Introduction
The identification of tissue-specific stem cells is
a key aim of current research and may potentially lead to the
development of treatments for various diseases, including
neurodegenerative disorders and cardiac diseases (1,2). In
addition, stem cells may have a vital role in the field of
regenerative medicine (3). In a
previous study, stem cell progeny were shown to divide at a rapid
rate, whereas the turnover time of stem cells in the bone marrow
and skin was comparably slow (4).
Furthermore, stem cells have been shown to contribute to wound and
organ repair processes following injury (5–7). Adult
stem cells and their niches have been well characterized in various
organs, including the bone marrow, intestines, skin,
gastrointestinal mucosa, liver, prostate and brain (8–12). A
single neural stem cell has been shown to differentiate into
astrocytes, glia and neurons (11).
However, the nature and function of kidney-specific stem cells
remain unclear, with certain researchers doubting the existence of
adult stem cells in the kidneys (13).
Previous studies reported that stem cells in the
adult kidneys of skate and freshwater teleosts were associated with
the formation of nephrons following a partial nephrectomy (14–16). In
addition, bone marrow-derived stem cells were shown to migrate to
the kidneys and specialize into tubular epithelial cells following
acute kidney injury (17–21). During embryonic development, stem
cells are present in the metanephric mesenchyme, which is the site
of origin of numerous structures belonging to the mature kidney,
with the exception of the collecting duct, interstitium and
vasculature (22,23). The present study aimed to identify
and isolate kidney-derived stem cells, and to determine their
function in the kidneys. In addition, the expression of
octamer-binding transcription factor 4 (Oct-4) was detected, since
it has previously been shown to be expressed in various types of
stem cells, including embryonic stem cells, primordial germ cells,
adult gonads, and stem cells isolated from umbilical cord blood,
the bone marrow, hair follicles, muscle, skin, breast tissue,
pancreas, liver, amniotic fluid, endothelial progenitor cells and
neural stem cells (24–41).
Materials and methods
Animals and model establishment
The animal use protocol in the present study was
approved by the Animal Care and Ethical Committee at the Second
Hospital of Shandong University (Jinan, China). A total of 12
Fischer 344 transgenic (Tg) rats (age, 3 days; weight, 12.5–13.5
kg), of which half were female, expressing the human diphtheria
toxin receptor (hDTR; RGD_ID1302921) were used in the present
study. The rats were maintained under a 12-h light/dark cycle, with
ad libitum access to food and water. Kidney injury was
stimulated in nine rats using the diphtheria toxin (DT;
Sigma-Aldrich, St. Louis, MO, USA). The remaining three rats were
used as the control. The minimum lethal dose of DT for humans has
been reported to be 100 ng/kg body weight (42,43). On
the basis of a standardization protocol (42,43), 10
ng/kg DT concentration was considered sufficient to induce kidney
injury in the Tg rats.
5-bromo-2′-deoxyuridine (BrdU)
labeling retention assay and immunohistochemical analysis
For the BrdU labeling retention assay, the Tg rats
were injected subcutaneously with 50 µg/g/d 5-bromo-2′-deoxyuridine
(BrdU; Sigma-Aldrich) for 3 days. At 60 days post-BrdU
administration, the rats were sacrificed by intraperitoneal
injection with 60 mg/kg body weight pentobarbital sodium
(Sigma-Aldrich). Immunohistochemical analyses was performed to
confirm that the BrdU was successfully incorporated into the rat
kidney tissues. In order to visualize BrdU incorporation, kidney
tissue sections (4 µm) were deparaffinized with xylene and
hydrated, during which the glass slides were dipped into isopropyl
alcohol and subsequently hydrated with water (all Sigma-Aldrich).
Endogenous peroxidase was inhibited using 10% methanol in 1X
phosphate-buffered saline (PBS; Sigma-Aldrich), and DNA was
denatured by incubating the tissue sections with 2 N HCl at 37°C
for 45 min. Nonspecific staining was blocked with 2% bovine serum
albumin (BSA; Sigma-Aldrich) for 1 h at ambient temperature. The
sections were then incubated overnight at 4°C with mouse anti-BrdU
monoclonal antibody (1:40; B8434; Sigma-Aldrich) and goat
anti-oct-4 polyclonal antibody (1:100; SAB2500713; Sigma-Aldrich).
Following incubation with primary antibody, tissue sections were
washed and incubated with secondary antibodies, including goat
anti-mouse fluorescein isothiocyanate-conjugated immunoglobulin
(Ig) G heavy and light chains (H&L; 1:10,000; ab6785; Abcam,
Cambridge, UK), donkey anti-goat Texas Red®-conjugated
IgG H&L (1:10,000; ab6883; Abcam) and donkey anti-mouse Texas
Red®-conjugated IgG H&L (1:10,000; ab6818; Abcam),
for 1 h at room temperature. The prepared slides were mounted with
DPX (Sigma-Aldrich) and observed under an Eclipse Ti-S fluorescent
microscope (Nikon Corporation, Tokyo, Japan). Immunohistochemical
analyses, using anti-Oct-4 and anti-BrdU antibodies, were performed
as described in a previous study (44). In the DT-treated rats, the rats were
injected with 10 ng/kg DT 60 days following BrdU administration. On
day 7 post-DT injection, the DT-treated rats were sacrificed by
intraperitoneal injection with 60 mg/kg body weight pentobarbital
sodium and damage to the kidneys was observed by analysis of the
rat urine with the Trypan blue dye (Sigma-Aldrich).
Cell culture experiments
Kidney-derived stem cells were isolated from the Tg
rat kidneys using the following culture protocol. The rats were
sacrificed by intraperitoneal injection with 60 mg/kg body weight
pentobarbital sodium, after which the kidneys were surgically
removed, minced and partially digested using collagenase in the
presence of a trypsin inhibitor (all Sigma-Aldrich). The resulting
cell suspension was washed and plated in a medium composed of 58%
Dulbecco's modified Eagle's medium-low glucose, 42% MCDB-201
medium, 1 insulin-transferrin-selenium, BSA (1 mg/ml), 0.05 M
dexamethasone, 0.1 mM ascorbic acid 2-phosphate, 100 U penicillin
and 1,000 U streptomycin with 2% fetal calf serum, 10 ng/ml
epidermal growth factor, 10 ng/ml platelet-derived growth factor-BB
and 10 ng/ml leukemia inhibitory factor (all Sigma-Aldrich). The
medium composition used for the present experiment is a slightly
modified version of a medium described in a previous study
(45). The cells were seeded on
fibronectin-coated culture flasks (BD Biosciences, San Jose, CA,
USA) at a low density (300 cells/cm2), to avoid
cell-cell contact and cultured at 37°C in the presence of 5%
CO2.
Immunostaining
Kidney-derived stem cells were fixed with 4%
paraformaldehyde, permeabilized with Triton X-100 (Sigma-Aldrich)
and blocked with 1% BSA in PBS for 1 h. The cells were incubated
with goat anti-oct-4 polyclonal antibody (1:100; SAB2500713;
Sigma-Aldrich) overnight at 4°C. The plates were washed in 1X PBS
and incubated with polyclonal horseradish peroxidase-conjugated
rabbit anti-goat IgG (1:10,000; ab97023; Abcam) for 1 h in the dark
at room temperature. The antibody complexes were visualized using
the 3,3′-diaminobenzidine (DAB) substrate (Sigma-Aldrich). The
processed plates were observed under the Nikon Ti-S fluorescent
microscope. In addition, unstained cells were observed using a
phase contrast microscope (ECLIPSE TS100/100-F; Nikon Corporation,
Tokyo, Japan).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was isolated from the kidney-derived
cultured stem cells using an RNeasy Mini Kit (Qiagen, Hilden,
Germany) following the standard manufacturer protocol, and stored
at −70°C. RNA was treated with DNase1 (Invitrogen, Carlsbad, CA,
USA), and the quality and quantity of the RNA were validated using
the NanoDrop 2000 (Thermo Fisher Scientific, Inc., Wilmington, DE,
USA), according to the manufacturer's protocol. Reverse
transcription was conducted using 0.5 µg total RNA as a template
and the ThermoScript™ RT-PCR System & Platinum®
Taq DNA Polymerase kit (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. PCR was performed on 1 µl cDNA using the
following primers: Oct-4, forward 5′-CTGTAACCGGCGCCAGAA-3′ and
reverse 5′-TGCATGGGAGAGCCCAGA-3′ (Sigma-Aldrich); Pax-2, the
SABiosciences RT2 PCR primer set (LOC293992; Qiagen Sciences, LLC);
and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward
5′-TGGAGAGGCCTGCCAAGTA-3′ and reverse 5′-AAGAGTGGGAGTTGCTGTTG-3′
(Sigma-Aldrich). PCR cycles were run under standard conditions in
the T100™ Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and were as follows: Initial denaturation at 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. PCR
products were resolved in 2% agarose gel containing ethidium
bromide (both Sigma-Aldrich), and were then observed and documented
using a gel documentation unit (Gel Doc™ EZ System; Bio-Rad
Laboratories, Inc.).
Results
BrdU labeling of Tg rats
The Tg rats were injected subcutaneously with 50
µg/g BrdU in order to detect cellular proliferation.
Immunohistochemical analysis at day 60 following BrdU labelling
suggested that BrdU was successfully incorporated into the Tg rat
kidney tissues, as demonstrated by the co-localization of anti-BrdU
and anti-oct-4 antibodies (data not shown).
Injection of DT toxin
The hDTR was specifically expressed in the podocytes
of the Tg rats. After 60 days, the Tg rats were injected with 10
ng/kg DT and the presence of dead cells in the urine was detected
on day 7 following DT injection (data not shown) by staining the
urine with the Trypan blue dye. The numbers of podocytes in the
kidney were markedly depleted following treatment with DT. Kidney
tissue samples were collected at day 10 following DT injection. Day
10 was selected as the appropriate time to collect the kidney
samples based on the preliminary standardization protocol.
Identification of BrdU and Oct-4
positive cells
Kidney samples were collected from the Tg rats
(including the control rats) and immunohistochemical analyses were
conducted using anti-BrdU and anti-Oct-4 antibodies. BrdU-positive
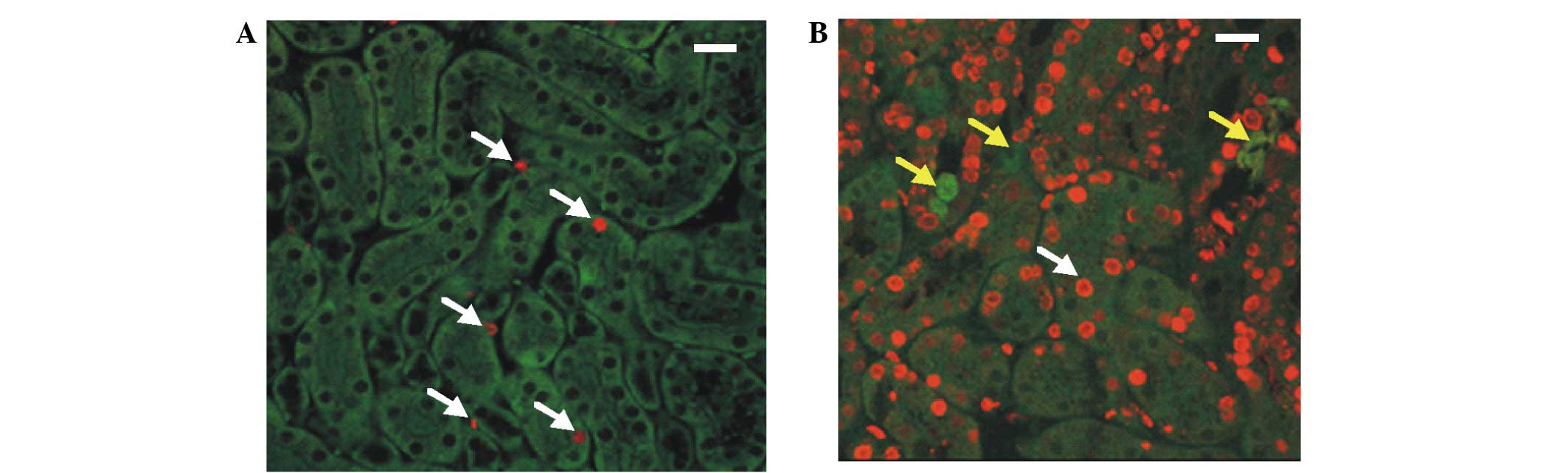
cells were detected in the control and DT-treated kidney tissue
sections; however, the number of BrdU-positive cells in the control
sections was markedly decreased, as compared with the DT-treated
kidney sections (Fig. 1A and B). In
addition, the DT-treated cells appeared to be renewed by the
BrdU-positive cells (Fig. 1B), and
these results were confirmed by immunohistochemical detection of
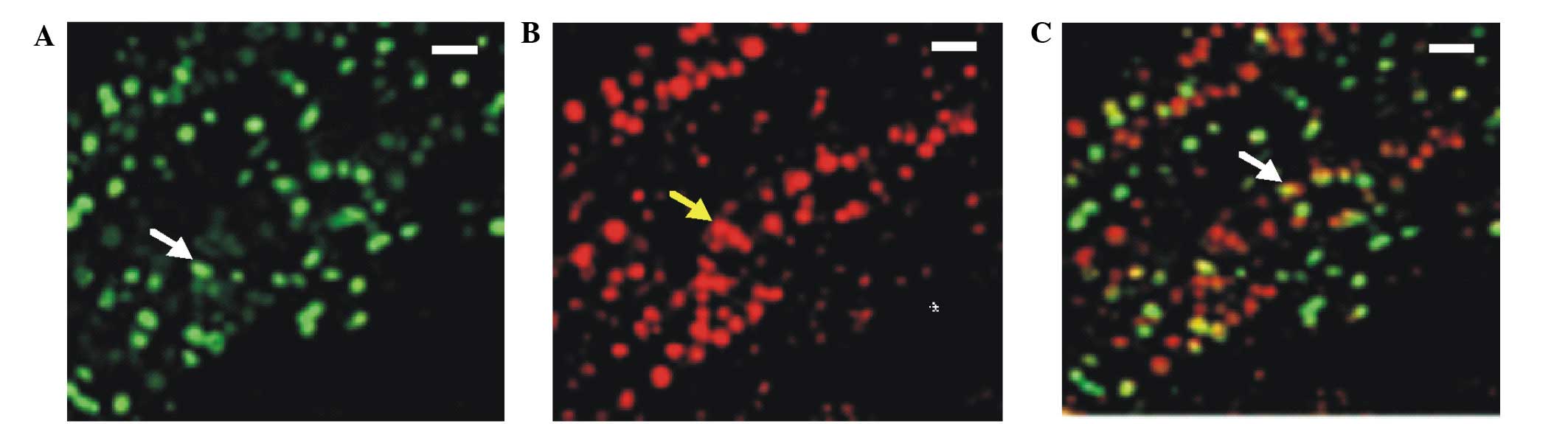
Oct-4 and BrdU co-expression. Therefore, these results suggested
that the majority of the BrdU-positive cells were kidney-derived
stem cells, since they also expressed Oct-4 (Fig. 2). These kidney-derived stem cells may
be involved in the restoration or renewal of the injured or
depleted cells.
Isolation and culture of
kidney-derived stem cells
In order to isolate the kidney-derived stem cells,
kidney samples from the Tg rat kidneys underwent cell culturing.
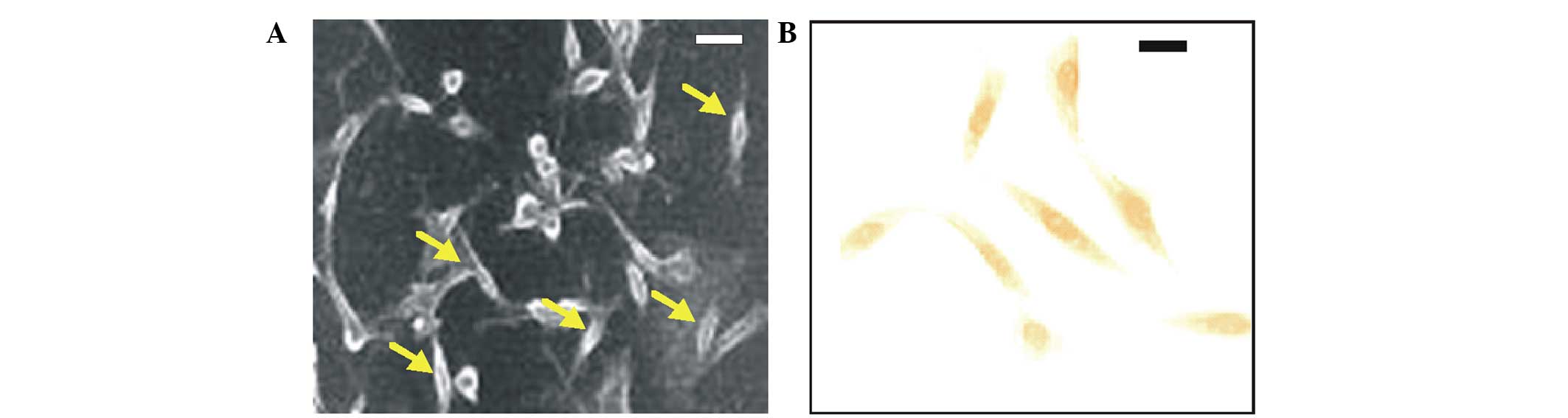
Following 5 weeks of culturing, the majority of the cell types were
non-viable. In addition, the cultured cells appeared monomorphic
and spindle-shaped when observed under a phase contrast microscope
(Fig. 3A).
Immunostaining and RT-PCR
analysis
In order to confirm that the isolated cells were
kidney-derived stem cells, immunostaining was conducted using the
anti-Oct-4 antibody. The Oct-4 positive cells exhibited a dark
brown coloration following staining with the DAB substrate
(Fig. 3B); thus suggesting that the
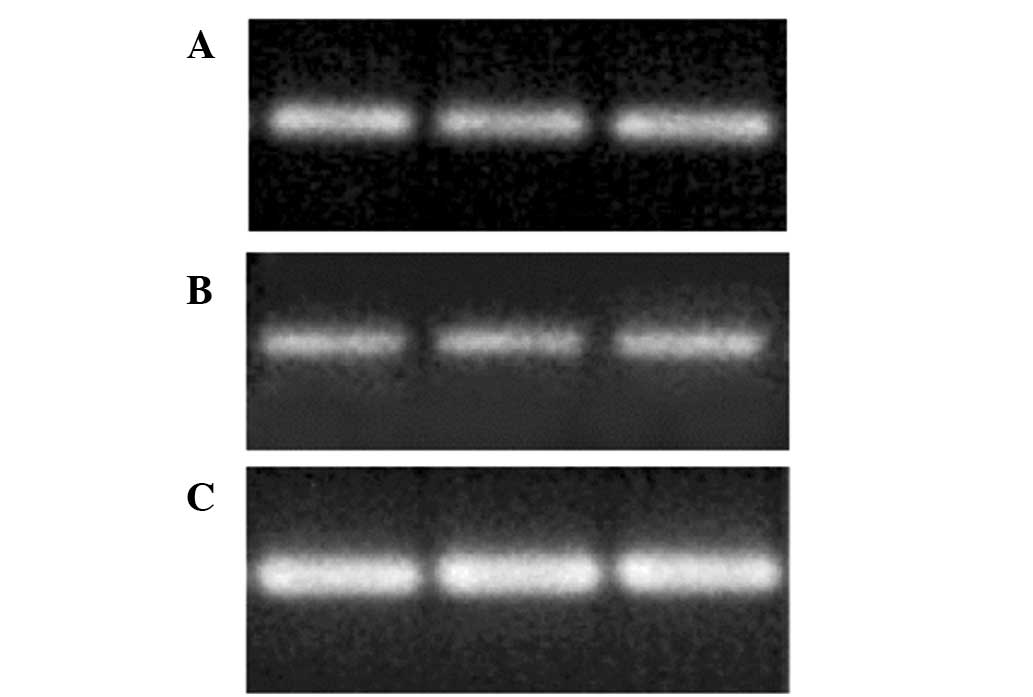
isolated cells were kidney-derived stem cells. Furthermore, RT-PCR
was performed in order to confirm whether the Oct-4 positive cells
had originated from the kidneys. Oct-4, Pax-2 and GAPDH mRNA
expression was detected in the cultured cells (Fig. 4). These results suggested that the
cultured unique cells were kidney-derived stem cells.
Discussion
Kidney injury and diseases are current a major
health concern. Regenerative medicine is the key to successful
treatment of those diseases. Basic scientific knowledge of tissue
specific stem cells is essential for the development of the field
of regenerative medicine (19).
Adult stem cells and their niches have been well characterized in
numerous organs, including bone marrow, the intestines, skin,
gastrointestinal mucosa, liver, prostate and brain (8–12).
However, the function of adult stem cells specific to the kidneys
remains unclear. In the present study, kidney-specific stem cells
were isolated and characterized using the stem cell markers Oct-4
and Pax-2.
In order to confirm the presence of kidney-specific
stem cells, the DT toxin was employed in the present experiments.
It is universally known that stem cells are involved in cellular
renewal and compensation when a specific tissue is under injury or
affected by diseases (3). In order
to establish a model of kidney injury, 10 ng/kg DT was administered
to the Tg rats. By contrast, a DT concentration of >50 µg/kg is
toxic and has been shown to result in rat mortality within 10 days
(43). Therefore, it was crucial to
standardize the lower minimum concentration of DT. Furthermore, it
has been reported that a DT concentration of 25 ng/kg resulted in
animal survival durations of up to 28 days (43). Hence, the DT concentration selected
for the present experiment was 10 ng/kg, based upon careful
standardization (43).
A BrdU labeling retention assay was conducted in
order to confirm the presence of kidney-derived stem cells, and the
results clearly indicated that the kidney samples contained
tissue-specific stem cells. However, the signals obtained by the
BrdU labeling retention assay suggested that not all of the
BrdU-positive cells were stem cells. Therefore, to confirm the
results of the preliminary BrdU labeling retention assay,
immunohistochemical analyses were conducted using anti-Oct-4 and
anti-BrdU antibodies. The results of the immunohistochemical
analyses indicated that the majority of the BrdU-positive cells
were also Oct-4 positive; thus confirming that the BrdU-positive
cells were stem cells. Therefore, on the basis of the preliminary
data and subsequent immunohistochemistry results, it was concluded
that the kidney tissues contained organ-specific stem cells.
Notably, the kidney tissue depletion caused by 10 ng/kg DT was
restored by the proliferation of the kidney-derived stem cells.
This recovery may be due to the low concentration of DT injected
into the Tg rats.
In order to isolate the kidney stem cells, in
vitro cell culturing was performed. Certain cells behaved like
stem cells following a prolonged interval. After 5 weeks of cell
culture, the majority of the cells were non-viable. However,
certain unique cell types were observed in the culture medium that
appeared monomorphic and spindle-like in shape when observed under
the phase contrast microscope. In order to confirm that these cells
were stem cells, immunostaining was conducted using an anti-Oct-4
antibody. The results of the immunostaining assay clearly indicated
that the unique cell types were stem cells. In order to validate
this, and to confirm that the unique cell types were kidney-derived
stem cells, RT-PCR was performed. The mRNA expression profile of
Oct-4 and Pax-2 in the unique cell types were evaluated. Pax-2 is a
transcription factor that is important for kidney development. Both
Oct-4 and Pax-2 were expresssed in the unique cell types; thus
suggesting that they were kidney-specific stem cells. On the basis
of these results, it was confirmed that the unique cell types were
kidney-derived stem cells.
A previous study reported the lack of a definitive
marker for kidney stem cells (13).
The identification of kidney-specific markers is therefore
required, which is crucial for the isolation of adult kidney stem
cells and the elucidation of their role within the organ. The
unique cells isolated in the present study may be used to identify
accurate kidney-specific stem cell markers in future studies.
In conclusion, the results of the present study
demonstrated that kidney stem cells may be involved in the
restoration of cells injured by DT. In addition, the unique cell
type was isolated and behaved in a manner resembling that of
kidney-derived stem cells. The characteristic and identity of the
unique cells was identified, and these cells may provide a useful
cell line for studying the fundamental characteristics of kidney
stem cells, as well as identifying kidney-specific stem cell
markers.
Acknowledgements
The present study was supported by the Youth
Foundation of the Second Hospital of Shandong University (grant no.
Y2013010012). The authors would like to thank the Department of
Nephrology at the Shandong Jiaotong Hospital (Jinan, China) for
supplying the rats and supporting the project.
References
|
1
|
Lindvall O, Kokaia Z and Martinez-Serrano
A: Stem cell therapy for human neurodegenerative disorders - how to
make it work. Nat Med. 10:S42–S50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Segers VFM and Lee RT: Stem-cell therapy
for cardiac disease. Nature. 451:937–942. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weissman IL: Stem cells: Units of
development, units of regeneration and units in evolution. Cell.
100:157–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cotsarelis G, Cheng SZ, Dong G, Sun TT and
Lavker RM: Existence of slow-cycling limbal epithelial basal cells
that can be preferentially stimulated to proliferate: Implications
on epithelial stem cells. Cell. 57:201–209. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taylor G, Lehrer MS, Jensen PJ, Sun TT and
Lavker RM: Involvement of follicular stem cells in forming not only
the follicle but also the epidermis. Cell. 102:451–461. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lavker RM and Sun TT: Epidermal stem
cells: Properties, markers, and location. Proc Natl Acad Sci USA.
97:13473–13475. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beltrami AP, Barlucchi L, Torella D, Baker
M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, et
al: Adult cardiac stem cells are multipotent and support myocardial
regeneration. Cell. 114:763–776. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alison MR, Poulsom R and Forbes SJ: Update
on hepatic stem cells. Liver. 21:367–373. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bernard-Kargar C and Ktorza A: Endocrine
pancreas plasticity under physiological and pathological
conditions. Diabetes. 50(Suppl 1): S30–S35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Forbes SJ, Poulsom R and Wright NA:
Hepatic and renal differentiation from blood-borne stem cells. Gene
Ther. 9:625–630. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morrison SJ, White PM, Zock C and Anderson
DJ: Prospective identification, isolation by flow cytometry and in
vivo self-renewal of multipotent mammalian neural crest stem cells.
Cell. 96:737–749. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wright NA: Epithelial stem cell repertoire
in the gut: Clues to the origin of cell lineages, proliferative
units and cancer. Int J Exp Pathol. 81:117–143. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gupta S and Rosenberg ME: Do stem cells
exist in the adult kidney? Am J Nephrol. 28:607–613. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Drummond IA, Mukhopadhyay D and Sukhatme
VP: Expression of fetal kidney growth factors in a kidney tumor
line: Role of FGF2 in kidney development. Exp Nephrol. 6:522–533.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elger M, Hentschel H, Litteral J, Wellner
M, Kirsch T, Luft FC and Haller H: Nephrogenesis is induced by
partial nephrectomy in the elasmobranch Leucoraja erinacea.
J Am Soc Nephrol. 14:1506–1518. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salice CJ, Rokous JS, Kane AS and
Reimschuessel R: New nephron development in goldfish (Carassius
auratus) kidneys following repeated gentamicin-induced
nephrotoxicosis. Comp Med. 51:56–59. 2001.PubMed/NCBI
|
|
17
|
Poulsom R, Forbes SJ, Hodivala-Dilke K,
Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M,
Cook T, et al: Bone marrow contributes to renal parenchymal
turnover and regeneration. J Pathol. 195:229–235. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gupta S, Verfaillie C, Chmielewski D, Kim
Y and Rosenberg ME: A role for extrarenal cells in the regeneration
following acute renal failure. Kidney Int. 62:1285–1290. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin F, Cordes K, Li L, Hood L, Couser WG,
Shankland SJ and Igarashi P: Hematopoietic stem cells contribute to
the regeneration of renal tubules after renal ischemia-reperfusion
injury in mice. J Am Soc Nephrol. 14:1188–1199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kale S, Karihaloo A, Clark PR, Kashgarian
M, Krause DS and Cantley LG: Bone marrow stem cells contribute to
repair of the ischemically injured renal tubule. J Clin Invest.
112:42–49. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szczypka MS, Westover AJ, Clouthier SG,
Ferrara JL and Humes HD: Rare incorporation of bone marrow-derived
cells into kidney after folic acid-induced injury. Stem Cells.
23:44–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herzlinger D, Koseki C, Mikawa T and
Al-Awqati Q: Metanephric mesenchyme contains multipotent stem cells
whose fate is restricted after induction. Development. 114:565–572.
1992.PubMed/NCBI
|
|
23
|
Oliver JA, Maarouf O, Cheema FH, Martens
TP and Al-Awqati Q: The renal papilla is a niche for adult kidney
stem cells. J Clin Invest. 114:795–804. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dyce PW, Zhu H, Craig J and Li J: Stem
cells with multilineage potential derived from porcine skin.
Biochem Biophys Res Commun. 316:651–658. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yeom YI, Fuhrmann G, Ovitt CE, Brehm A,
Ohbo K, Gross M, Hübner K and Schöler HR: Germline regulatory
element of Oct-4 specific for the totipotent cycle of embryonal
cells. Development. 122:881–894. 1996.PubMed/NCBI
|
|
26
|
Mitalipov SM, Kuo HC, Hennebold JD and
Wolf DP: Oct-4 expression in pluripotent cells of the rhesus
monkey. Biol Reprod. 69:1785–1792. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kehler J, Tolkunova E, Koschorz B, Pesce
M, Gentile L, Boiani M, Lomelí H, Nagy A, McLaughlin KJ, Schöler HR
and Tomilin A: Oct4 is required for primordial germ cell survival.
EMBO Rep. 5:1078–1083. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang Y, Vaessen B, Lenvik T, Blackstad M,
Reyes M and Verfaillie CM: Multipotent progenitor cells can be
isolated from postnatal murine bone marrow, muscle and brain. Exp
Hematol. 30:896–904. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schwartz RE, Reyes M, Koodie L, Jiang Y,
Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS and Verfaillie CM:
Multipotent adult progenitor cells from bone marrow differentiate
into functional hepatocyte-like cells. J Clin Invest.
109:1291–1302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reyes M, Dudek A, Jahagirdar B, Koodie L,
Marker PH and Verfaillie CM: Origin of endothelial progenitors in
human postnatal bone marrow. J Clin Invest. 109:337–346. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu H, Fang D, Kumar SM, Li L, Nguyen TK,
Acs G, Herlyn M and Xu X: Isolation of a novel population of
multipotent adult stem cells from human hair follicles. Am J
Pathol. 168:1879–1888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baal N, Reisinger K, Jahr H, Bohle RM,
Liang O, Münstedt K, Rao CV, Preissner KT and Zygmunt MT:
Expression of transcription factor Oct-4 and other embryonic genes
in CD133 positive cells from human umbilical cord blood. Thromb
Haemost. 92:767–775. 2004.PubMed/NCBI
|
|
34
|
D'Ippolito G, Diabira S, Howard GA, Menei
P, Roos BA and Schiller PC: Marrow-isolated adult multilineage
inducible (MIAMI) cells, a unique population of postnatal young and
old human cells with extensive expansion and differentiation
potential. J Cell Sci. 117:2971–2981. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Davis SF, Hood J, Thomas A and Bunnell BA:
Isolation of adult rhesus neural stem and progenitor cells and
differentiation into immature oligodendrocytes. Stem Cells Dev.
15:191–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Romagnani P, Annunziato F, Liotta F,
Lazzeri E, Mazzinghi B, Frosali F, Cosmi L, Maggi L, Lasagni L,
Scheffold A, et al: CD14+ CD34 low cells with stem cell phenotypic
and functional features are the major source of circulating
endothelial progenitors. Circ Res. 97:314–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Romero-Ramos M, Vourc'h P, Young HE, Lucas
PA, Wu Y, Chivatakarn O, Zaman R, Dunkelman N, el-Kalay MA and
Chesselet MF: Neuronal differentiation of stem cells isolated from
adult muscle. J Neurosci Res. 69:894–907. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Trosko JE and Tai MH: Adult stem cell
theory of the multi-stage, multi-mechanism theory of
carcinogenesis: Role of inflammation on the promotion of initiated
stem cells. Contrib Microbiol. 13:45–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tsai MS, Hwang SM, Tsai YL, Cheng FC, Lee
JL and Chang YJ: Clonal amniotic fluid-derived stem cells express
characteristics of both mesenchymal and neural stem cells. Biol
Reprod. 74:545–551. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiao J, Nan Z, Motooka Y and Low WC:
Transplantation of a novel cell line population of umbilical cord
blood stem cells ameliorates neurological deficits associated with
ischemic brain injury. Stem Cells Dev. 14:722–733. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou YF, Fang F, Fu JR, Dong YS, Ye DY,
Shu SN, Zhen H and Li G: An experimental study on astrocytes
promoting production of neural stem cells derived from mouse
embryonic stem cells. Chin Med J (Engl). 118:1994–1999.
2005.PubMed/NCBI
|
|
42
|
Pappenheimer AM Jr: The story of a toxic
protein, 1888–1992. Protein Sci. 2:292–298. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wharram BL, Goyal M, Wiggins JE, Sanden
SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman
LB and Wiggins RC: Podocyte depletion causes glomerulosclerosis:
Diphtheria toxin-induced podocyte depletion in rats expressing
human diphtheria toxin receptor transgene. J Am Soc Nephrol.
16:2941–2952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maeshima A, Sakurai H and Nigam SK: Adult
kidney tubular cell population showing phenotypic plasticity,
tubulogenic capacity and integration capability into developing
kidney. J Am Soc Nephrol. 17:188–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gupta S, Verfaillie C, Chmielewski D, Kren
S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y,
et al: Isolation and characterization of kidney-derived stem cells.
J Am Soc Nephrol. 17:3028–3040. 2006. View Article : Google Scholar : PubMed/NCBI
|