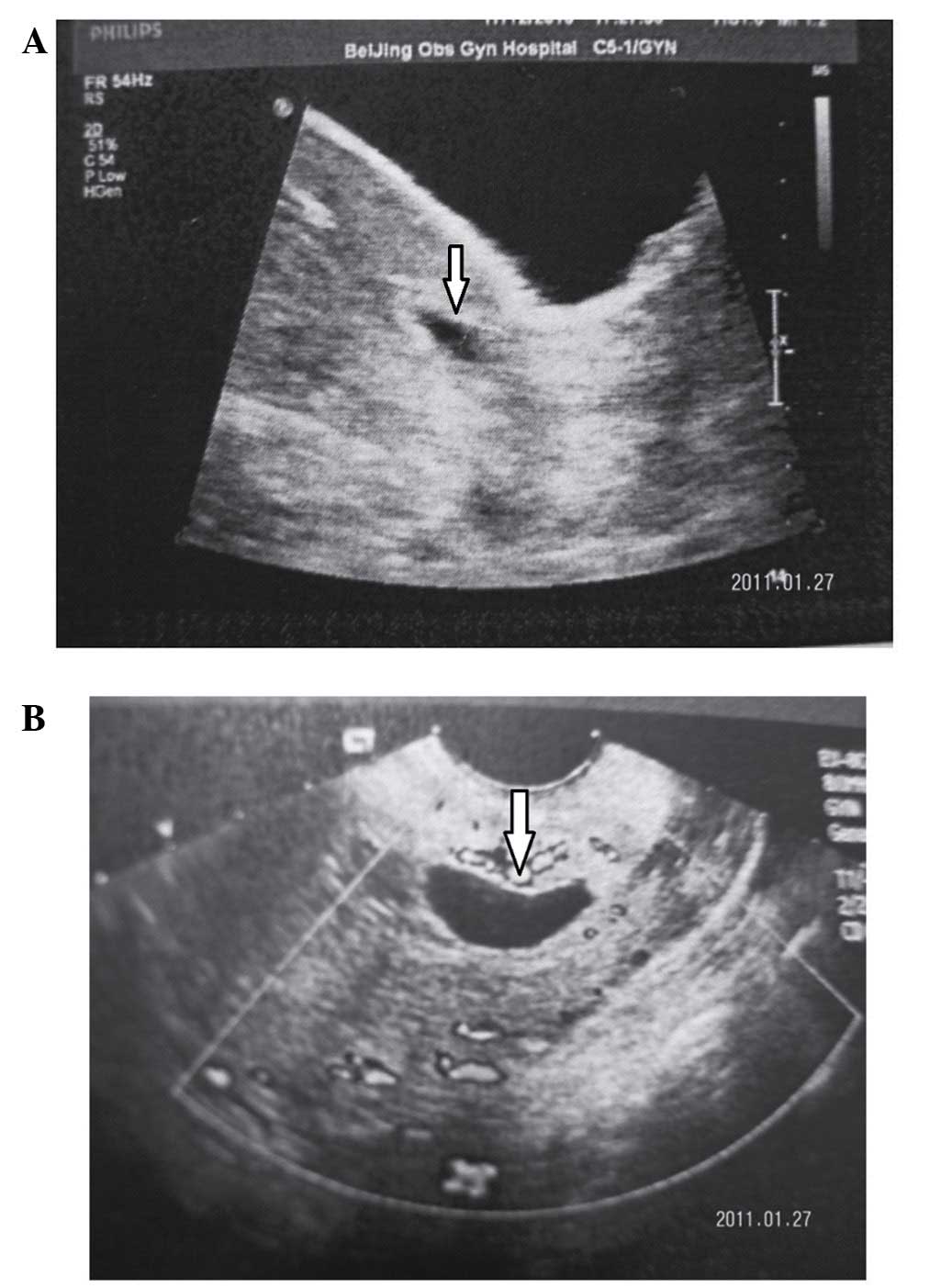
scar. The diagnosis of CSP in the first trimester was determined
based on the following ultrasonographic criteria: i) an empty
uterus with a clearly visualized endometrium; ii) an empty cervical
canal; iii) an anteriorly located gestational sac with a decreased
myometrium layer between the bladder and the sac; and iv) a reduced
or absent myometrium between the gestational sac and bladder on a
sagittal view of the uterus (this was observed to be <5 mm in
two-thirds of cases) (11) (Fig. 1).
Written informed consent was obtained prior to UAE
from each participant with CSP. Approval for the study was obtained
from the ethics committee of the Beijing Obstetrics and Gynaecology
Hospital.
Procedure
Twenty-five patients with a clear diagnosis of CSP
were offered prophylactic UAE prior to uterine curettage. A right
transfemoral approach was used for artery access, and each uterine
artery was selectively catheterized with a 4- or 5-F Roberts
uterine catheter. Prior to UAE, 50 mg of MTX was dissolved in 20 ml
of physiologic saline solution. This dose was separated between the
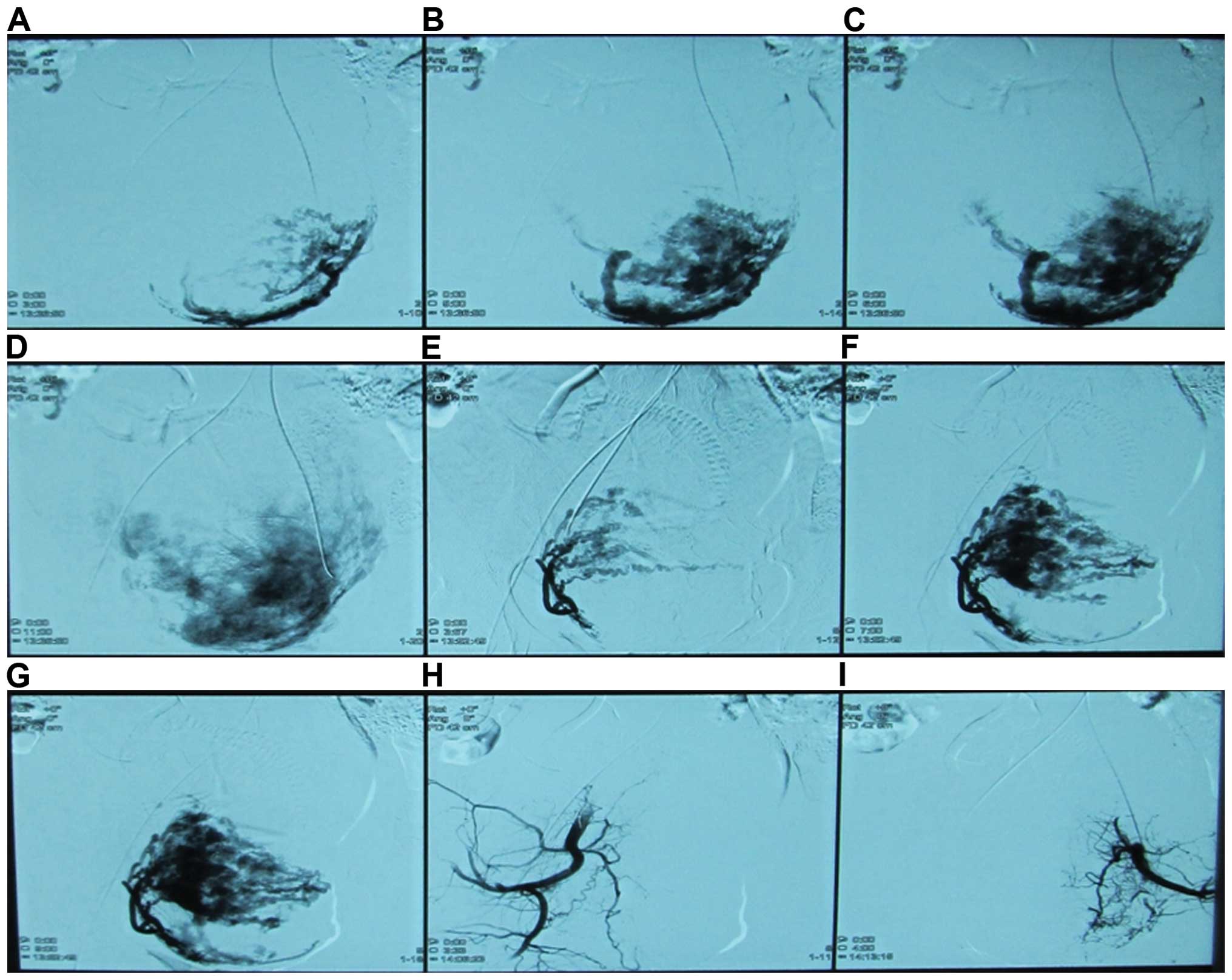
two uterine arteries and infused via the arterial catheter. UAE was
performed by an experienced radiologist with the use of gelfoam
particles (500–1,000 µm in diameter) mixed with nonionic contrast
medium. Angiography was performed after UAE to confirm that the
bilateral uterine arteries were occluded (Fig. 2). After 24–48 h, uterine curettage
was performed under ultrasound-guided or laparoscopy-guided
curettage to confirm the complete removal or destruction of the CSP
mass. The other five patients, who were misdiagnosed from other
hospitals as having an intrauterine pregnancy were treated with
emergency UAE because of uncontrollable massive haemorrhage within
48 h after uterine curettage. Prophylactic anti-infection drugs
were administered to prevent infection. Serum HCG levels were
measured every three days.
Follow-up
The patients were recommended to use contraception
for the first year postoperatively. They were followed up by
telephone interview between three months and one year depending on
serum HCG results, ultrasonographic findings and menstrual cycle
recovery.
Statistical analysis
Statistical analysis was undertaken using SPSS
version 13.0 software (Chicago, IL, USA). Measurement data were
presented as the means and standard deviations. The significance of
between-group differences was tested using analysis of variance or
Chi-square tests. P<0.05 was considered statistically
significant. The data were normally distributed (P>0.05).
Results
Patients
A total of 30 women were included in the current
study. Clinical presentation was described as follows: mild vaginal
bleeding (11 patients); mild abdominal pain (1 patient); both of
the above present (5 patients) and asymptomatic (8 patients;
Table II). Twenty-five patients
diagnosed for CSP by abdominal and TVS were approached to
participate in the study. The patients received prophylactic UAE
combined with MTX followed by uterine curettage and all 25 patients
recovered without complications. The remaining five patients, who
were misdiagnosed as having an intrauterine pregnancy in another
hospital, were treated with suction curettage initially complicated
by uncontrolled vaginal bleeding. Four of the five patients were
treated successfully with UAE in our hospital. Five days after UAE,
the patients underwent ultrasound-guided repeat uterine curettage
because of a slow decrease in serum β-HCG or continued vaginal
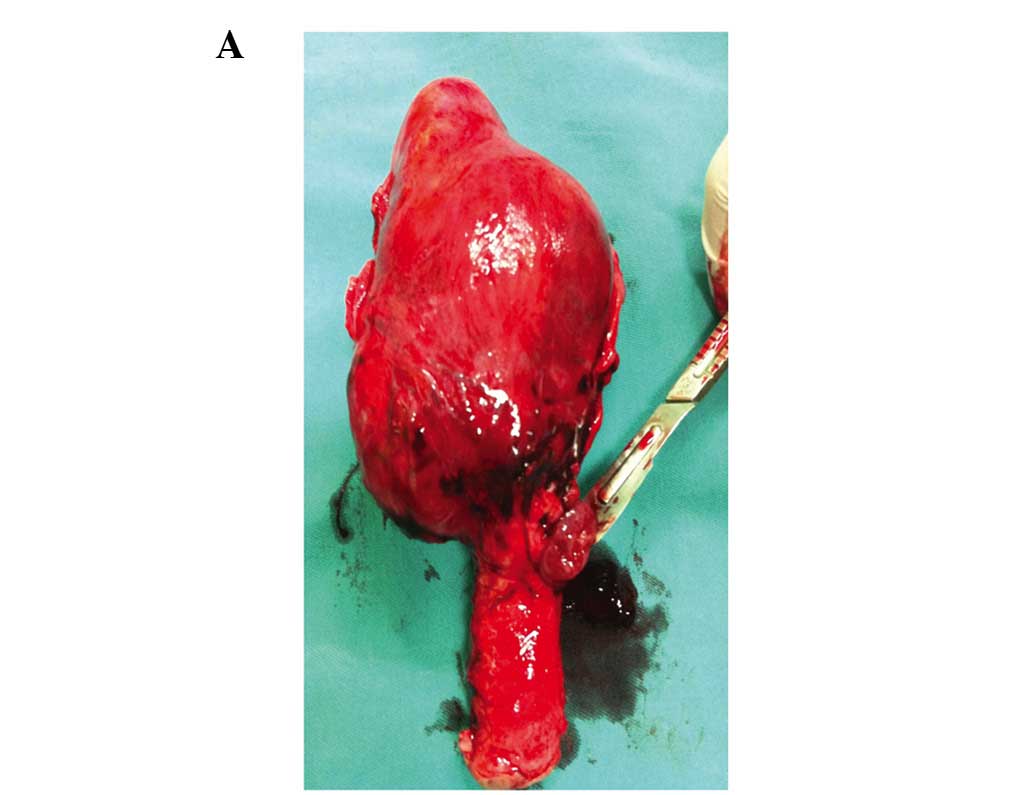
bleeding. The last patient underwent laparotomy and hysterectomy
after an expanding haematoma was demonstrated between the uterus
and bladder by transabdominal ultrasound (Fig. 3).
 | Table II.A comparison of the different types of
clinical cases. |
Table II.
A comparison of the different types of
clinical cases.
| Clinical
presentation | Vaginal bleeding | Abdominal pain | Vaginal bleeding
abdominal pain | Asymptomatic | Massive bleeding
after curettage |
|---|
| Case | 11 | 1 | 5 | 8 | 5 |
| Percentage | 36.67% | 3.33% | 16.67% | 26.67% | 16.67% |
Of the 25 patients who received preventive UAE
combined with MTX, there were no reports of irregular menstrual or
serious adverse effects. Two patients developed postoperative fever
but responded to anti-infective treatment administered at the
hospital. Of the 25 patients, 12 had laparoscopy-guided curettage
and 13 had ultrasound-guided curettage. Of the patients who
received laparoscopy-guided curettage, three patients chose the
surgical option due to a desire for tube ligation. During follow
up, no serious adverse effects were observed in these patients.
However, serum β-HCG levels decreased more rapidly with
ultrasound-guided curettage than with laparoscopy-guided curettage.
The decreased rate was 84.3±10.5 and 76.3±15.23%, respectively
(Table III). In the patients
undergoing laparoscopy and ultrasound-guided curettage, the serum
β-HCG levels returned to normal after 25 and 34 days. The mean
curettage time was 15±5.3 min and mean blood loss was 16±3.8 ml.
Ultrasound examination was performed prior to discharge from the
hospital to ensure that the caesarean scar had disappeared, and
that no abnormal ultrasound findings were present. Follow-up
interviews indicated that a normal menstrual cycle had returned
36.9±2.9 days postoperatively, with no reports of abnormal vaginal
bleeding. None of the patients had normal intrauterine pregnancies
during the follow-up period.
 | Table III.A comparison of operative outcomes
between laparoscopy-guided curettage and ultrasound-guided
curettage. |
Table III.
A comparison of operative outcomes
between laparoscopy-guided curettage and ultrasound-guided
curettage.
|
| No. of previous
caesarean sections | No. of previous
abortion | Apart time of
previous caesarean section (years) | Blood loss volume
(ml) | Menstrual cycle
recovery (days) | Rate of serum β-HCG
reduction (%)a |
|---|
| Ultrasound-guided
curettage | 1.14±0.3 | 1.86±1.03 | 3.71±3.36 | 17.50±4.16 | 37.14±3.79 | 88.43±10.50 |
| Laparoscopy-guided
curettage | 1.09±0.30 | 2.82±1.83 | 4.01±2.59 | 15.91±3.36 | 36.64±1.36 | 76.31±15.23 |
| P-value | 0.70 | 0.14 | 0.80 | 0.83 | 0.43 | 0.04 |
Discussion
CSP is a rare form of ectopic pregnancy. The
etiology of CSP is unclear, but it is generally thought that CSP
occurs when a blastocyst is implanted on fibrous scar tissue within
a wedge-shaped myometrial defect in the anterior lower uterine
segment at the site of a prior caesarean scar (12,13). The
pathological examination of excised CSP alone and in hysterectomy
specimens demonstrated clusters of trophoblast cells as well as
scattered syncytiotrophoblast cells invading the myometrium through
a microscopic dehiscent tract created by a previous caesarean
section procedure or other uterine surgery (12,13).
CSP can present at any time from implantation to
term but has been reported to present more commonly in the first
trimester. The common presenting symptoms are vaginal bleeding and
abdominal pain, but at least one-third of patients are asymptomatic
(6,14). The results of the present study
indicate one-fourth of patients. In the present study, five
patients were misdiagnosed for having an intrauterine pregnancy and
had uncontrolled massive hemorrhage after uterine curettage.
Therefore, true diagnosis was crucial as a large number of
complications caused by misdiagnosis may lead to inappropriate
interventions. TVS was the reference standard for the diagnosis of
CSP in the first trimester with a reported 86.4% sensitivity
(12,14). Maymon et al (15) recommended using combined TVS and
transabdominal sonography (TAS) with a full bladder. Thus a
‘panoramic view’ of the uterus is provided with accurate
measurement of the distance between the gestational sac and bladder
(15). In the present study, all 30
patients were diagnosed via a combination of TVS and TAS.
Two different types of CSP have been proposed
(16). The first type is an
implantation on the prior scar with progression towards the
cervico-isthmic space or the uterine cavity. Such a pregnancy may
progress to viability with the risk of massive haemorrhage. The
second type is a deep implantation into the caesarean scar defect
growing towards the bladder and abdominal cavity and more prone to
scar rupture (11,12).
Successful births have been described with the
appropriate management, but the prognosis for an uneventful term
pregnancy is poor. The hysterectomy rate in these cases is 71%
because of the increased risk of placental previa/accreta and
massive hemorrhage (12–17). Termination of the diagnosed cases by
surgical or medical means may improve the outcomes by allowing
preservation of the uterus and future fertility. It is anticipated
that delay in treatment of scar pregnancies can lead to uterine
rupture, hysterectomy and significant maternal morbidity (11,18).
Various medical and surgical treatments have been attempted,
however, there is no consensus on the optimal mode of treatment
(19). Many medical and surgical
approaches have been attempted with the aim of eliminating the
gestational sac and preserving fertility, including systemic MTX or
local injection into the gestation sac of MTX, potassium chloride,
hyperosmolar glucose or prostaglandins. The surgical methods
included dilatation and curettage, UAE, hysteroscopic resection,
laparoscopic resection or even hysterectomy. Local, systemic and
combined treatments with MTX have been described. However, fibrous
tissue within the scar around the gestational sac can delay
systemic MTX absorption into the sac (20). Local injection of embryocides under
sonographic guidance may decrease the need of additional
interventions compared to systemic MTX (11,12,14).
However, close monitoring of the patient after intervention is
required as hemorrhage may still occur. Therefore, bilateral UAE
should be considered (13,15).
Surgical management with laparoscopy or laparotomy
with excision of the pregnancy may be optima for women who do not
respond to conservative medical treatments or are late to present
for medical attention (19).
However, surgery carries the risk of postoperative adhesions that
may impair future fertility, increased size of surgical wounds, a
longer hospital stay and recovery and possibly an increased risk of
future placental previa/accreta (8,12,19).
Dilation and curettage should not be considered as the first choice
of therapy. This is because the majority of the villi are implanted
in the myometrium and it seems unlikely that the gestational sac
can be expelled by curettage without perforating the uterine wall
or damaging the urinary bladder and may also cause life-threatening
haemorrhage (8,12). Isolated suction curettage was used as
the initial treatment on five of our patients. Four patients
suffered life-threatening haemorrhage and one patient suffered
uterine damage that finally led to hysterectomy. Previous findings
suggest that selective UAE can temporarily block uterine perfusion
and minimize hemorrhage and they have used this technique before or
after curettage (21).
UAE treatment of CSP was first reported in 1999 and
has been used widely to control haemorrhage and preserve the uterus
(22,23). However, isolated UAE without
eliminating the gestational sac results in the gradual decrease of
serum β-HCG levels and irregular menstrual bleeding (7,12).
Therefore, uterine curettage should be performed after UAE. Gelfoam
appears to promote clotting via physical effects by supporting
thrombus development. Vascular occlusion with gelfoam causes acute
necrotizing arteritis. This inflammatory process eventually leads
to breakdown of the gelfoam within 1–3 weeks after embolization,
with subsequent vascular recanalization (24). Because of its temporary nature,
uterine curettage should be carried out as soon as possible. In
published studies, uterine curettage was usually carried out within
24–72 h after UAE (20). In
accordance with our study, the results demonstrated that UAE
followed by uterine curettage may be an effective and safe
treatment for CSP. In the pathological sample of CSP, trophoblast
cells were found to have invaded the myometrium, adhered, implanted
and even penetrated the myometrium (13). For this reason, 50 mg of MTX was
given by infusing the arterial catheter to manage the possibility
of residual villi in the scar tissue. Previous studies reported
that MTX alone followed by suction curettage requires more
hospitalization time and causes greater bleeding volumes compared
with UAE (9). Therefore, curettage
after UAE combined with MTX (50 mg) is a safe and effective means
of treating CSP.
Of note, suction curettage may also be used to treat
CSP, unlike ordinary curettage, because ultrasonography of CSP
displays a thinned or absent myometrium between the gestational sac
and bladder (<5 mm in two-thirds of cases). Thus, careful
monitoring of curettage is necessary to avoid damaging the uterus.
Compared with laparoscopy-guided and ultrasound-guided curettage,
vaginal bleeding and length of stay in hospital showed no
significant difference, although serum β-HCG levels decreased more
rapidly with ultrasound-guided curettage than with
laparoscopy-guided curettage. The rate of decline was 84.3±5.5 and
76.3±10.2%, respectively. The reason may be that the sonogram shows
more clearly the location of the gestational sac and thickness of
the myometrium between the gestational sac and bladder. When
curettage is monitored by ultrasound, the operators can remove the
ectopic sac under direct vision and have clear views of the
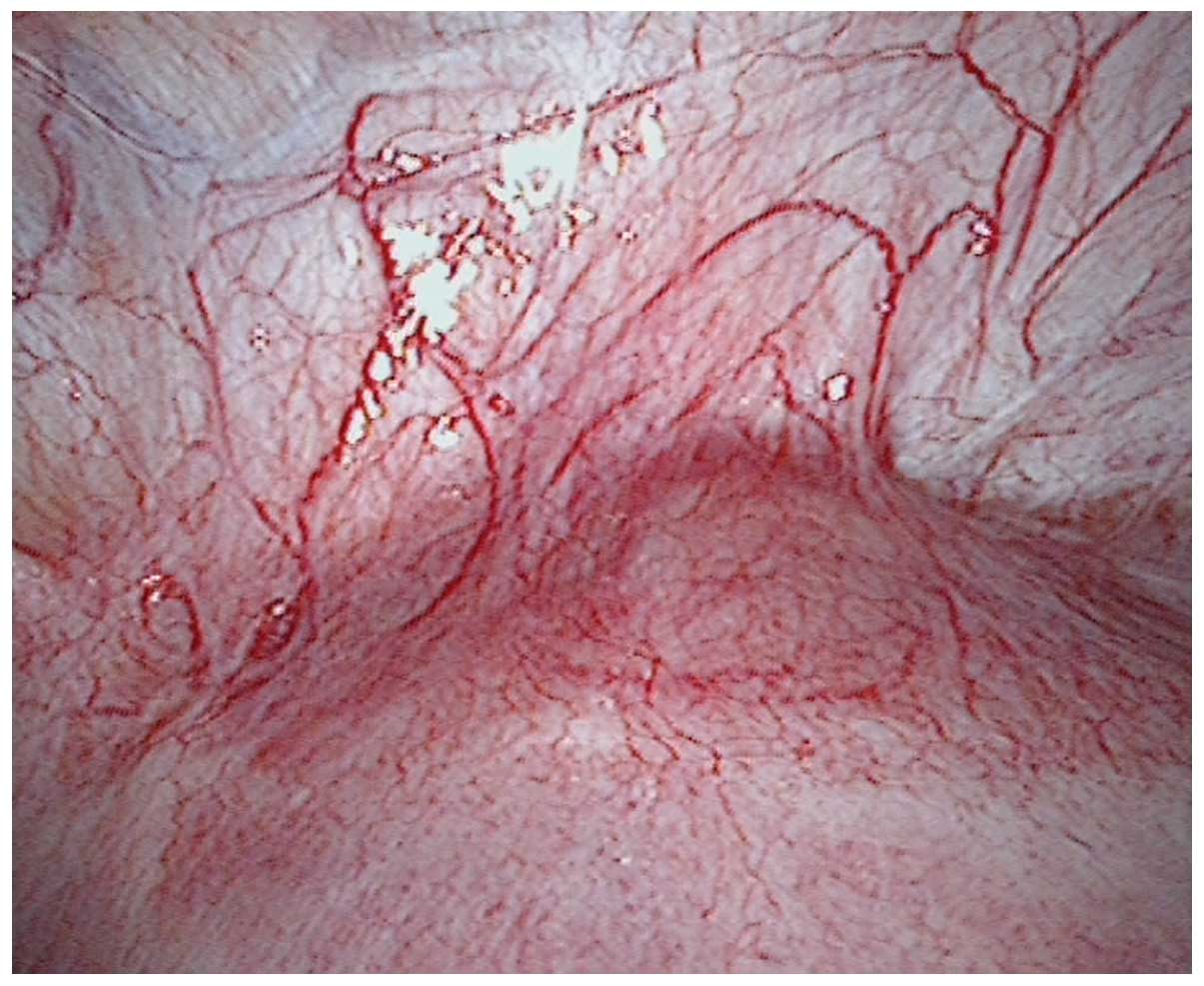
endometrium and myometrium. The advantage of laparoscopy-guided
curettage is observing the appearance of the uterus under direct
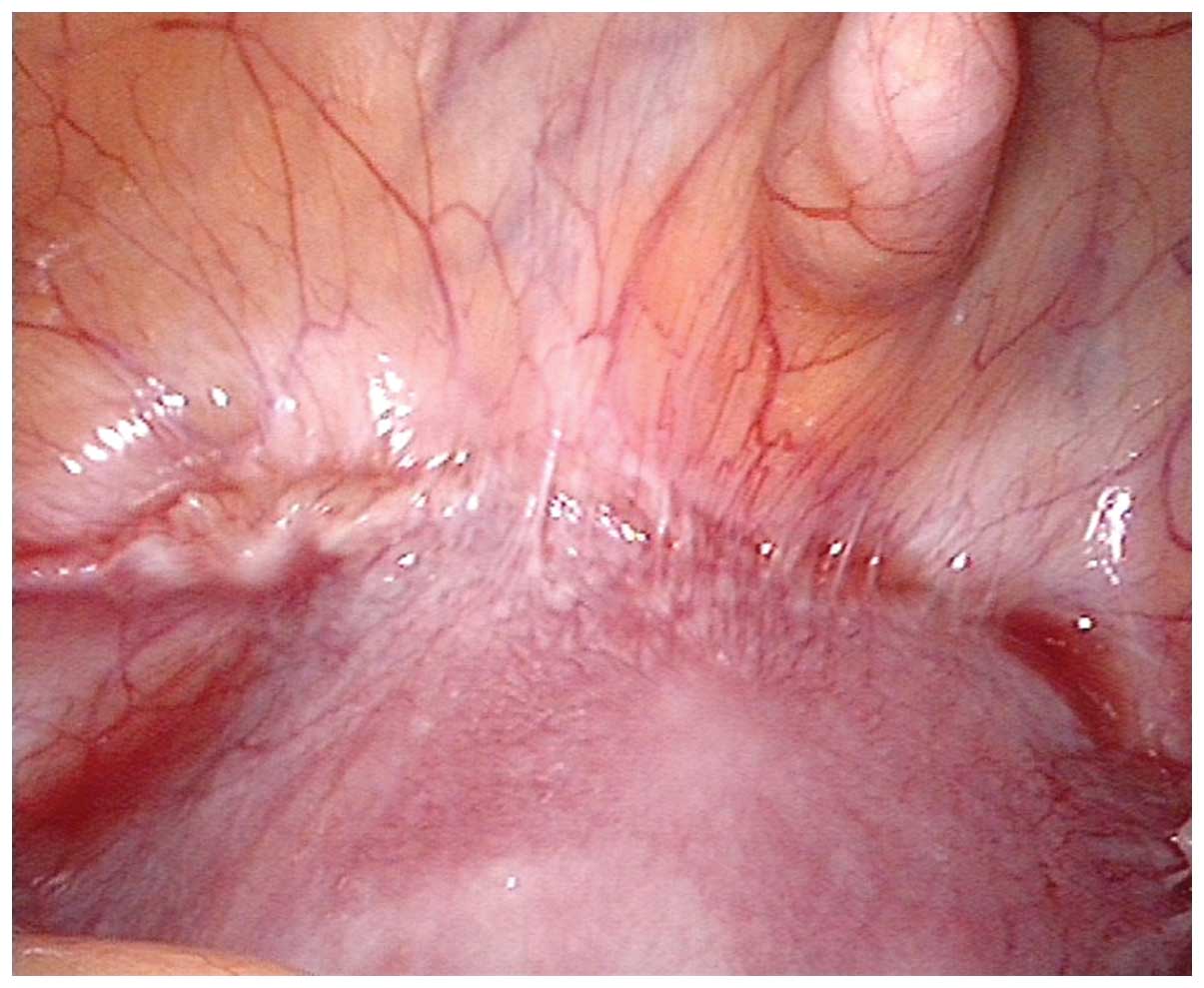
vision during exploration. The topical purple bulge was usually
seen in the lower segment over the caesarean scar. The blood
vessels show hyperplasia in the same area (Fig. 4). However, in the current study,
eight of the laparoscopy-guided cases showed the uterine segment
had some heavy adhesions with the surrounding tissues and caesarean
scars were not easy to expose (Fig.
5).
Separating the adhesion may increase the risk of
tissue damage. Furthermore, laparoscopic surgery may require
anaesthesia and increase the costs and risk of surgery. Therefore,
we considered whether ultrasound-guided curettage may be
appropriate in women who are hemodynamically stable with an
unruptured CSP of a myometrial thickness of >2 mm. Medical
treatment including local, systemic and combined treatment with MTX
may be appropriate in women with an unruptured CSP of <8 weeks'
gestation and a myometrial thickness of <2 mm (6,12).
There is no consensus on time period required for
the following pregnancy or the risk of future pregnancies. The
present study results indicated that, none of the patients had
normal intrauterine pregnancies during the follow-up period,
although the patients were followed up for only one year and the
long-term consequences of the treatment are unknown. A recent study
of 24 women successfully treated for prior CSP without surgical
correction of the scar reported favourable reproductive outcomes
and a recurrence rate of only 5% (17). It showed that 88% of woman conceived
naturally, and 95% of the pregnancies were intrauterine while 65%
appeared normal. Thirty-five percent ended in spontaneous abortion.
Findings of that study showed that, fertility was successfully
preserved in the women who opted for first-trimester termination of
CSP (17). However, the findings
require further validation in a larger group of cases.
In conclusion, CSP is an uncommon but dangerous
occurrence because of the increased risk of uterine rupture and
massive haemorrhage. The precise localization of early pregnancies
by sonography and early recognition of the typical sonographic
findings are critical. In diagnosing CSP, TVS and TAS should be
used in combination to obtain an accurate diagnosis. Based on the
present findings it may be concluded that UAE combined with MTX
followed by ultrasound-guided curettage may be an effective and
safe treatment for CSP. More importantly, emergency UAE should be
recommended as the first choice to treat uncontrollable massive
haemorrhage of CSP and preserve the uterus and future
fertility.
Acknowledgements
The present study was supported by the Joint Funds
of National Key Clinical Departments Foundation of China. The
authors thank Mrs. Ruilian Chen and Dr Daqing Ma for their help and
critical comments during manuscript preparation.
References
|
1
|
Larsen JV and Solomon MH: Pregnancy in a
uterine scar sacculus: an unusual case of postabortal hemorrhage. S
Afr Med. 53:142–143. 1978.
|
|
2
|
Tinelli A, Tinelli R and Malvasi A:
Laparoscopic management of cervical-isthmic pregnancy: a proposal
method. Fertil Steril. 92:829.e3–829.e6. 2009. View Article : Google Scholar
|
|
3
|
Zhang B, Jiang ZB, Huang MS, Guan SH, Zhu
KS, Qian JS, Zhou B, Li MA and Shan H: Uterine artery embolization
combined with methotrexate in the treatment of cesarean scar
pregnancy: results of a case series and review of the literature. J
Vasc Interv Radiol. 23:1582–1588. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Köroğlu M, Kayhan A, Soylu FN, Erol B,
Schmid-Tannwald C, Gürses C, Karademir İ, Ernst R, Yousuf A and Oto
A: MR imaging of ectopic pregnancy with an emphasis on unusual
implantation sites. Jpn J Radiol. 31:75–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL
and Hwang JL: Cesarean scar pregnancy: issues in management.
Ultrasound Obstet Gynecol. 23:247–253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maymon R, Halperin R, Mendlovic S,
Schneider D, Vaknin Z, Herman A and Pansky M: Ectopic pregnancies
in Caesarean section scars: the 8 year experience of one medical
centre. Hum Reprod. 19:278–84. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sadeghi H, Rutherford T, Rackow BW,
Campbell KH, Duzyj CM, Guess MK, Kodaman PH and Norwitz ER:
Cesarean scar ectopic pregnancy: case series and review of the
literature. Am J Perinatol. 27:111–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jurkovic D, Hillaby K, Woelfer B, Lawrence
A, Salim R and Elson CJ: First-trimester diagnosis and management
of pregnancies implanted into the lower uterine segment Cesarean
section scar. Ultrasound Obstet Gynecol. 21:220–227. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhuang Y and Huang L: Uterine artery
embolization compared with methotrexate for the management of
pregnancy implanted within a cesarean scar. Am J Obstet Gynecol.
201:152.e1–152.e3. 2009. View Article : Google Scholar
|
|
10
|
Sugawara J, Senoo M, Chisaka H, Yaegashi N
and Okamura K: Successful conservative treatment of a cesarean scar
pregnancy with uterine artery embolization. Tohoku J Exp Med.
206:261–265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fylstra DL: Ectopic pregnancy within a
cesarean scar: a review. Obstet Gynecol Surv. 57:537–543. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ash A, Smith A and Maxwell D: Caesarean
scar pregnancy. BJOG. 114:253–263. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang CB and Tseng CJ: Primary evacuation
therapy for Cesarean scar pregnancy: three new cases and review.
Ultrasound Obstet Gynecol. 27:222–226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McKenna DA, Poder L, Goldman M and
Goldstein RB: Role of sonography in the recognition, assessment,
and treatment of cesarean scar ectopic pregnancies. J Ultrasound
Med. 27:779–783. 2008.PubMed/NCBI
|
|
15
|
Maymon R, Halperin R, Mendlovic S,
Schneider D and Herman A: Ectopic pregnancies in a Caesarean scar:
review of the medical approach to an iatrogenic complication. Hum
Reprod Update. 10:515–523. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vial Y, Petignat P and Hohlfeld P:
Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol.
16:592–593. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ben Nagi J, Ofili-Yebovi D, Marsh M and
Jurkovic D and Jurkovic D: First-trimester cesarean scar pregnancy
evolving into placenta previa/accreta at term. J Ultrasound Med.
24:1569–1573. 2005.PubMed/NCBI
|
|
18
|
Osborn DA, Williams TR and Craig BM:
Cesarean scar pregnancy: sonographic and magnetic resonance imaging
findings, complications, and treatment. J Ultrasound Med.
31:1449–1456. 2012.PubMed/NCBI
|
|
19
|
Wang YL, Su TH and Chen HS: Laparoscopic
management of an ectopic pregnancy in a lower segment cesarean
section scar: a review and case report. J Minim Invasive Gynecol.
12:73–79. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu X, Zhang X, Zhu J and Di W: Caesarean
scar pregnancy: comparative efficacy and safety of treatment by
uterine artery chemoembolization and systemic methotrexate
injection. Eur J Obstet Gynecol Reprod Biol. 161:75–79. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiao LZ, Zhao J, Wan XR, Liu XY, Feng FZ,
Ren T and Xiang Y: Diagnosis and treatment of cesarean scar
pregnancy. Chin Med Sci J. 23:10–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akbayir O, Gedikbasi A, Akyol A, Ucar A,
Saygi-Ozyurt S and Gulkilik A: Cesarean scar pregnancy: a rare
cause of uterine arteriovenous malformation. J Clin Ultrasound.
39:534–538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang F and He J: Methotrexate-based
bilateral uterine arterial chemoembolization for treatment of
cesarean scar pregnancy. Acta Obstet Gynecol Scand. 89:1592–1594.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jander HP and Russinovich NA:
Transcatheter gelfoam embolization in abdominal, retroperitoneal,
and pelvic hemorrhage. Radiology. 136:337–344. 1980. View Article : Google Scholar : PubMed/NCBI
|