Introduction
Acute myocardial infarction (AMI) is a costly
cardiovascular disease with a high mortality rate (1). The average hospitalization costs are
high (2). Furthermore, the
morbidity, disability and mortality of heart failure following
myocardial infarction severely affects the quality of life of the
patient and community health care resources. Therefore, measures
that can effectively hinder the damage associated with heart
failure are important. AMI is the predominant cause of myocardial
infarction, and left ventricular remodelling (LVM) occurs at a
later stage (3). LVM refers to the
pathological changes in the immune activation state of the body
caused by large amounts of cytokines secreted by myocardial cells,
under the influence of injury, ventricular wall stress, oxidative
stress and partial activation of the neuroendocrine system, which
induces myocardial collagen deposition and changes in collagen type
and collagen degradation (4). Recent
studies on LVM reported the presence of stem cells in the
myocardium, which have the ability to differentiate into various
cells (5,6). A small number of stem cells are able to
express c-kit and nanog surface markers. These cardiac stem cells
can differentiate are able to cardiomyocytes both in vitro
and in vivo (7), and cardiac
stem cells have been suggested to provide inherent damage repair
and reserve ability in myocardial cells. However, analyses on the
expression of cardiac stem cells, as well as the regenerative
capacity of the myocardium and cardiac-associated factors in
ventricular remodelling are limited (8,9).
Furthermore, whether the number of myocardial stem cells increase
or decrease subsequent to heart failure, and whether myocardial
regeneration is enhanced or diminished remains to be elucidated.
The present study established a theoretical basis for potential
myocardial regeneration and gene therapy following heart failure by
examining the pathological changes and dynamic expression of c-kit-
and nanog-positive stem cells.
Materials and methods
Animal groups
A total of 40 Sprague-Dawley male rats, weighing
230–250±2.5 g, were provided by the Animal Centre of the Xinxiang
Medial University (Xinxiang, China). The rats were randomly divided
into a normal control (CON) group (n=15) and a heart failure
model group (n=25). The present study was performed in
strict accordance with the recommendations in the eighth edition
2010 Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health (10,11). The
animal use protocol was reviewed and approved by the Institutional
Animal Care and Use Committee of the Xinxiang Central Hospital
(Xinxiang, China).
Heart failure model
Rats in the heart failure group were
intraperitoneally administered with 4 mg/kg adriamycin (ADR; Sangon
Biotech Co., Ltd., Shanghai, China) weekly for six weeks. Rats in
the CON group were given the equivalent amount of saline. After
eight weeks, both groups were subjected to echocardiography using
an ACUSON SC2000 (Siemens AG, Munich, Germany), with left
ventricular ejection fraction (LVEF) <45% being the criterion of
heart failure (12).
Material collection
The rats identified to have successfully developed
heart failure following treatment with ADR were anesthetized using
10% chloral hydrate (0.3 ml/100 g [lethal dose); Sangon Biotech
Co., Ltd.] and then immediately subjected to thoracotomy. The rat
hearts were rapidly eviscerated, and residual blood was removed
using ice-cold phosphate-buffered saline (PBS) buffer. The large
blood vessels and surrounding tissues were removed with scissors.
Approximately one half of the hearts were packed in cryogenic vials
(Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China),
rapidly frozen with liquid nitrogen and transferred to a −80°C
refrigerator. The remaining half was placed in 4% paraformaldehyde
(Sangon Biotech Co., Ltd.) for fixation for 12 h, followed by
gradient dehydration with 20 and 30% sucrose (myocardial cells can
sink to the bottom) purchased from Sangon Biotech Co., Ltd. Tissue
sections (thickness, 10 µm using a microtome from Dingguo
Changsheng Biotechnology Co., Ltd.) were frozen using a cryostat
frozen section machine (CM1900; Leica Microsystems GmbH, Wetzlar,
Germany), and used for masson staining (cat no. HT15-1KT;
Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 30 min,
immunohistochemistry and immunofluorescence.
Immunohistochemistry
The frozen tissue sections were heated in an oven
for 30 min at 37°C, washed three times with PBS for 5 min each
time, maintained for 15 min in 0.3% Triton-X-100 PBS liquid and
soaked in a mixture of 30% hydrogen peroxide and methanol (1:50
ratios) all Sangon Biotech Co., Ltd) for 30 min to block endogenous
peroxidase activity. The tissue sections were treated at room
temperature for 20 min with goat serum (Sijiqing, Hangzhou, China)
containing rabbit anti-rat nanog polyclonal antibody (1:200; cat
no. sc-376915; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
mouse anti-rat c-kit monoclonal antibody (1:100; cat no. sc-17806;
Santa Cruz Biotechnology, Inc.). The serum was subsequently
centrifuged at 5,000 × g for 3 min at 37°C. The tissue sections
were incubated overnight at 4°C in a wet box (Dingguo Changsheng
Biotechnology Co., Ltd.) and then rewarmed for 30 min at room
temperature. The tissue sections were treated with reagent B,
incubated for 15 min at 37°C, washed three times with PBS for 5 min
each time, treated with reagent C (both reagents purchased from
Dingguo Changsheng Biotechnology Co., Ltd.), incubated for 15 min
at 37°C and washed three times with PBS for 5 min each time. The
sections were then treated with diaminobenzidine (Sangon Biotech
Co., Ltd.) at room temperature, thoroughly rinsed with distilled
water and counterstained in hematoxylin (Sangon Biotech Co., Ltd.).
Images were captured using an Olympus AX80 (Olympus Corporation,
Tokyo, Japan) following dehydration by gradient elution using
ethanol, and neutral gum mounting (Sangon Biotech Co., Ltd.). The
CON group was performed by a dilution of primary antibody instead
of primary antibody during immunohistochemistry.
Immunofluorescence
Frozen tissue sections were heated in an oven for 30
min at 37°C. Following three washes with PBS for 5 min each time,
the sections were maintained for 15 min in 0.3% Triton-X-100 PBS
liquid, treated at room temperature for 20 min with goat serum
containing the rabbit anti-rat nanog polyclonal antibody (1:200)
and the mouse anti-rat c-kit monoclonal antibody (1:100), and
incubated overnight at 4°C in a wet box. The tissue sections were
subsequently rewarmed for 20 min at room temperature in buffer
(Sangon Biotech Co., Ltd.) containing cyanine 3-labelled goat
anti-rabbit (1:200; cat no. A0516; Beyotime Institute of
Biotechnology, Haimen, China) and fluorescein
isothiocyanate-labelled goat anti-mouse (1:100; cat no. P0196;
Beyotime Institute of Biotechnology, Shanghai, China), and
incubated for 1 h at 37°C. The nuclei were counterstained in DAPI
(Sangon Biotech Co., Ltd.) for 5 min. The tissue sections were then
mounted with antifade mounting medium (Dingguo Changsheng
Biotechnology Co., Ltd.), and observed under a fluorescence
microscope (Olympus AX80; Olympus Corporation). The CON group was
performed by a dilution of primary antibody instead of primary
antibody during immunohistochemistry.
Reverse transcription-polymerase chain
reaction
Refrigerated myocardial tissue samples (200 mg) were
obtained from the same site of heart tissues cut in both groups and
then homogenized (LM12-XSB-88; Xihuayi, Beijing, China) with liquid
nitrogen. This process was conducted in strict accordance with the
manufacturer's protocol in Reverse Transcription kit (cat no.
RR036A; Takara Biotechnology Co., Ltd., Dalian, China).
Thermocycling conditions included preliminary denaturation at 95°C
for 2 min, 35 cycles each involving denaturation at 95°C for 30
sec, annealing at 60°C for 30 sec, extension at 72°C for 3 min. Gel
electrophoresis (Tocan, Shanghai, China). Total RNA Extraction kit
(Takara Biotechnology Co., Ltd.) was used for RNA extraction (50 µg
total RNA was extracted). Target gene and reference gene primers
(Sangon Biotech Co., Ltd.) were as follows: c-kit forward,
5′-CTAGCCAGAGACATCAGGA-3′, and reverse, 5′-CCATAGGACCAGACATCAC-3′;
nanog forward, 5′-AGAAGATGCGGACTGTGTTC-3′, and reverse,
5′-GCTCAGGTTCAGAATGGTAGA-3′; and GAPDH forward,
5′-ATGACTCTACCCACGGCAAG-3′, and reverse,
5′-TACTCAGCACCAGCATCACC-3′. The results were observed and analyzed
using a Tocan 820 gel imaging system (Tocan), which was based on
the integrated absorbance value. c-kit/GAPDH and nanog/GAPDH refer
to the relative expression levels of c-kit and nanog mRNA,
respectively. The results were based on the integrated absorbance
value, and the 2−ΔΔcq method was used for quantification
of the results (13).
Statistical analysis
The data were expressed as the mean ± standard
deviation. All data analyses were carried out using SPSS version
17.0 (SPSS, Inc., Chicago, IL, USA). Student's paired t-test was
used to determine the statistical differences in both groups.
P<0.05 was considered to indicate a statistically significant
result.
Results
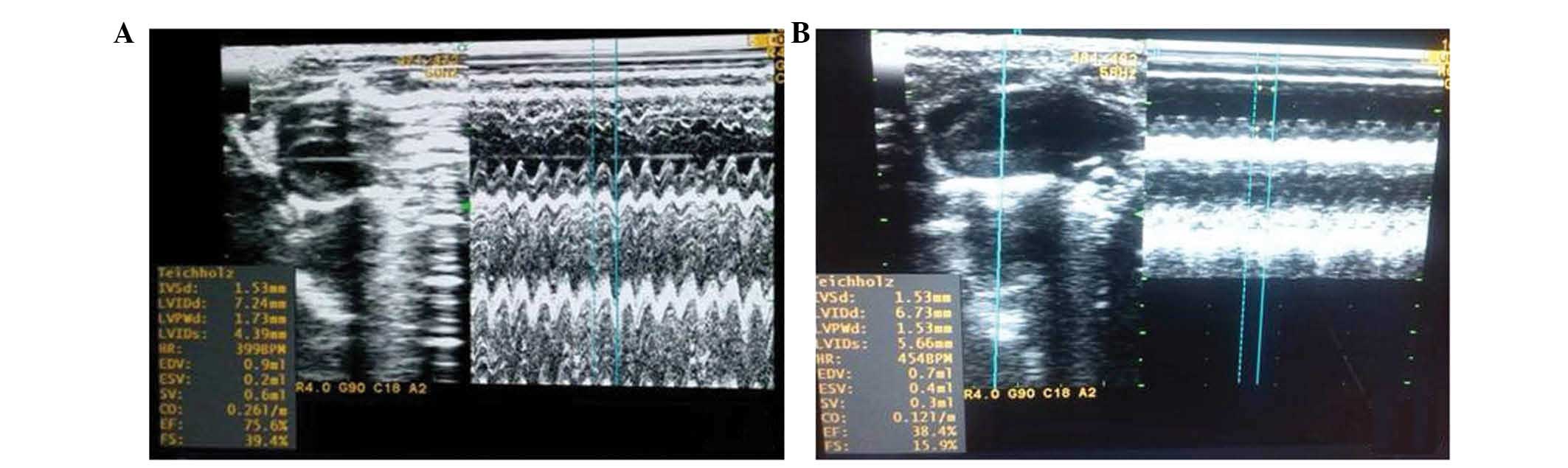
Changes in cardiac function in rats
with heart failure
At the end of the eighth week, the rats in both the
ADR and CON groups were subjected to echocardiography. The results
demonstrated that cardiac function in the ADR group significantly
decreased. Compared with the CON group, left ventricular diastolic
diameter and left ventricular systolic diameter were significantly
higher (P<0.05), whereas LVEF and left ventricular fractional
shortening were significantly lower (P<0.05), which indicated
left ventricular systolic dysfunction (Table I). The ADR group proved to be a
successful model of heart failure compared with the CON group, as
shown in Fig. 1.
 | Table I.Echocardiographic indices of cardiac
function of the rats in both the ADR and CON groups at the end of
the eighth week of treatment with ADR (results are expressed as
means ± standard deviation). |
Table I.
Echocardiographic indices of cardiac
function of the rats in both the ADR and CON groups at the end of
the eighth week of treatment with ADR (results are expressed as
means ± standard deviation).
| Group | LVDD (mm) | LVSD (mm) | LVEF (%) | LVFS (%) |
|---|
| CON (n=15) | 3.972±1.463 | 2.102±1.421 | 70.94±1.126 | 34.94±1.122 |
| ADR (n=30) |
6.726±0.523a |
5.73±0.610a |
34.64±9.105a |
14.76±3.595a |
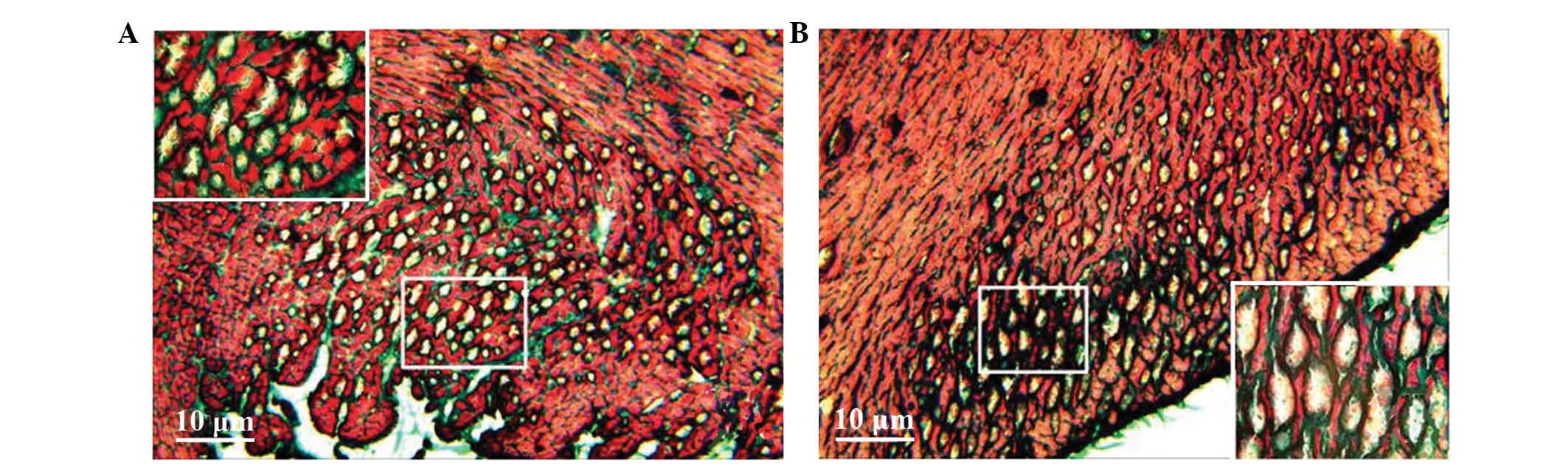
Pathological changes in the myocardium
of rats with heart failure
Masson staining showed myocardial cell disarray, a
small number of fractures and an increase in collagen fibres in the
intercellular matrix in the heart failure model group. Furthermore,
numerous vacuoles of various sizes appeared in the intercellular
matrix of the subendocardial myocardium and subepicardial
myocardium, and a small number of vacuoles were similar with lumen
sections (Fig. 2). Conversely,
normal myocardial cells exhibited less interstitial collagen fibres
and almost no vacuolation in the subendocardial myocardium and
subepicardial myocardium (Fig.
2).
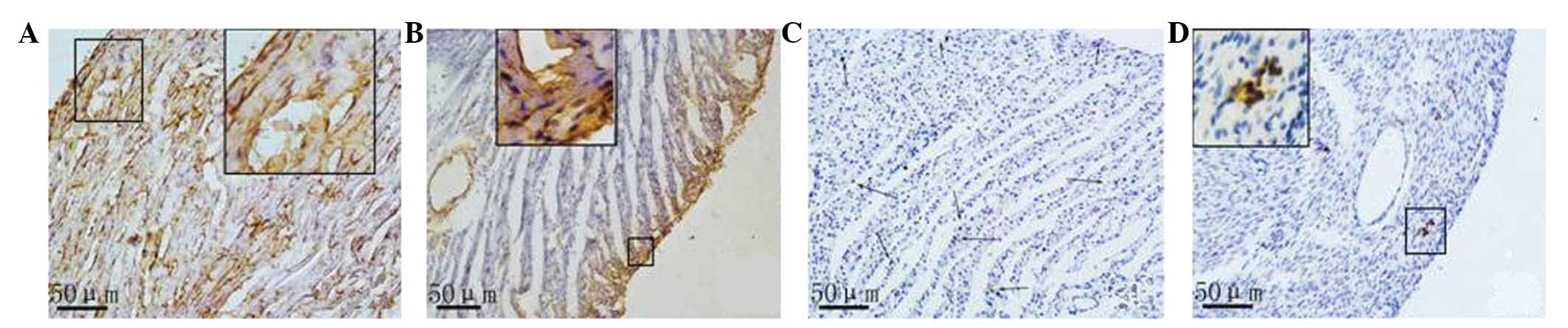
Immunohistochemical analysis of the
protein expression of c-kit and nanog
Based on the results of the immunohistochemical
analysis, a small amount of nanog and c-kit proteins were expressed
in the rat myocardial tissue samples both in the CON and ADR
groups. Nanog expression in the normal myocardial tissue samples
was lower compared with that in the cardiomyocytes and vascular
endothelial cells in the control group. A smaller amount of nanog
expression in normal myocardial tissue existed in cardiomyocytes,
endothelial cells and stem cells. Myocardial cells with positive
nanog expression were scattered or in clusters was observed in the
CON group. Nanog was expressed in the majority of the vascular
endothelial cells. Nanog-positive stem cells were often single or
in clusters, and they were located between myocytes or around the
small blood vessels. In the myocardial tissue samples of the rats
with heart failure, three types of nanog-positive cells
(cardiomyocytes, endothelial cells, small and round stem cells)
decreased, and the subendocardial myocardium and subepicardial
myocardium presented a positive distribution (Fig. 3).
In the myocardial tissue samples of both rats with
normal hearts and those with heart failure, c-kit-positive cells
were expressed predominantly in the small round or oval cells that
were present in small clusters. No positive c-kit expression was
observed in cardiomyocytes or endothelial cells. Compared with the
normal myocardium, the number of c-kit-positive cells was markedly
reduced in the myocardium of rats with heart failure.
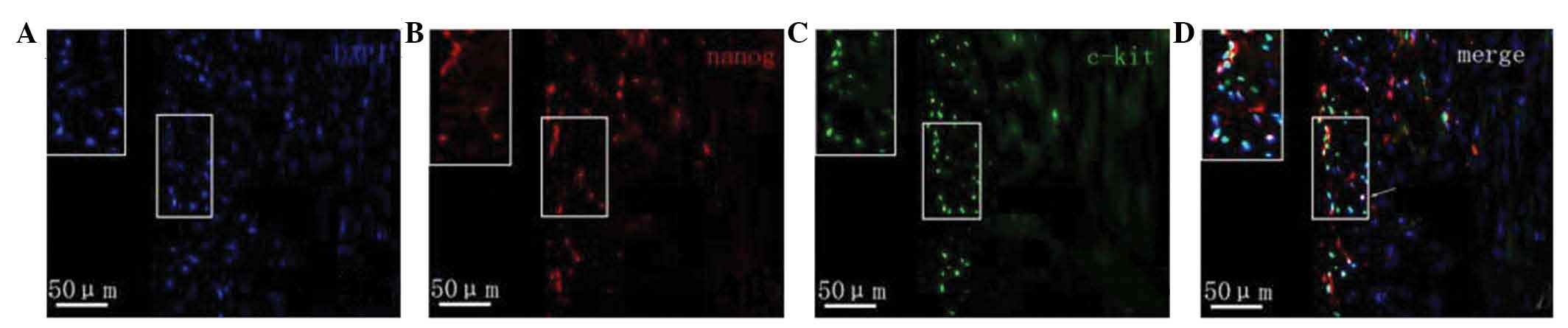
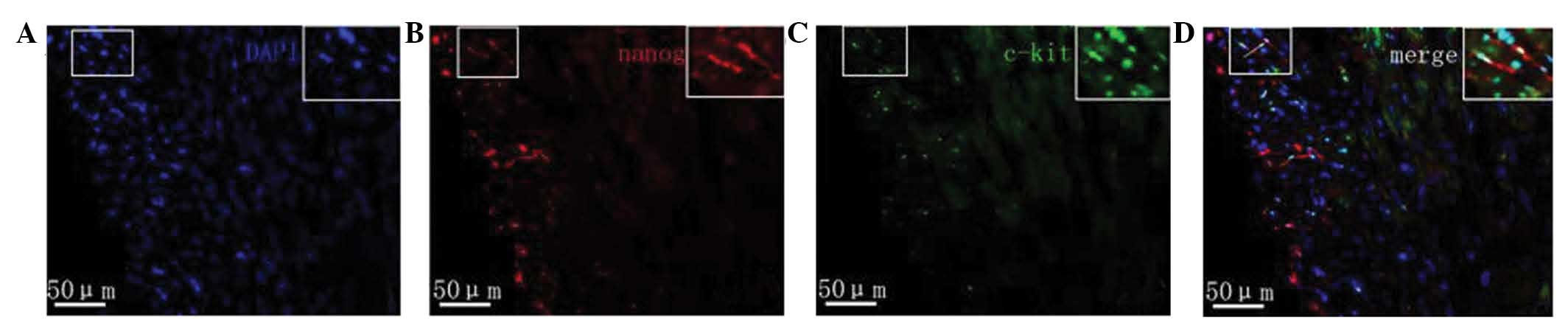
Protein expression of c-kit and nanog
as determined by immunofluorescence
Fluorescence microscopy revealed that in the
myocardial tissue samples of the normal group nanog protein
expression was increased, specifically in the cardiomyocytes,
vascular endothelium and small round cells. Cells with strong
positive expression were unevenly distributed. Nanog-positive cells
in the myocardial tissue samples of rats with heart failure were
predominantly distributed in the subendocardial myocardium and
subepicardial myocardium. The expression of nanog in the
subendocardial myocardium and subepicardial myocardium was markedly
lower compared with that of the normal myocardium, and its
expression in the subendocardial myocardium was downregulated
compared with that in the subepicardial myocardium (Fig. 4). The c-kit protein was predominantly
expressed in small cells, which were often in clusters distributed
in the myocardium rather than evenly distributed. c-kit-positive
cells were predominantly located in the myocardium of the
subepicardial myocardium. Compared with normal myocardial tissue,
the number of c-kit-positive cells in the rats with heart failure
was markedly reduced. c-kit fluorescence was not observed in
cardiomyocytes and endothelial cells. Furthermore, a small number
of cells co-expressed both the c-kit and nanog genes (Fig. 5).
Discussion
Heart failure, which has high incidence, morbidity
and mortality rates, is the final stage of the majority of
cardiovascular diseases (14,15).
Therefore, the treatment of chronic heart failure remains a severe
challenge faced by medical workers. In recent years, a growing
number of studies have demonstrated that cell proliferation exists
in myocardial tissues, and that cardiac stem cells capable of
differentiation are also isolated from myocardial tissues (16,17).
These stem cells have the ability to differentiate both in
vitro and in vivo into cardiomyocytes, providing a
possible therapeutic strategy for heart failure treatment. Ishikawa
et al (18) reported the
mitosis phenomenon of cardiac myocytes in normal adult rats was
found, and c-kit was one of the heart surface markers, and can be
found in embryonic, neonatal and adult mammalian hearts. The study
(18) also demonstrated that the
number of c-kit may reflect the developmental or physiological
stage of such settlement cells (normal cells which exist in certain
parts of the bodies) (19).
Recently, c-kit-positive cells have been used in clinical trials
for the preliminary treatment of heart diseases (20), including myocardial regeneration
originated from coronary heart ball cell following myocardial
infarction (21), and functional
analysis of cardiac stem cells in ischemic myocardium (22). These stem cells have been used for
the preliminary treatment of heart diseases. In 2003, Chambers
et al (23) and Mitsui et
al (24) reported that the nanog
gene, which is predominantly expressed in undifferentiated
embryonic stem cells, partial adult cells and tumor cells,
facilitates embryonic stem cell self-renewal, maintains its
undifferentiated state and promotes its proliferation (25). Furthermore, the nanog gene may also
be used for regulating and promoting myocardial cells from
re-entering the division cycle (26).
The results of the present study demonstrated that
the number of c-kit- and nanog-positive cells was reduced in rats
with heart failure, the corresponding proteins and mRNA levels of
positive c-kit and nanog in myocardial tissue samples in normal
adult rats and rats with heart failure were also decreased. The
results of the present study also demonstrated that c-kit and nanog
were correlated with the degree of ventricular remodelling, which
demonstrated that neutrophils are able to secrete a large number of
inflammatory cells (27), and
inhibit the expression of c-kit and nanog during early inflammation
(28,29). In the present study, this
pathological process was verified from the molecular and genetic
level. These results were concordant with those of Senyo et
al (22), who reported that the
annual renewal rate of the aforementioned stem cells in adult
hearts is 4–10%, whereas the inhibition, while the inhibition of
c-kit and nanog expression levels could decrease this renewal rate.
Therefore, cardiac stem cells with c-kit and nanog surface markers
are important for cardiac self-renewal and self-repair (30,31).
The immunofluorescence analysis demonstrated that a
small number of cells co-expressed c-kit and nanog, results which
were concordant with those of our previous studies (32,33).
Heart failure not only severely damages the heart muscle cells, it
also reduces the number of c-kit-positive cells, nanog-positive
cells and stem cells (34,35). These results may be observed for the
following reasons: i) Environmental changes in the myocardium
following heart failure can result in conditions that are no longer
suitable for stem cell growth and proliferation in rats; ii) cell
apoptosis may occur following heart failure; iii) ADR may be toxic
for the modelling of cardiac stem cells (36). Regardless of these factors, the
reduction of both cardiac stem cells and associated substances that
promote the formation, development and transformation of stem
cells, are not conducive to myocardial injury repair. However,
whether the small number of cells with the two genetic materials
(c-kit and nanog) reflects the diversity of stem cell function or
represents two different cardiac stem cells requires further
investigation.
References
|
1
|
Khan R and Ly HQ: Transradial percutaneous
coronary interventions in acute coronary syndrome. Am J Cardiol.
114:160–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amarenco P, Abboud H, Labreuche J, Arauz
A, Bryer A, Lavados PM, Massaro A, Munoz Collazos M, Steg PG,
Yamout BI and Vicaut E: OPTIC Registry Investigators: Impact of
living and socioeconomic characteristics on cardiovascular risk in
ischemic stroke patients. Int J Stroke. 9:1065–1072. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rivera-Fernández R, Arias-Verdú MD,
García-Paredes T, Delgado-Rodríguez M, Arboleda-Sánchez JA,
Aguilar-Alonso E, Quesada-García G and Vera-Almazán A: Prolonged QT
interval in ST-elevation myocardial infarction and mortality: New
prognostic scale with QT, Killip and age. J Cardiovasc Med
(Hagerstown). 17:11–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith AJ, Lewis FC, Aquila I, Waring CD,
Nocera A, Agosti V, NadalGinard B, Torella D and Ellison GM:
Isolation and characterization of resident endogenous c-Kit+
cardiac stem cells from the adult mouse and rat heart. Nat Protoc.
9:1662–1681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DiStefano R, Felice F, Pini S, Mazzotta G,
Bovenzi FM, Bertoli D, Abelli M, Borelli L, Cardini A, Lari L, et
al: Impact of depression on circulating endothelial progenitor
cells in patients with acute coronary syndromes: A pilot study. J
Cardiovasc Med (Hagerstown). 15:353–359. 2014.PubMed/NCBI
|
|
6
|
Wöhrle J, von Scheidt F, Schauwecker P,
Wiesneth M, Markovic S, Schrezenmeier H, Hombach V, Rottbauer W and
Bernhardt P: Impact of cell number and microvascular obstruction in
patients with bone-marrow derived cell therapy: Final results from
the randomized, double-blind, placebo controlled intracoronary Stem
Cell therapy in patients with Acute Myocardial Infarction (SCAMI)
trial. Clin Res Cardiol. 102:765–770. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo H, Li Q, Pramanik J, Luo J and Guo Z:
Nanog expression in heart tissues induced by acute myocardial
infarction. Histol Histopathol. 29:1287–1293. 2014.PubMed/NCBI
|
|
8
|
Carvalho JL, Braga VB, Melo MB, Campos AC,
Oliveira MS, Gomes DA, Ferreira AJ, Santos RA and Goes AM: Priming
mesenchymal stem cells boosts stem cell therapy to treat myocardial
infarction. J Cell Mol Med. 17:617–625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rahbarghazi R, Nassiri SM, Ahmadi SH,
Mohammadi E, Rabbani S, Araghi A and Hosseinkhani H: Dynamic
induction of pro-angiogenic milieu after transplantation of
marrow-derived mesenchymal stem cells in experimental myocardial
infarction. Int J Cardiol. 173:453–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malick M, Gilbert K, Barry M, Godbout R
and Rousseau G: Desvenlafaxine reduces apoptosis in amygdala after
myocardial infarction. Brain Res Bull. 109:158–163. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naaijkens BA, van Dijk A, Meinster E,
Kramer K, Kamp O, Krijnen PA, Niessen HW and Juffermans LJ: Wistar
rats from different suppliers have a different response in an acute
myocardial infarction model. Res Vet Sci. 96:377–379. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chong JJ, Chandrakanthan V, Xaymardan M,
Asli NS, Li J, Ahmed I, Heffernan C, Menon MK, Scarlett CJ,
Rashidianfar A, et al: Adult cardiac-resident MSC-like stem cells
with aproepicardial origin. Cell Stem Cell. 9:527–540. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reuther S, Reiter M, Raabe A and Dikomey
E: Effect of irradiation on the expression of DNA repair genes
studied in human fibroblasts by real-time qPCR using three methods
of reference gene validation. Radiat Environ Biophys. 52:463–469.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anders B, Alonso A, Artemis D, Schäfer A,
Ebert A, Kablau M, Fluechter S, Findeisen P, Hennerici MG and Fatar
M: What does elevated high-sensitive troponin I in stroke patients
mean: Concomitant acute myocardial infarction or a marker for
high-risk patients? Cerebrovasc Dis. 36:211–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Regnault V, Lagrange J, Pizard A, Safar
ME, Fay R, Pitt B, Challande P, Rossignol P, Zannad F and Lacolley
P: Opposite predictive value of pulse pressure and aortic pulse
wave velocity on heart failure with reduced left ventricular
ejection fraction: Insights from an Eplerenone Post-Acute
Myocardial Infarction Heart Failure Efficacy and Survival Study
(EPHESUS) substudy. Hypertension. 63:105–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crafts TD, Jensen AR, BlocherSmith EC and
Markel TA: Vascular endothelial growth factor: Therapeutic
possibilities and challenges for the treatment of ischemia.
Cytokine. 71:385–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Przybyt E, Krenning G, Brinker MG and
Harmsen MC: Adipose stromal cells primed with hypoxia and
inflammation enhance cardiomyocyte proliferation rate in vitro
through STAT3 and Erk1/2. J Transl Med. 11:392013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishikawa K, Fish K, Aguero J, YanizGalende
E, Jeong D, Kho C, Tilemann L, Fish L, Liang L and Eltoukhy AA:
Stem cell factor gene transfer improves cardiac function after
myocardial infarction in swine. Circ Heart Fail. 8:167–174. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malliaras K, Makkar RR, Smith RR, Cheng K,
Wu E, Bonow RO, Marbán L, Mendizabal A, Cingolani E, Johnston PV,
et al: Intracoronary cardiosphere-derived cells after myocardial
infarction: Evidence of therapeutic regeneration in the final
1-year results of the CADUCEUS trial (Cardiosphere-Derived
autologous stem Cells to reverse ventricular dysfunction). J Am
Coll Cardiol. 63:110–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galiger C, Kostin S, Golec A, Ahlbrecht K,
Becker S, Gherghiceanu M, Popescu LM, Morty RE, Seeger W and
Voswinckel R: Phenotypical and ultrastructural features of
Oct4-positive cells in the adult mouse lung. J Cell Mol Med.
18:1321–1333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mendjan S, Mascetti VL, Ortmann D, Ortiz
M, Karjosukarso DW, Ng Y, Moreau T and Pedersen RA: NANOG and CDX2
pattern distinct subtypes of human mesoderm during exit from
pluripotency. Cell Stem Cell. 15:310–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Senyo SE, Steinhauser ML, Pizzimenti CL,
Yang VK, Cai L, Wang M, Wu TD, GuerquinKern JL, Lechene CP and Lee
RT: Mammalian heart renewal by pre-existing cardiomyocytes. Nature.
493:433–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chambers I, Colby D, Robertson M, Nichols
J, Lee S, Tweedie S and Smith A: Functional expression cloning of
Nanog, a pluripotency sustaining factor in embryonic stem cells.
Cell. 113:643–655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mitsui K, Masamune T, Okuyama K, Oguchi T,
Furuya A, Iwashita H, Ishiyama T and Matsukawa T: A case of severe
hypotension caused by external cardiac compression by tumor and
doctor's hand in a patient with mediastinal tumor. Masui.
62:204–208. 2013.PubMed/NCBI
|
|
25
|
Lahm H, Doppler S, Dreßen M, Werner A,
Adamczyk K, Schrambke D, Brade T, Laugwitz KL, Deutsch MA,
Schiemann M, et al: Live fluorescent RNA-based detection of
pluripotency gene expression in embryonic and induced pluripotent
stem cells of different species. Stem Cells. 33:392–402. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kajstura J, Rota M, Cappetta D, Ogórek B,
Arranto C, Bai Y, Ferreira-Martins J, Signore S, Sanada F, Matsuda
A, et al: Cardiomyogenesis in the aging and failing human heart.
Circulation. 126:1869–1881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cieslik KA, Trial J, Crawford JR, Taffet
GE and Entman ML: Adverse fibrosis in the aging heart depends on
signaling between myeloid and mesenchymal cellRole of inflammatory
fibroblasts. J Mol Cell Cardiol. 70:56–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duran JM, Makarewich CA, Sharp TE,
Starosta T, Zhu F, Hoffman NE, Chiba Y, Madesh M, Berretta RM, Kubo
H, et al: Bone-derived stem cells repair the heart after myocardial
infarction through transdifferentiation and paracrine signaling
mechanisms. Circ Res. 113:539–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wehman B, Sharma S, Mishra R, Guo Y,
Colletti EJ, Kon ZN, Datla SR, Siddiqui OT, Balachandran K and
Kaushal S: Pediatric End-Stage Failing Hearts Demonstrate Increased
Cardiac Stem Cells. Ann Thorac Surg. 100:615–622. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hariharan N, Quijada P, Mohsin S, Joyo A,
Samse K, Monsanto M, De La Torre A, Avitabile D, Ormachea L,
McGregor MJ, et al: Nucleostemin rejuvenates cardiac progenitor
cells and antagonizes myocardial aging. J Am Coll Cardiol.
65:133–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chugh AR, Beache GM, Loughran JH, Mewton
N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima
JA, et al: Administration of cardiac stem cells in patients with
ischemic cardiomyopathy: The SCIPIO trial: Surgical aspects and
interim analysis of myocardial function and viability by magnetic
resonance. Circulation. 126(11): Suppl 1. S54–S64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ellison GM, Galuppo V, Vicinanza C, Aquila
I, Waring CD, Leone A, Indolfi C and Torella D: Cardiac stem and
progenitor cell identification: Different markers for the same
cell? Front Biosci (Schol Ed). 2:641–652. 2010.PubMed/NCBI
|
|
33
|
Xue C, Zhang J, Lv Z, Liu H, Huang C, Yang
J and Wang T: Angiotensin II promotes differentiation of mouse
c-kit-positive cardiac stem cells into pacemaker-like cells. Mol
Med Rep. 11:3249–3258. 2015.PubMed/NCBI
|
|
34
|
Fortini C, Cesselli D, Beltrami AP,
Bergamin N, Caragnano A, Moretti L, Cecaro F, Aquila G, Rizzo P,
Riberti C, et al: Alteration of Notch signaling and functionality
of adipose tissue derived mesenchymal stem cells in heart failure.
Int J Cardiol. 174:119–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Igura K, Okada M, Kim HW and Ashraf M:
Identification of small juvenile stem cells in aged bone marrow and
their therapeutic potential for repair of the ischemic heart. Am J
Physiol Heart Circ Physiol. 305:H1354–H1362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jasmin, Jelicks LA, Tanowitz HB, Peters
VM, Mendez-Otero R, Campos de Carvalho AC and Spray DC: Molecular
imaging, biodistribution and efficacy of mesenchymal bone marrow
cell therapy in a mouse model of Chagas disease. Microbes Infect.
16:923–935. 2014. View Article : Google Scholar : PubMed/NCBI
|