Introduction
Autism spectrum disorders (ASDs) are
neurodevelopmental disorders with deficits in social interaction
and social communication, in addition to stereotyped behaviors with
restricted interests (1). Children
with a mother who was exposed to anti-epileptic drug valproic acid
(VPA) during pregnancy are more prone to have ASDs (2). As a result, a single administration of
VPA to pregnant rats during the gestation period has been
extensively used as an animal model of autism (3–5).
Neuropathological studies suggest that autistic subjects have
decreased numbers of cerebellar Purkinje cells, and impaired
cortical migration (6). This
indicates that cerebellum and cortex disorders may be involved in
the VPA model of autism. There is also evidence of decreased levels
of essential fatty acids in the red blood cells of autistic
children and altered serum levels of polyunsaturated fatty acids
(PUFAs) in the valproate-induced autism model (7,8).
Furthermore, Zhao et al detected reduced expression levels
of associated liver metabolic enzymes ∆5-desaturase, ∆6-desaturase
and elongase (Elovl2) (7). These
observations indicate that fatty acid and liver metabolic enzymes
may be involved in the pathogenesis of autism.
When regarding the liver metabolic enzymes of fatty
acids, it is important to take the process of fatty acid synthesis
into consideration. Fatty acid synthesis involves the creation of
fatty acids from acetyl-coenzyme A (CoA) and malonyl-CoA precursors
through the catalytic action of fatty acid synthase (FASN).
Acetyl-CoA can be transformed into malonyl-CoA by acetyl-CoA
carboxylase (ACC), which is the rate-limiting enzyme during the
fatty acid synthesis process. Researchers have shown that lipogenic
enzymes, such as FASN and ACC, are highly expressed in the rodent
brain during the early neonatal period and decline thereafter
(9,10). This suggests a neurodevelopmental
role of FASN and ACC. Since ASDs are neurodevelopmental disorders,
the aim of the present study was to detect the expression of FASN
and ACC in the prefrontal cortex and cerebellum in the VPA-induced
animal model of autism in order to determine the role of the fatty
acid synthesis pathway in the pathogenesis of autism.
Materials and methods
Animals and treatment
A total of 26 female (weight, 270–310 g) and 13 male
(weight, 310–340 g) Sprague-Dawley rats were obtained from
Laboratory Animal Research Center (Shanghai, China). The animals
were maintained in a light- (lights on at 7:00 and lights off at
19:00), temperature- (23±2°C) and humidity-controlled (55±10%)
environment and allowed ad libitum food and water. The
present study was performed in accordance with the guidelines of
the National Institutes of Health (NIH) Guide for the Care and Use
of Laboratory Animals (8th edition, 2011) and was approved by the
Animal Care and Use Committee of Shanghai Jiao Tong University
School of Medicine (Shanghai, China). The estrous cycle was
monitored and the rats were mated overnight. The first day of
gestation was considered to be when spermatozoa were found in the
vaginal smear. Female rats in the experimental group were given a
single intraperitoneal injection of VPA (600 mg/kg, 250 mg/ml) on
the 12.5th day of pregnancy, whereas control females were given the
same amount of physiological saline at the same time (3,11). The
offspring rats of the experimental females were marked as the VPA
group, and of the control females were marked as the control group.
Pups were anesthetized using sodium pentobarbital (100 mg/kg body
weight) and sacrificed by decapitation at postnatal day 7. The
brains were removed and prefrontal cortex and cerebellum samples
were kept at −80°C until the assays were performed.
Western blot analysis
Samples of the prefrontal cortex and cerebellum from
the mouse brains were homogenized (1–2 min) in cold buffer (10%
w/v) consisting of 50 mM Tris-HCl (pH 7.4), 8.5% sucrose, 2.0 mM
ethylenediamine tetraacetic acid, 10 mM β-mercaptoethanol and a
protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA).
The protein concentration was quantified by the Bradford method
using a protein assay kit from Bio-Rad Laboratories, Inc.
(Hercules, CA, USA). The homogenate samples were mixed with 2X
concentrated Laemmeli buffer (125 mM Tris-HCl, pH 6.8, 4% sodium
dodecyl sulfate (SDS), 20% glycerol, 2% β-mercaptoethanol, and
0.005% bromophenol blue) and separated by 8% SDS-polyacrylamide gel
electrophoresis. The separated proteins were transferred onto a
polyvinylidene fluoride membrane for 1 h at 100 V at 4°C. The
membranes were then incubated at room temperature for 1 h with
blocking buffer, including 5% non-fat dry milk in Tris-buffered
saline with 0.1% Tween 20 to block nonspecific reactions. After
blocking, the blots were incubated with primary antibody overnight
at 4°C. The primary antibodies were rabbit antibodies targeting
FASN (Gly46) (1:1,000; #3180), ACC (1:1,000; #3676), phospho (p)ACC
(Ser 79) (1:1,000; #11818), phosphatidylinositol-4,5-bisphosphate
3-kinase (PI3K p110; 1:1,000; #4255), Akt (1:1,000; #4691) and
adenosine 5′-monophosphate-activated protein kinase (AMPK; 1:1,000;
#5831), acquired from Cell Signaling Technology, Inc. (Danvers, MA,
USA), followed by an anti-rabbit IgG, horseradish peroxide-linked
secondary antibody incubation for 1 h at room temperature (1:2,000;
#7074; Cell Signaling Technology, Inc.). β-Actin rabbit mAb (D6A8;
#8457) was used as a loading control. The immunoblots were
developed by enhanced chemiluminescence according to the
manufacturer's protocol (Merck Millipore, Darmstadt, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the cortex and
cerebellum tissues using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol, and was used as the template for cDNA synthesis. All RNA
preparation and handling steps were done under RNAse-free
conditions. Then, 1 µg total RNA from each sample were treated with
DNase I (Fermentas; Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. Reverse transcription was
performed using M-MLV. The following primer sets were used for
qPCR: PI3K (sense 5′-AACACAGAAGACCAATACTC-3′, anti-sense
5′-TTCGCCATCTACCACTAC-3′), Akt (sense 5′-GTGGCAAGATGTGTATGAG-3′,
anti-sense 5′-CTGGCTGAGTAGGAGAAC-3′), AMPK (sense
5′-GACCTCGGTCAAGTGTCG-3′, anti-sense 5′-TGGGTTATCAACGGGCTA-3′), ACC
(sense 5′-CGCTGCGGTCAAGTGT-3′, anti-sense
5′-CGTTGGCGTAGTTGTTATT-3′), FASN (sense 5′-ATGAAGAGGGACCATAAA-3′,
anti-sense 5′-ACAGGTGGGAACAAGG-3′) and β-actin (sense
5′-CACCCGCGAGTACAACCTT-3′, anti-sense 5′-CCCATACCCACCATCACACC-3′).
SYBR green-based qPCR assays were used for the gene expression
analysis of PI3K, Akt, AMPK, ACC, FASN and the housekeeping gene
β-actin. qPCR was performed using a Bio-Rad iQ5 Real-Time PCR
system (Bio-Rad Laboratories, Inc.). The relative expression levels
of the signaling molecules were calculated using the
2−ΔΔCq method with β-actin as the endogenous reference
gene (12).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Blot densities were quantified using Image J software
(National Institutes of Health, Bethesda, MD, USA). Comparisons
between the VPA group and control group were performed by
independent-samples t-test using IBM SPSS Statistics 20.0 software
(IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
FASN expression is increased in the
prefrontal cortex and cerebellum in the VPA model of autism
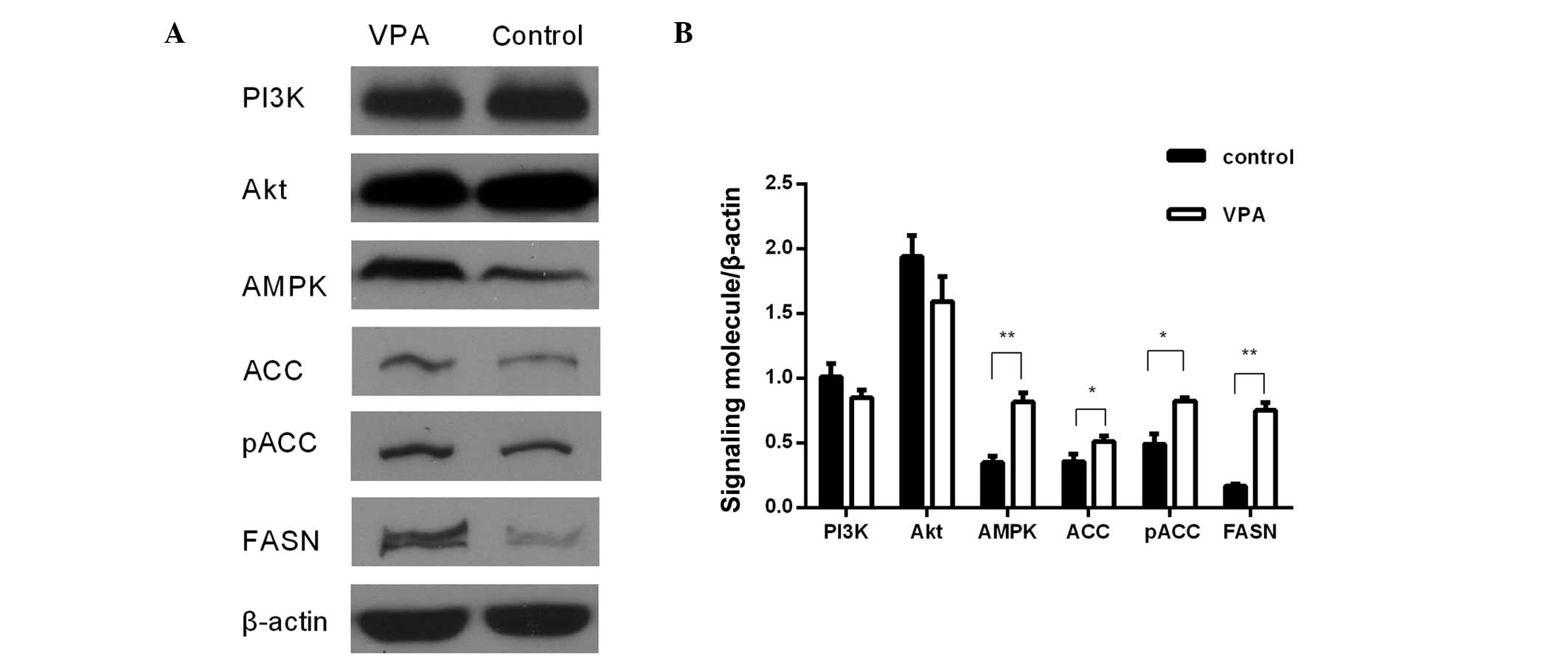
To evaluate the expression changes of FASN in
autism, a VPA model of autism was generated. Western blot analysis
showed a 4-fold increase in FASN protein expression in the
prefrontal cortex and a 2-fold increase in FASN expression in the
cerebellum in the VPA group. The relative expression values
(FASN/β-actin) were 0.76±0.02 for the VPA group and 0.17±0.01 for
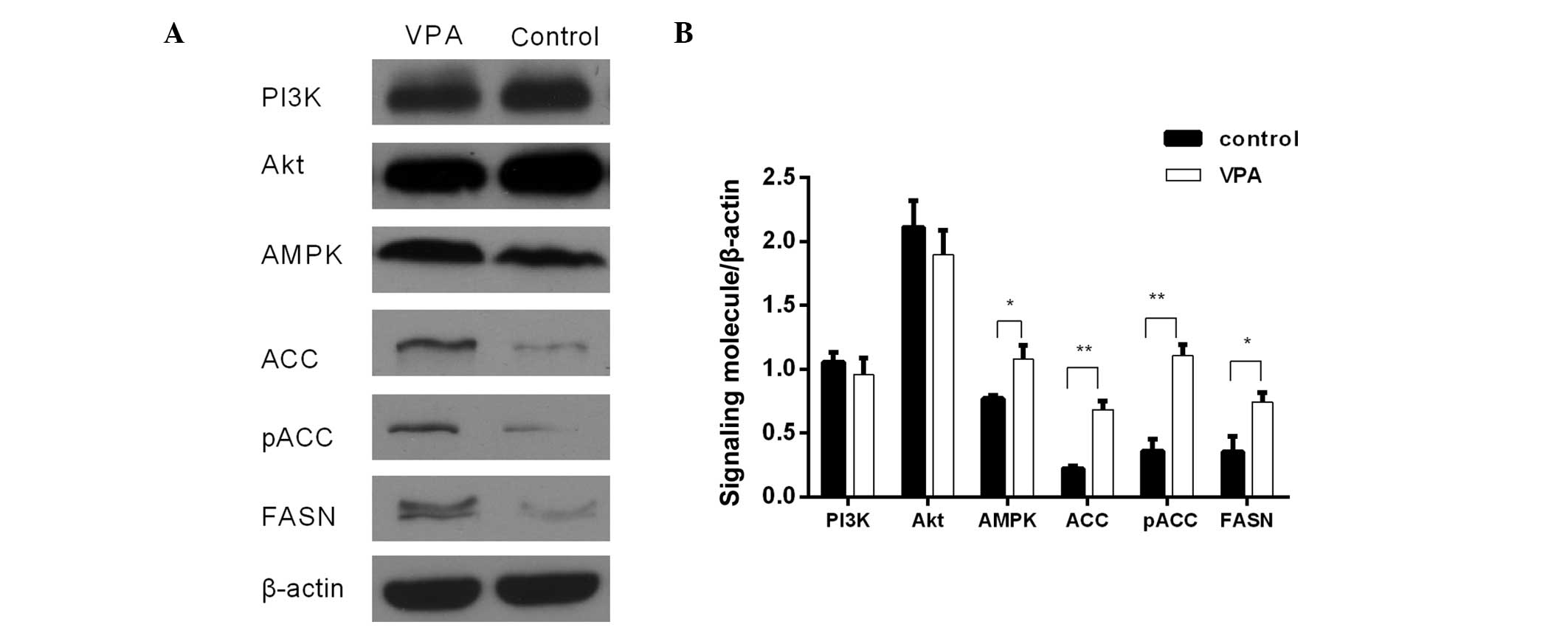
the control group in the prefrontal cortex (Fig. 1), and 0.76±0.06 for the VPA group and
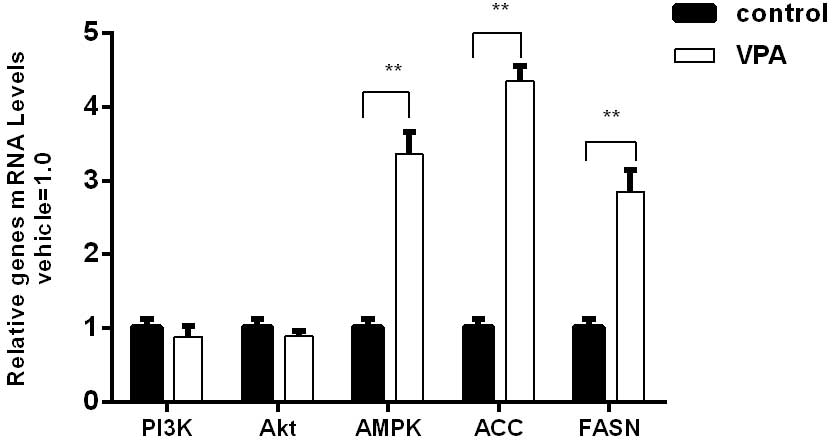
0.33±0.03 for the control group in the cerebellum (Fig. 2). Using RT-qPCR, it was detected that
the mRNA level of FASN in the VPA group was 6.1-fold higher than
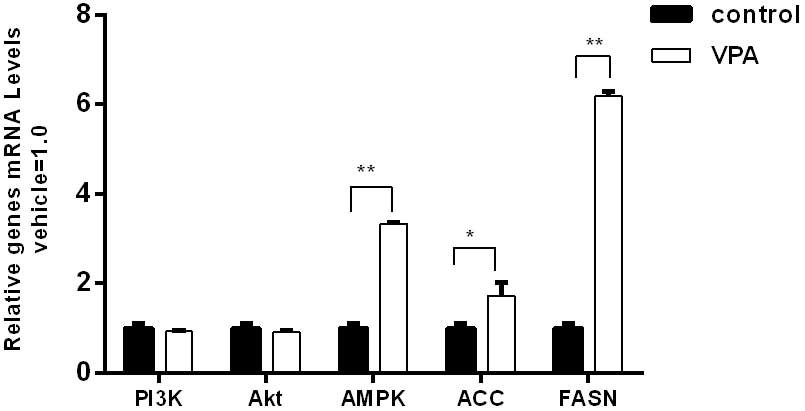
that of the control group in the prefrontal cortex (Fig. 3) and 2.8-fold higher than that of the
control group in the cerebellum (Fig.
4).
ACC expression is increased in the
prefrontal cortex and cerebellum in the VPA model of autism
Western blot analysis was performed in order to
evaluate the protein expression of ACC, the key enzyme of fatty
acid synthesis. A significant increase in ACC expression was
detected in the prefrontal cortex and cerebellum in the VPA model
of autism. The relative expression values (ACC/β-actin) were
0.52±0.02 for the VPA group and 0.29±0.06 for the control group in
the prefrontal cortex (Fig. 1) and
0.64±0.03 for the VPA group and 0.25±0.01 for the control group in
the cerebellum (Fig. 2). Using
RT-qPCR, it was detected that the mRNA level of ACC in the VPA
group was 1.7-fold higher than that of the control group in the
prefrontal cortex (Fig. 3) and
4.3-fold higher than that of the control group in the cerebellum
(Fig. 4).
pACC expression is increased in the
prefrontal cortex and cerebellum in the VPA model of autism
To further elucidate the activity of ACC, the
expression levels of pACC were detected. Phosphorylation by AMPK at
Ser79 can inhibit the enzymatic activity of ACC (13). Compared with the control group, pACC
expression in the prefrontal cortex and cerebellum in the VPA group
was significantly upregulated. The relative expression levels
(pACC/β-actin) in the VPA and control groups were 0.94±0.04 and
0.51±0.04, respectively, in the prefrontal cortex (Fig. 1), and 1.34±0.13 and 0.38±0.03,
respectively, in the cerebellum (Fig.
2).
AMPK expression is increased in the
prefrontal cortex and cerebellum in the VPA model of autism
In order to investigate the role of AMPK in autism,
the expression of AMPK was detected at the protein and mRNA levels.
Western blot analysis showed that AMPK protein expression was
significantly increased by 143.7% in the prefrontal cortex
(P<0.01; Fig. 1) and by 36.7% in
the cerebellum (P<0.05; Fig. 2)
of the VPA-induced rats as compared with the controls. RT-qPCR
analysis showed that the AMPK mRNA expression level in the VPA
group was 3-fold higher than that in the control group in the
prefrontal cortex (Fig. 3) and also
in the cerebellum (Fig. 4).
PI3K and Akt expression are unchanged
in the prefrontal cortex and cerebellum in the VPA model of
autism
The expression of PI3K and Akt exhibited no
significant differences between the VPA group and the control group
at the protein and mRNA levels (Figs.
1–4). However, the protein and
mRNA levels of PI3K and Akt in the VPA group manifested slightly
reductions compared with the control group levels.
Discussion
The involvement of fatty acids in the pathogenesis
of autism has previously been reported. Schultz et al found
that children who were not breastfed or were fed infant formula
without docosahexaenoic acid or arachidonic acid were more likely
to have autism (14). Furthermore,
Meguid et al reported that PUFA supplementation may play an
important role in ameliorating the behavior of autistic children
(15). Long chain acyl-CoA
dehydrogenase (LCAD) is a key mitochondrial enzyme in the
β-oxidation of branched chain and unsaturated fatty acids (16). Cox et al found that children
with autism exhibit significant elevations of unsaturated fatty
acid metabolites, in a similar manner to LCAD-deficient mice
(17). Other metabolic
abnormalities, including alterations of ammonia detoxification,
reduced synthesis of ω-3 docosahexaenoic acid, and abnormal
cholesterol metabolism have also been detected in autistic children
(18).
FASN is an enzyme encoded by the FASN gene in
humans. Its main function is to catalyze the synthesis of palmitate
from acetyl-CoA and malonyl-CoA in the presence of nicotinamide
adenine dinucleotide (NADPH), and into long-chain saturated fatty
acids (19). Furthermore, Veigel
et al reported that FASN is a metabolic marker of cell
proliferation (20). The results of
the present study show that the protein expression of FASN is
increased in the prefrontal cortex and cerebellum in the VPA model
of autism. The upregulation of FASN suggests that cell
proliferation is increased in autism. Consistently, increased
progenitor proliferation and premature cell cycle withdrawal, which
occur as a consequence of alterations affecting the Ras/Raf/MEK
extracellular signal-regulated kinase (ERK) pathway, have been
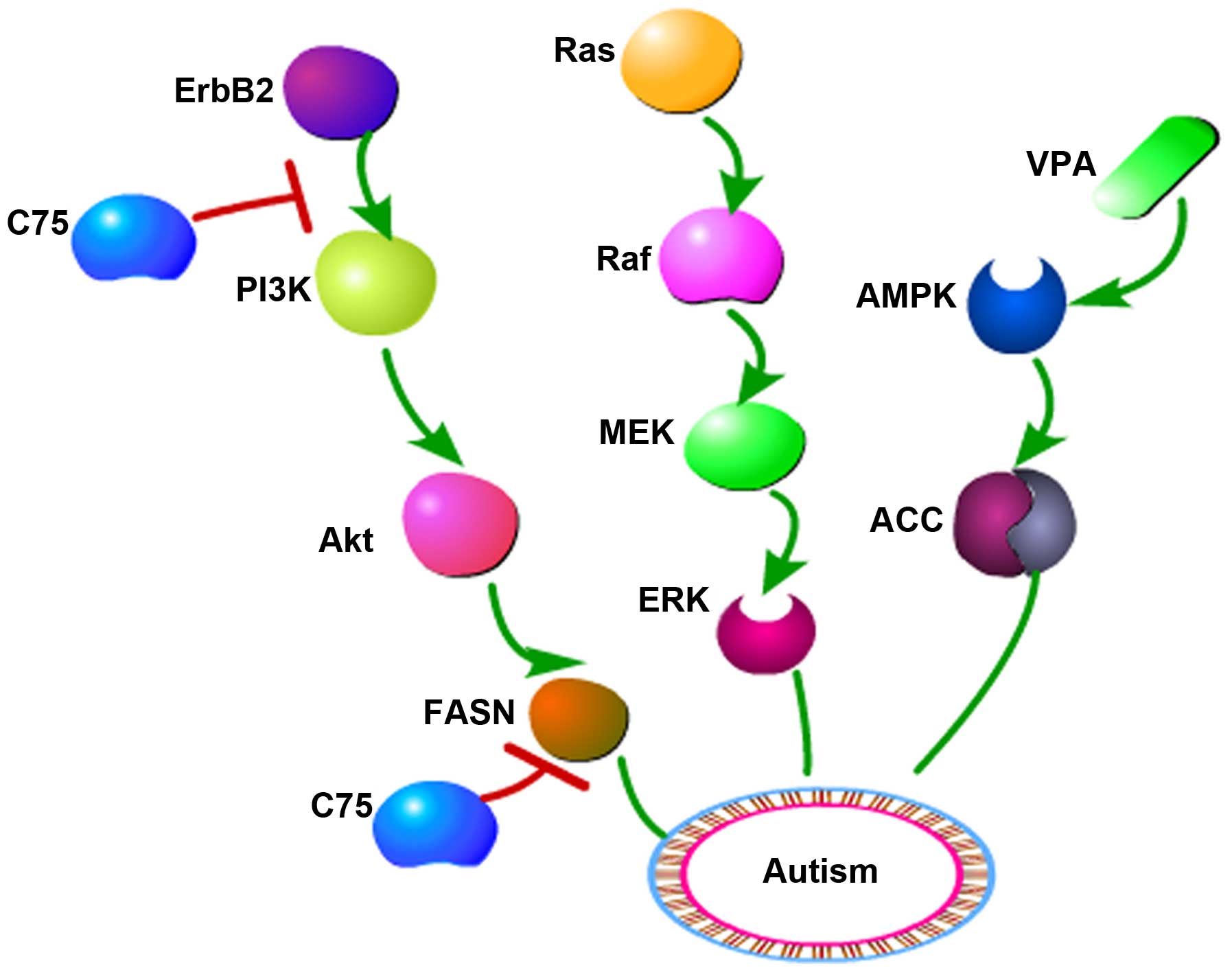
observed in a 16p11.2 deletion murine model of autism (21), as shown in Fig. 5. Previous studies by the authors of
the present study have also indicated that upregulation of the
Ras/Raf/MEK/ERK pathway may be involved in autism (22,23). In
addition, enhanced progenitor proliferation and cell cycle exit can
lead to premature depletion of progenitor pools and alteration of
the number and frequency of cortical neurons (21). FASN catalyzes the formation of
phospholipids for membrane microdomains that accommodate receptor
tyrosine kinases, including ErbB1 and ErbB2. The FASN inhibitor C75
can repress the expression of FASN, ErbB1, ErbB2 and Akt,
suggesting that FASN may participate in the ErbB/Akt pathway
(24). The ErbB2 inhibitor lapatinib
is able to suppress the activity of the ErbB2/PI3K/Akt/FASN pathway
(25). Dysregulation of the PI3K/Akt
pathway has been shown to have an association with autism (26); therefore, it is speculated that
alterations of the ErbB2/PI3K/Akt/FASN signaling pathway may be
involved in autism.
ACC catalyzes the irreversible carboxylation of
acetyl-CoA to produce malonyl-CoA, a building block for new fatty
acids (27). Malonyl-CoA is also
able to inhibit the combination of fatty acids and carnitine to
prevent fatty acids from entering the mitochondria for oxidation
and degradation (28). Reduced NADH
oxidase activity in lymphocytic mitochondria has been detected in
children with autism (29). Elevated
plasma pyruvate levels, increased mitochondrial rates of hydrogen
peroxide production and mitochondrial DNA over-replication have
been observed in autistic subjects (29). In addition, there have been studies
demonstrating elevations in short-chain and long-chain
acyl-carnitines in propionic acid-induced rodent autism models
(30,31). ACC can be phosphorylated and
inactivated at Ser79 by AMPK (13).
VPA is an activator of AMPK (32).
The results of the present study indicate that the intraperitoneal
injection of VPA increased the protein expression of ACC, which may
suggest upregulation of the AMPK/ACC pathway in the VPA animal
model of autism. The AMPK/ACC pathway regulates cellular survival
or apoptosis and energy homeostasis in the hypothalamus (33). Therefore, it is speculated that an
apoptosis pathway may be involved in the development of autism. At
present, the clinical treatment of autism remains a big challenge.
Researchers have demonstrated that a ketogenic diet (KD) plays a
potential therapeutic role and may attenuate social and metabolic
alterations identified in the VPA model of autism (34). Furthermore, the treatment effect of
the KD may be caused by a reduction in the activity of AMPK/ACC
pathway and heat shock protein 70 to decrease kainic acid-induced
hippocampal cell death (35).
In this context, the results of the present study
reveal important alterations in the protein expression levels of
FASN and ACC in the prefrontal cortex and cerebellum in the VPA
model of autism. These results suggest that ErbB2/PI3K/Akt/FASN
and/or AMPK/ACC pathway disorders are likely to be involved in the
induction of autistic symptoms. Therefore, it is hypothesized that
fatty acid synthesis may participate in autism through
ErbB2/PI3K/Akt/FASN and AMPK/ACC pathways.
Acknowledgements
This study was supported by funding for an ‘oriental
scholar’ distinguished professor from the Shanghai Mental Health
Center, Shanghai Jiao Tong University School of Medicine and the
National Nature Science Foundation of China (grant nos. 81371491
and 8152803).
References
|
1
|
American Psychiatric Association:
Diagnostic and Statistical Manual of Mental Disorders (DSM-5)
(5th). American Psychiatric Publishing. Arlington, VA: 2013.
|
|
2
|
Ornoy A: Valproic acid in pregnancy: How
much are we endangering the embryo and fetus? Reprod Toxicol.
28:1–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rodier PM, Ingram JL, Tisdale B and Croog
VJ: Linking etiologies in humans and animal models: Studies of
autism. Reprod Toxicol. 11:417–422. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chomiak T and Hu B: Alterations of
neocortical development and maturation in autism: Insight from
valproic acid exposure and animal models of autism. Neurotoxicol
Teratol. 36:57–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Foley AG, Gannon S, Rombach-Mullan N,
Prendergast A, Barry C, Cassidy AW and Regan CM: Class I histone
deacetylase inhibition ameliorates social cognition and cell
adhesion molecule plasticity deficits in a rodent model of autism
spectrum disorder. Neuropharmacology. 63:750–760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bauman ML: Microscopic neuroanatomic
abnormalities in autism. Pediatrics. 87:791–796. 1991.PubMed/NCBI
|
|
7
|
Zhao G, Gao J, Liang S, Wang X, Sun C, Xia
W, Hao Y, Li X, Cao Y and Wu L: Study of the serum levels of
polyunsaturated fatty acids and the expression of related liver
metabolic enzymes in a rat valproate-induced autism model. Int J
Dev Neurosci. 44:14–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brigandi SA, Shao H, Qian SY, Shen Y, Wu
BL and Kang JX: Autistic children exhibit decreased levels of
essential fatty acids in red blood cells. Int J Mol Sci.
16:10061–10076. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saito M, Chakraborty G, Mao RF and Vadasz
C: Developmental profiles of lipogenic enzymes and their regulators
in the neonatal mouse brain. Neurochem Res. 34:1945–1954. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spencer EB, Bianchi A, Widmer J and
Witters LA: Brain acetyl-CoA carboxylase: Isozymic identification
and studies of its regulation during development and altered
nutrition. Biochem Biophys Res Commun. 192:820–825. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schneider T and Przewłocki R: Behavioral
alterations in rats prenatally exposed to valproic acid: Animal
model of autism. Neuropsychopharmacology. 30:80–89. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ha J, Daniel S, Broyles SS and Kim KH:
Critical phosphorylation sites for acetyl-CoA carboxylase activity.
J Biol Chem. 269:22162–22168. 1994.PubMed/NCBI
|
|
14
|
Schultz ST, Klonoff-Cohen HS, Wingard DL,
Akshoomoff NA, Macera CA, Ji M and Bacher C: Breastfeeding, infant
formula supplementation and autistic disorder: The results of a
parent survey. Int Breastfeed J. 1:162006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meguid NA, Atta HM, Gouda AS and Khalil
RO: Role of polyunsaturated fatty acids in the management of
Egyptian children with autism. Clin Biochem. 41:1044–1048. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lea W, Abbas AS, Sprecher H, Vockley J and
Schulz H: Long-chain acyl-CoA dehydrogenase is a key enzyme in the
mitochondrial beta-oxidation of unsaturated fatty acids. Biochim
Biophys Acta. 1485:121–128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cox KB, Hamm DA, Millington DS, Matern D,
Vockley J, Rinaldo P, Pinkert CA, Rhead WJ, Lindsey JR and Wood PA:
Gestational, pathologic and biochemical differences between very
long-chain acyl-CoA dehydrogenase deficiency and long-chain
acyl-CoA dehydrogenase deficiency in the mouse. Hum Mol Genet.
10:2069–2077. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clark-Taylor T and Clark-Taylor BE: Is
autism a disorder of fatty acid metabolism? Possible dysfunction of
mitochondrial beta-oxidation by long chain acyl-CoA dehydrogenase.
Med Hypotheses. 62:970–975. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Persson B, Kallberg Y, Bray JE, Bruford E,
Dellaporta SL, Favia AD, Duarte RG, Jörnvall H, Kavanagh KL,
Kedishvili N, et al: The SDR (short-chain dehydrogenase/reductase
and related enzymes) nomenclature initiative. Chem Biol Interact.
178:94–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Veigel D, Wagner R, Stübiger G, Wuczkowski
M, Filipits M, Horvat R, Benhamú B, López-Rodríguez ML, Leisser A,
Valent P, et al: Fatty acid synthase is a metabolic marker of cell
proliferation rather than malignancy in ovarian cancer and its
precursor cells. Int J Cancer. 136:2078–2090. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pucilowska J, Vithayathil J, Tavares EJ,
Kelly C, Karlo JC and Landreth GE: The 16p11.2 deletion mouse model
of autism exhibits altered cortical progenitor proliferation and
brain cytoarchitecture linked to the ERK MAPK pathway. J Neurosci.
35:3190–3200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang K, Cao F, Sheikh AM, Malik M, Wen G,
Wei H, Brown WT and Li X: Up-regulation of Ras/Raf/ERK1/2 signaling
impairs cultured neuronal cell migration, neurogenesis, synapse
formation, and dendritic spine development. Brain Struct Funct.
218:669–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao FJ, Zhang X, Liu T, Li XW, Malik M and
Feng SQ: Up-regulation of Ras/Raf/ERK1/2 signaling in the spinal
cord impairs neural cell migration, neurogenesis, synapse
formation, and dendritic spine development. Chin Med J (Engl).
126:3879–3885. 2013.PubMed/NCBI
|
|
24
|
Grunt TW, Wagner R, Grusch M, Berger W,
Singer CF, Marian B, Zielinski CC and Lupu R: Interaction between
fatty acid synthase- and ErbB-systems in ovarian cancer cells.
Biochem Biophys Res Commun. 385:454–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Long XH, Zhang GM, Peng AF, Luo QF, Zhang
L, Wen HC, Zhou RP, Gao S, Zhou Y and Liu ZL: Lapatinib alters the
malignant phenotype of osteosarcoma cells via downregulation of the
activity of the HER2-PI3K/AKT-FASN axis in vitro. Oncol Rep.
31:328–334. 2014.PubMed/NCBI
|
|
26
|
Chen J, Alberts I and Li X: Dysregulation
of the IGF-I/PI3K/AKT/mTOR signaling pathway in autism spectrum
disorders. Int J Dev Neurosci. 35:35–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abu-Elheiga L, Matzuk MM, Abo-Hashema KA
and Wakil SJ: Continuous fatty acid oxidation and reduced fat
storage in mice lacking acetyl-CoA carboxylase 2. Science.
291:2613–2616. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nelson DL and Cox MM: Lehninger Principles
of Biochemistry (5th). W. H. Freeman and Company. London:
8062008.
|
|
29
|
Giulivi C, Zhang YF, Omanska-Klusek A,
Ross-Inta C, Wong S, Hertz-Picciotto I, Tassone F and Pessah IN:
Mitochondrial dysfunction in autism. JAMA. 304:2389–2396. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Frye RE, Melnyk S and Macfabe DF: Unique
acyl-carnitine profiles are potential biomarkers for acquired
mitochondrial disease in autism spectrum disorder. Transl
Psychiatry. 3:e2202013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thomas RH, Foley KA, Mepham JR, Tichenoff
LJ, Possmayer F and MacFabe DF: Altered brain phospholipid and
acylcarnitine profiles in propionic acid infused rodents: Further
development of a potential model of autism spectrum disorders. J
Neurochem. 113:515–529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Avery LB and Bumpus NN: Valproic acid is a
novel activator of AMP-activated protein kinase and decreases liver
mass, hepatic fat accumulation and serum glucose in obese mice. Mol
Pharmacol. 85:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wen JP, Liu CE, Hu YT, Chen G and Lin LX:
Globular adiponectin regulates energy homeostasis through
AMP-activated protein kinase-acetyl-CoA carboxylase (AMPK/ACC)
pathway in the hypothalamus. Mol Cell Biochem. 344:109–115. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ahn Y, Narous M, Tobias R, Rho JM and
Mychasiuk R: The ketogenic diet modifies social and metabolic
alterations identified in the prenatal valproic acid model of
autism spectrum disorder. Dev Neurosci. 36:371–380. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jeon BT, Lee DH, Kim KH, Kim HJ, Kang SS,
Cho GJ, Choi WS and Roh GS: Ketogenic diet attenuates kainic
acid-induced hippocampal cell death by decreasing AMPK/ACC pathway
activity and HSP70. Neurosci Lett. 453:49–53. 2009. View Article : Google Scholar : PubMed/NCBI
|