Introduction
Ventricular arrhythmias of the right ventricular
outflow tract (RVOT), including premature ventricular contraction
(PVC) and ventricular tachycardia (VT), are usually common in
healthy individuals without structural heart disease and appear to
be benign with a good prognosis (1).
However, previous studies have reported that malignant arrhythmias,
such as spontaneous ventricular fibrillation (VF) and polymorphic
ventricular tachycardia, are occasionally initiated by ventricular
extrasystoles arising from RVOT (2,3).
Generally, the majority of PVCs and VTs originating from RVOT can
be effectively eliminated by catheter radiofrequency ablation (RFA)
(4) targeting the initiating
ventricular extrasystole. It is thought that the majority of RFA
failures can be attributed to inappropriate mapping and location of
the target (5). Cardiac metastatic
tumors are uncommon and usually appear in the pericardium (6). Malignant tumor-associated myocardial
infiltration is even more rarely observed in clinical practice and
may potentially form arrhythmogenic foci (6). Presented herein is the case of a
25-year-old male patient who suffered refractory monomophic VT of
RVOT origin and underwent two unsuccessful RFAs under the guidance
of a CARTO system prior to hospitalization. A series of
examinations, including echocardiography, chest computed tomography
(CT), cardiac magnetic resonance and intracardiac
electrophysiological study (EPS), revealed that cardiac metastatic
extraskeletal mesenchymal chondrosarcomas (ESMCs) of RVOT origin
gave rise to VT, which was resistant to many antiarrhythmic drugs
(AAD), and was eventually resolved by RFA therapy.
Case report
A 25-year-old male farmer who did not present any
structural heart disease was admitted to the General Hospital of
PLA (Beijing, China) in April 2014 following recurrent palpitations
and shortness of breath for 16 months. On admission, no
abnormalities were detected following a routine 12-lead surface
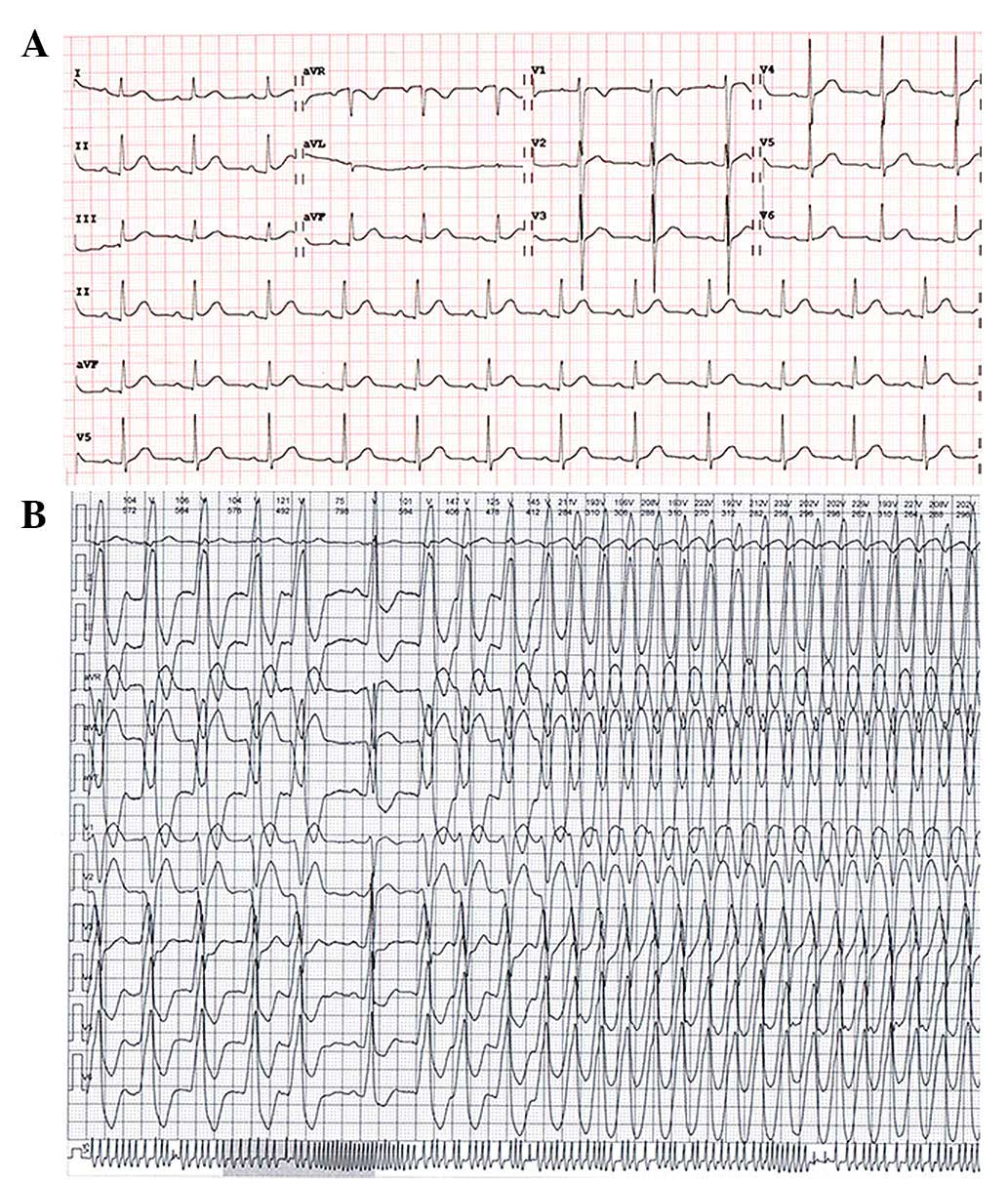
electrocardiography (ECG; Fig. 1A).
Holter monitoring indicated frequent PVC and recurrent monomophic
VT (Fig. 1B), which originated from
RVOT as determined by a traditional VT localization algorithm
(7). However, although the patient
underwent two rounds of RFA localized to the RVOT using a
three-dimensional mapping system (CARTO) in the Henan Province
Hospital, ventricular arrhythmia reoccurred 3 and 6 days after each
procedure, respectively. Two weeks prior to hospitalization at the
General Hospital of PLA in April 2014, the number of VT episodes
markedly increased and occasionally the VT of the patient
spontaneously accelerated to >200 beats/min. In addition, VT
degenerated into a sustainable pattern resistant to many AADs,
which resulted in hemodynamic instability and severely impacted the
quality of life of the patient. The ECG morphology of the PVC and
VT showed a simple positive R wave in lead II, III, AVF and QS in
lead V1, as well as a positive R wave in lead V6 with R/S
transition zone (first precordial lead with R/S ratio >1) in
lead V3, which was similar to the ECG morphology of the left bundle
branch block. These ECG characteristics fulfilled the diagnostic
criteria of idiopathic VT of RVOT origin, however, compared with
idiopathic VT manifesting constant ECG morphology, ambulatory ECG
records indicated intermittent small morphological changes of VT in
many leads. A notched R wave in lead III and AVF, and a higher R
wave in lead I intermittently appeared. Simultaneously, an R/S
transition zone in precordial leads fluctuated between V3 and V4..
Holter monitoring indicated that the ventricular rates of VT were
markedly altered at 90–210 beats/min (Fig. 1B). Physical examination and the
results of the laboratory tests carried out using Cobas 6000 and
Modular P800 analyzers (Roche Diagnositics, Inc., Rotkreuz,
Switzerland), including 0.3 mg/dl C-reactive protein (normal range,
0–0.8 mg/dl), 2 mm/h erythrocyte sedimentation rate (normal range,
0–20 mm/h), cardiac-specific enzymes including 0.008 ng/ml TNT
(normal range, 0–0.1 ng/ml) and CK-MB 0.67 ng/ml (normal range,
0–6.5 ng/ml), 0.3 mg/l D-dimmer (normal range, <0.5 mg/l), 143.8
ng/ml Pro-B-type natriuretic peptide (normal range, <150 ng/ml),
serum electrolytes including 4.1 mmol/l potassium (normal range,
3.5–5.5 mmol/l), 138 mmol/l natrium (normal range, 135–145 mmol/l)
and 0.9 mmol/l magnesium (normal range, 0.7–1.2 mmol/l) were within
normal ranges.
The same patient with VT was previously diagnosed
with ESMC in 2012, and underwent tumor surgical resection from the
retroperitoneum at the Henan Province Hospital. The patient did not
receive chemotherapy or radiotherapy, and did not undergo routine
follow-up. Following admission of the patient to the General
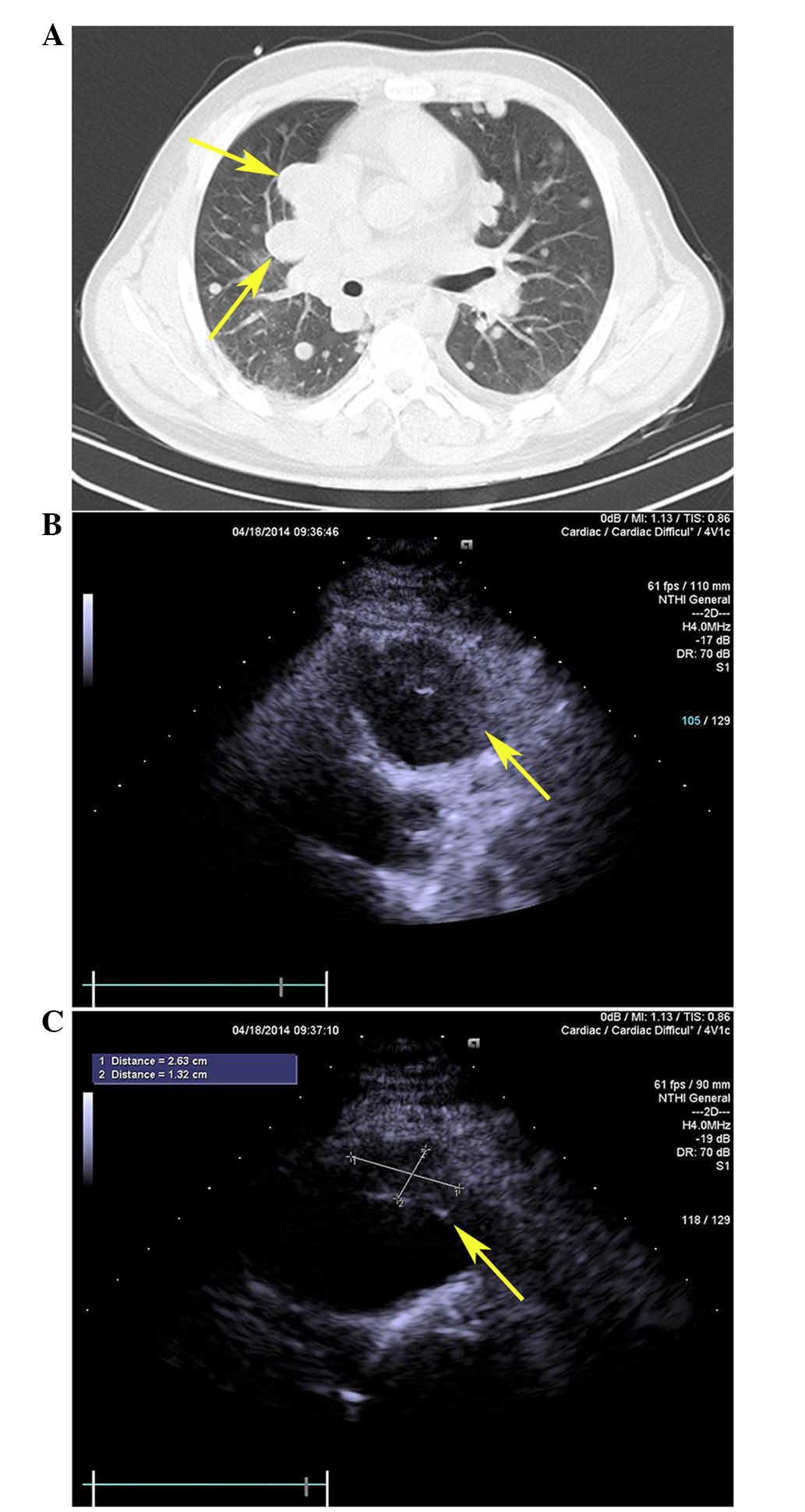
Hospital of PLA on 14th April, 2014, an enhanced chest CT scan
revealed extensive nodular mass infiltration in the pleura,
pericardium, lung and mediastinum of the patient (Fig. 3A). These results suggested the tumor
had metastasized and involved the cardiovascular system. Subsequent
trans-thoracic echocardiograghy indicated the presence of a tissue
mass attached to the anterior free wall of the RVOT (Fig. 3B and C). These results were further
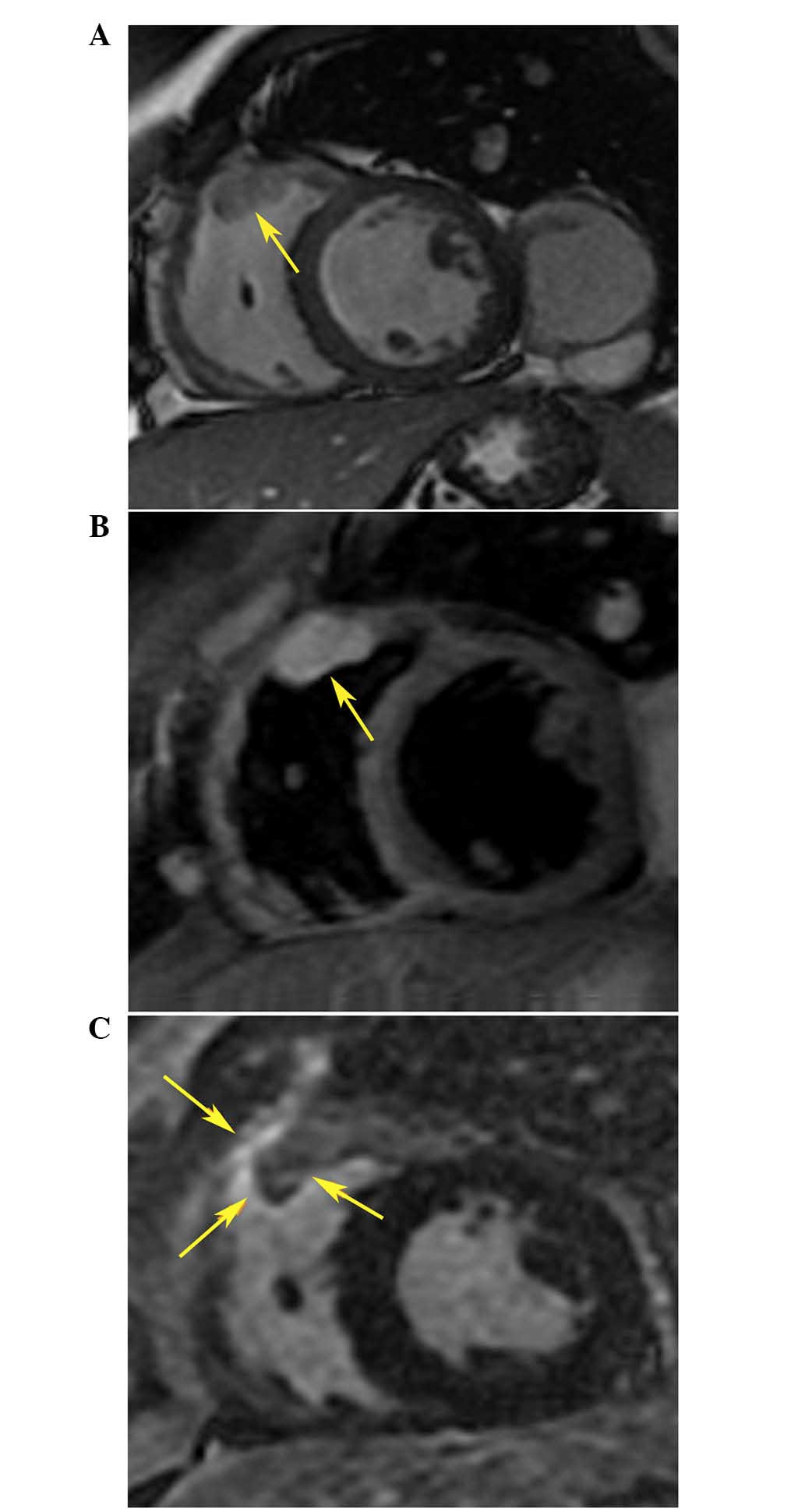
confirmed by cardiac magnetic resonance (CMR), which detected an
irregular oval mass infiltrated in the myocardium of the RVOT and
protruding into the right ventricular chamber (Fig. 4A and B). Based on these clinical
findings, it was suggested that the refractory ventricular
arrhythmia of the patient may be closely associated with or may
directly result from a metastatic tumor within the RVOT. However,
due to the patient's frequent episodes of VT, surgical resection of
the tumor, chemical therapy and radiation therapy were not
recommended as a treatment strategy. Due to the relatively stable
general condition of the patient who had a likely life expectancy
of >1 year, and due to the fact that sustained VT could further
impair heart function and degenerate into malignant
life-threatening arrhythmia, a third RFA procedure was attempted
with the aim to control ventricular arrhythmia.
The present study was approved by the ethical
committee of the general hospital of PLA. The patient provided
informed consent and underwent an electrophysiological study
following fasting and under sedation; amiodarone (Sanofi, Gentilly,
France) and metoprolol (AstraZeneca, London, UK), which had been
taken by the patient for 6 months prior to admission to the General
Hospital of PLA, were not discontinued with the purpose of reducing
the heart rate in the presence of VT. Intracardiac electrograms
were recorded using an electrophysiology system (CardioLab; GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA). A decapolar mapping
catheter (Biosense Webster, Inc., Diamond Bar, CA, USA) was
positioned in the apex of the right ventricle, and a 3.5 mm
saline-irrigated catheter (NAVISTAR ThermoCool®;
Biosense Webster, Inc.) was positioned into the RVOT via the right
femoral vein using a standard Brockenbrough technique (1). The mapping and RFA procedures were
performed under the guidance of a CARTO system (Biosense Webster,
Inc.). A heparinized saline solution was continuously infused
through the sheath (2 ml/min) to avoid formation of thrombi or air
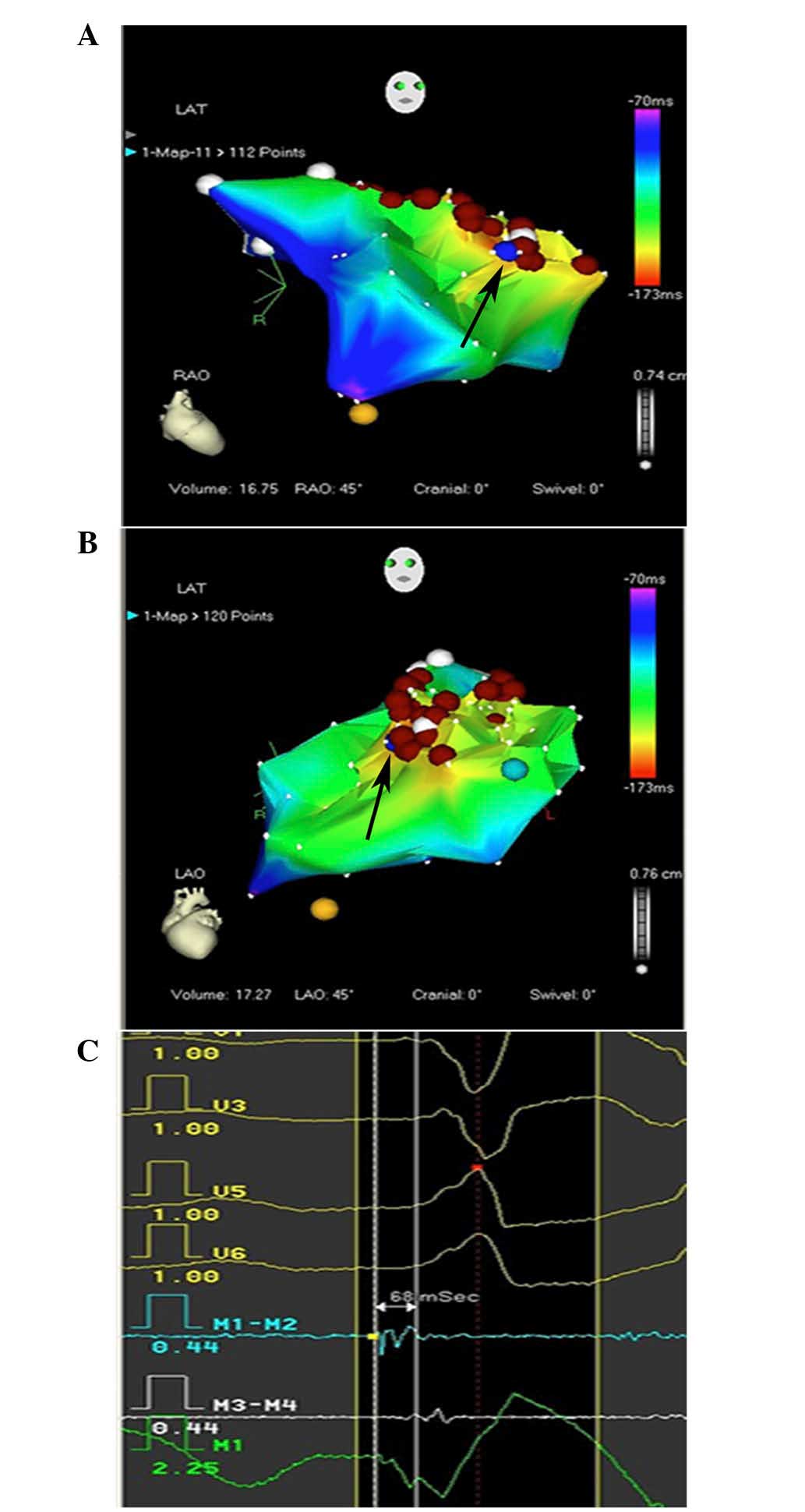
emboli. Systemic point-by-point activation mapping was applied in
the presence of VT, the earliest bipolar activity and recording of
a local unipolar QS pattern (Fig.
5C) were used to identify the target. The earliest local
ventricular activation recorded from the distal electrode pair of
the ablation catheter should precede the onset of the surface QRS
complex by 15–45 ms. As the RVOT was infiltrated with tumors, and
VT originated from numerous sites within the RVOT, the earliest
activation site changed according to the various VT origins,
ranging from the anterior ventricular septum to the anterior free
wall of the RVOT (Fig. 5A and B). An
RF current was delivered to a target point at a target temperature
of 45°C, a maximum power of 35 W and an infusion rate of 17 ml/min
(Stocker Generator; Biosense Webster Inc.). The target point met
the following criteria: The local activation of the distal
electrode pair of the ablation catheter preceded the onset of
surface ECG QRS by ≥15 ms; acceleration of the tachycardia followed
by gradual slowing during ablation were observed; abrupt
termination occurred during ablation; rapid intrinsic deflection in
the unipolar QS pattern was observed.
It was revealed that the majority of successful
targets were located between the anterior ventricular septum and
the free wall, which was compatible with the mapping results. As VT
originated from various adjacent sites, a target point with the
earliest activation was successfully eliminated during the process
of RFA, and another burst of VT with various rates and morphologies
appeared a few seconds later, which was mapped near the previous
target points. Following extensive RFA in the area of interest
comprising at least 30 target points, the VT episode subsided and
gradually disappeared. The end point of the procedure occurred upon
the disappearance of spontaneous VT and following non-inducibility
of VT by programmed electrical stimulation and/or administration of
isoproterenol at the rate of 0.2–0.6 µg/min for 30 min after
ablation.
Following the procedure, the patient no longer
complained of palpitations or dyspnea. Ambulatory Holter monitoring
revealed one episode of accelerated ventricular rhythm similar to
RVOT VT 2 days after ablation. In addition, a CMR scan showed the
presence of an enhanced area surrounding the metastatic tumor
within the RVOT (Fig. 4C), which was
compatible with ablation points. AADs were discontinued 1 month
after ablation, and no ventricular arrhythmia was recorded by
Holter monitoring in the 8 months follow-up.
Discussion
Ventricular arrhythmias arising from the RVOT
usually occur in the absence of apparent structural heart disease,
which is idiopathic and rarely life-threatening, and can be
effectively eliminated by RFA with a high success rate (4). The mechanism underlying RVOT VT is
thought to be adenosine-sensitive and triggered by cyclic adenosine
monophosphate (8). Symptoms vary
based on the severity of the arrhythmia and the sensitivity of
patients to the rates and duration of VT, and the majority of
patients experienced mild to moderate symptoms (9). However, in the present study, the
patient exhibited self-perpetuating refractory VT leading to severe
persistent symptoms, which were markedly different from common
idiopathic RVOT VT, and led to the risk of heart failure and sudden
death. The patient underwent two rounds of RFA at the Henan
Province Hospital; however, VT recurred 3 and 6 days, respectively,
after the two procedures. In terms of successful ablation site, the
majority of patients with idiopathic VT of RVOT origin have only
one site, however, the patient had two different target sites which
were not adjacent to each other, which was not compatible with
idiopathic VT of RVOT origin. In addition, during the episode of
VT, VT manifested various morphologies and rates, which indicated
the underlying mechanism may be multiple exits of VT or multiple
origins of VT. During the process of EPS, the earliest activation
site did not localize to one point, and instead fluctuated between
the free wall and septum within the RVOT, depending on the various
morphologies and rates of VT. When VT manifested as a notched R
wave in inferior leads, higher amplitude R waves in lead I, and
early precordial R/S transition zone (V3) with rapid heart rate,
the earliest activation point could be recorded near the anterior
free wall; however, narrow R waves in inferior leads with lower
amplitude R waves and later R/S transition zones (V4), as well as a
relatively slow heart rate were ECG characteristics associated with
the earliest activation site in the anterior septum. These results
strongly supported that the VT of the patient may originate from
various sites within the RVOT (10,7).
On admission to the General Hospital of PLA, the
patient underwent a series of examinations that suggested that a
metastatic tumor had infiltrated the RVOT, which may be responsible
for the refractory VT and explained the multiple origins of VT. It
has previously been reported that cardiac metastatic tumors may
result in complete atrioventricular block (11,12) and
VT (13,14). Myocardium surrounded or infiltrated
by metastatic tumor may manifest abnormal electrophysiological
properties (15) and manifested as
increased automaticity due to triggered activity, however, severity
of myocardial infiltration surrounding the metastatic tumor was not
homogeneous and resulted in complicated form of VT with variant ECG
morphology and heart rate. The dominant activation pattern was
irregular and randomly fluctuated between the anterior free wall
and the septum of the RVOT. During VT mapping, if local bipolar
activation precedes the QRS of surface ECG by ≥15 ms with a QS
pattern of unipolar recording, the site mapped was considered as
the ideal target for RFA ablation; it was observed that VT
accelerated during the initial period of ablation and gradually
decelerated or terminated within 30 sec, which were typical
characteristics of successful target ablation by RF (16,17).
After a total of 30 points surrounding the tumor were successfully
ablated within the RVOT, VT was not induced by programmed right
ventricular stimulation or isoproterenol infusion.
Original cardiac tumors are rarely seen in clinical
practice with an incidence rate of 0.02% (6). However, cardiac metastatic tumors are
20–40 times more frequent than primary cardiac tumors, and
predominantly affect the pericardium rather than the myocardium
(18). It was reported that 10–12%
cases of malignant tumors were heart-associated at autopsy
(18). The patient presented in the
current case report was therefore initially underdiagnosed and
thought to have idiopathic RVOT-VT; a history of ESMC was not
correlated with ventricular arrhythmia. Following hospitalization,
a routine chest CT scan indicated the presence of extensive
pulmonary metastasis of the primary tumor. It has previously been
reported that the heart is the cause of 23% of systemic metastasis
cases (6), which led to the
hypothesis that the VT of the patient may result from myocardial
infiltration by a tumor. This hypothesis was further confirmed by
ECG and CMR scans which outlined the site and extent of the
metastatic tumor within the RVOT.
The general condition and past history of patients
should be carefully monitored if typical RVOT-VT could not be
ablated or recurs following several procedures. In these cases the
RVOT may be affected by malignant metastatic tumors and may become
an arrhythmogenic foci. CMR is particularly useful for identifying
cardiac metastases due to its ability to provide tissue
characterization (19,20); the mass-infiltrated RVOT of the
patient emitted a low signal intensity on the T1-weighted CMR image
and a high signal intensity on the T2-weighted CMR image, which was
compatible with the CMR characteristics of malignant tumors and
ESMCs (19,20).
Chondrosarcoma is an invasive skeletal tumor with
various grades of malignancy, and represents ~11% of all primary
malignant bone tumors (20). This
type of tumor rapidly evolves and has a strong tendency to
metastasize, and is resistant to chemotherapy (21). Chondrosarcomas usually occur in
patients aged 40–80 years, and are more frequent in men (22). 90% of chondrosarcomas are
conventional, and the remaining 10% include dedifferentiated, clear
cell, myxoid and mesenchymal chondrosarcomas (22), with myxoid being the most common
subtype. The present case was determined to be a mesenchymal
chondrosarcoma as confirmed by pathological examination. ESMC may
appear in any location that contains mesenchymal cells; primary
sites include limb extremities, the torso, the head and neck, and
rarely the heart (23–25). Compared with the myxoid subtype, the
ESMC subtype is rare, more aggressive and with poor prognosis due
to the high probability of metastases (26), which may occur several years
following the initial treatment. Cardiac metastasis of ESMCs is
infrequent; it has been reported that the most common site of
cardiac metastasis are the right atrium and left intracavity,
leading to pulmonary embolism and systemic embolism (27,28).
Dyspnea and pleuritic chest pain are the most common symptoms
associated with cardiac metastases (29). To our knowledge, RVOT involvement is
rarely observed and refractory ventricular arrhythmia has not
previously been described as a primary symptom. In the present
study, metastatic ESMC-associated VT was effectively treated with
RFA rather than surgical resection. During the 8 month follow-up
period, the patient did not report any further palpitations, and
Holter monitoring confirmed the absence of VT recurrence. Previous
studies have reported that the median time from the initiation of
cardiac symptoms to the time at which the patient succumbs to the
disease was 2 months in the case of cardiac metastases (30,31).
However, the general health of the patient reported was relatively
stable 8 months following the RFA procedure, and the patient is
currently undergoing further treatment for ESMC. The results of the
present study suggested that the treatment of VT by RFA targeting
following ESMC cardiac metastasis may significantly increase
patient lifespan.
References
|
1
|
Calvo N, Jongbloed M and Zeppenfeld K:
Radiofrequency catheter ablation of idiopathic right ventricular
outflow tract arrhythmias. Indian Pacing Electrophysiol J.
13:14–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kurosaki K, Nogami A, Shirai Y and Kowase
S: Positive QRS complex in lead i as a malignant sign in right
ventricular outflow tract tachycardia: Comparison between
polymorphic and monomorphic ventricular tachycardia. Circ J.
77:968–974. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimizu W: Arrhythmias originating from
the right ventricular outflow tract: How to distinguish ‘malignant’
from ‘benign’? Heart Rhythm. 6:1507–1511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lamba J, Redfearn DP, Michael KA, Simpson
CS, Abdollah H and Baranchuk A: Radiofrequency catheter ablation
for the treatment of idiopathic premature ventricular contractions
originating from the right ventricular outflow tract: A systematic
review and meta-analysis. Pacing Clin Electrophysiol. 37:73–78.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yokokawa M, Good E, Crawford T, Chugh A,
Pelosi F Jr, Latchamsetty R, Jongnarangsin K, Ghanbari H, Oral H,
Morady F and Bogun F: Reasons for failed ablation for idiopathic
right ventricular outflow tract-like ventricular arrhythmias. Heart
Rhythm. 10:1101–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarjeant JM, Butany J and Cusimano RJ:
Cancer of the heart: Epidemiology and management of primary tumors
and metastases. Am J Cardovasc Drugs. 3:407–421. 2003. View Article : Google Scholar
|
|
7
|
Ito S, Tada H, Naito S, Kurosaki K, Ueda
M, Hoshizaki H, Miyamori I, Oshima S, Taniguchi K and Nogami A:
Development and validation of an ECG algorithm for identifying the
optimal ablation site for idiopathic ventricular outflow tract
tachycardia. J Cardiovasc Electrophysiol. 14:1280–1286. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilber DJ, Baerman J, Olshansky B, Kall J
and Kopp D: Adenosine-sensitive ventricular tachycardia. Clinical
characteristics and response to catheter ablation. Circulation.
87:126–134. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakagawa M, Takahashi N, Nobe S, Ichinose
M, Ooie T, Yufu F, Shigematsu S, Hara M, Yonemochi H and Saikawa T:
Gender differences in various types of idiopathic ventricular
tachycardia. J Cardiovasc Electrophysiol. 13:633–638. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dixit S, Gerstenfeld EP, Callans DJ and
Marchlinski FE: Electrocardiographic patterns of superior right
ventricular outflow tract tachycardias: Distinguishing septal and
free-wall wits of origin. J Cardiovasc Electrophysiol. 14:1–7.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mocini D, Longo R, Colivicchi F, Morabito
A, Gasparini G and Santini M: A complete atrioventricular block
secondary to myocardial metastases of lung cancer. A case report.
Ital Heart J. 6:931–932. 2005.PubMed/NCBI
|
|
12
|
Soon CY, Singh D and Ong HY: Myocardial
metastatic tumor from a primary oropharyngeal carcinoma presenting
as severe 3rd-degree atrioventricular block. Tex Heart Inst J.
36:182–183. 2009.PubMed/NCBI
|
|
13
|
Leak D: Amiodarone for control of
recurrent ventricular tachycardia secondary to cardiac metastasis.
Tex Heart Inst J. 25:198–200. 1998.PubMed/NCBI
|
|
14
|
Kinoshita K, Hanibuchi M, Kishi M,
Kanematsu T, Nishioka Y and Sone S: Case of squamous cell lung
cancer with myocardial metastasis complicated with ventricular
tachycardia. Nihon Kokyuki Gakkai Zasshi. 47:817–822. 2009.(In
Japanese). PubMed/NCBI
|
|
15
|
Miyake CY, Del Nido PJ, Alexander ME,
Cecchin F, Berul CI, Triedman JK, Geva T and Walsh EP: Cardiac
tumors and associated arrhythmias in pediatric patients, with
observations on surgical therapy for ventricular tachycardia. J Am
Coll Cardiol. 58:1903–1909. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soejima Y, Aonuma K, Iesaka Y and Isobe M:
Ventricular unipolar potential in radiofrequency catheter ablation
of idiopathic non-reentrant ventricular outflow tachycardia. Jpn
Heart J. 45:749–760. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bogun F, Taj M, Ting M, Kim HM, Reich S,
Good E, Jongnarangsin K, Chugh A, Pelosi F, Oral H and Morady F:
Spatial resolution of pace mapping of idiopathic ventricular
tachycardia/ectopy originating in the right ventricular outflow
tract. Heart Rhythm. 5:339–344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wada A, Winner M III and Houmsse M:
Metastatic melanoma of the right ventricular outflow tract as a
cause of ventricular tachycardia. Tex Heart Inst J. 41:103–104.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sparrow PJ, Kurian JB, Jones TR and
Sivananthan MU: MR imaging of cardiac tumors. Radiographics.
25:1255–1276. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Belhocine TZ, Scott AM, Even-Sapir E,
Urbain JL and Essner R: Role of nuclear medicine in the management
of cutaneous malignant melanoma. J Nucl Med. 47:957–967.
2006.PubMed/NCBI
|
|
21
|
Douis H and Saifuddin A: The imaging of
cartilaginous bone tumours. II. Chondrosarcoma. Skeletal Radiol.
42:611–626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pontes HA, Pontes FS, de Abreu MC, de
Carvalho PL, de Brito Kato AM, Fonseca FP, de Freitas Silva BS and
Neto NC: Clinicopathological analysis of head and neck
chondrosarcoma: Three case reports and literature review. Int J
Oral Maxillofac Surg. 41:203–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsing CT, Oh SY, Lee S, Kwon HC, Kim SH,
Park TH, Woo JS, Na SH and Kim HJ: Extraskeletal mesenchymal
chondrosarcoma of the heart responded to systemic chemotherapy: A
case report. Cancer Res Treat. 39:131–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parmar C, Jojo A, Vachhani KC and Vijayan
SN: Primary chondrosarcoma of the heart. Eur J Cardiothorac Surg.
33:513–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Izzo P, Ricci N, Capolupo R, et al: A rare
care of primary chondrosarcoma of the heart. J Cardiovasc Med.
8:210–213. 2007. View Article : Google Scholar
|
|
26
|
Hu HJ, Liao MY and Xu LY: Primary
retroperitoneal extraskeletal mesenchymal chondrosarcoma involving
the vena cava: A case report. Oncol Lett. 7:1970–1974.
2014.PubMed/NCBI
|
|
27
|
Fichaux O, de Muret A, Dessenne X, Rosset
P, Pacouret G, Pagot O and Charbonnier B: Cardiac metastasis of
chondrosarcoma: A case report. Ann Cardiol Angeiol (Paris).
47:165–168. 1998.(In French). PubMed/NCBI
|
|
28
|
Oizumi H, Tanaka R, Shimura H, Sasaki K,
Koike H, Hattori N and Tanaka S: A case of cerebral embolism with
metastatic chondrosarcoma in the left atrium. J Stroke Cerebrovasc
Dis. 20:79–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kwon JW, Choi JA, Kwack KS, Oh JH, Chung
JH and Kang HS: Myxoid chondrosarcoma in the calcaneus: A case
report with MR imaging findings. Skeletal Radiol. 36(Suppl 1):
S82–S85. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leung CY, Cummings RG, Reimer KA and Lowe
JE: Chondrosarcoma metastatic to the heart. Ann Thorac Surg.
45:291–295. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nesi G, Pedemonte E and Gori F:
Extraskeletal mesenchymal chondrosarcoma involving the heart:
Report of a case. Ital Heart J. 1:435–437. 2000.PubMed/NCBI
|