Introduction
Oral cancers such as squamous cell carcinoma have a
high rate of morbidity and poor survival, and their incidence has
been increasing worldwide (1).
Surgery, radiotherapy and chemotherapy, or combinations of those
treatments are generally performed to treat patients with oral
cancer. The National Institutes of Health Development Consensus
Conference recommended oral assessment and management of patients
during cancer therapy to improve the quality of life (2). However, therapy-induced complications,
including surgical site infection and aspiration pneumonia, remain
as significant problems requiring clinical treatment to prevent
infectious complications (3,4). Training to improve cough effectiveness
and swallowing is considered effective to reduce the incidence of
aspiration pneumonia in cancer patients. Oral health care has been
recognized as important to reduce postoperative complications in
these and other cancer patients (5,6). In
addition, it has been demonstrated that professional oral care
inhibits the development of oral mucositis in patients with
esophageal cancer who are undergoing chemoradiotherapy (7).
The present team began performing oral health care
procedures, including professional teeth cleaning and instructions
for self care by a dental hygienist for patients with oral cancer
in April 2008, as pre- and post-operative oral care is considered
to decrease the number of oral bacteria, resulting in inhibition of
inflammation at the surgical site. In addition, proper instructions
for self care are considered to motivate patients with oral cancer
to maintain oral health. It may be hypothesized that oral health
care could reduce infectious disease in oral cancer patients.
However, few reports have been revealed a clear correlation between
preoperative oral health care and a reduction in postoperative
complications. In the present study, the ability of preoperative
oral care to influence the inflammatory response and reduce
infectious complications was investigated. The objective of the
present study was to clarify the association between preoperative
oral health care and postoperative complications, including
infectious disease and surgical site infection, in patients with
oral cancer.
Patients and methods
Patients
The records of 70 patients (37 males and 33 females;
mean age, 65.5 years; age range, 34–85 years) with oral cancer who
underwent surgical treatment at the Department of Oral and
Maxillofacial Surgery at Hiroshima University Hospital (Hiroshima,
Japan) between 2008 and 2014 were reviewed. Those who received
preoperative chemotherapy or radiotherapy and suffered from tumor
recurrence were excluded in order to exclude the effects of those
treatments on postoperative conditions. The present retrospective
study was approved by the ethics committee of Hiroshima University
and informed consent was obtained from all participants.
Tumors were classified using the TNM staging system
according to the 5th edition of the General Rules for Clinical
Studies on Head and Neck Cancer (Japan Society for Head and Neck
Cancer, 2012) (8). A total of 25
oral cancer tumors were identified to arise from the tongue, 16
from the lower gingiva, 12 from the upper gingiva, 6 from the
buccal mucosa, 6 from the hard palate and 5 from the mouth floor.
In terms of patient history, 10 patients suffered from diabetes, 2
from respiratory disorders and 4 from cardiovascular disorders.
Furthermore, 22 of these patients received preoperative oral care,
whereas 48 patients did not. Furthermore, the surgical treatments
received were divided into two categories in terms of surgical
damage. Surgical resection with or without skin transplantation was
performed in 51 patients, who were categorized into the minimally
invasive surgery group. Surgical resection with neck dissection was
performed in 7 patients, and a combination of surgical resection
with neck dissection and free flap transplantation or pectoralis
major myocutaneous flap transplantation was performed in 12
patients. These 19 patients were categorized into the severely
invasive surgery group. The clinicopathological factors of the
present oral cancer patients are summarized in Table I.
 | Table I.Clinicopathological factors of oral
cancer patients. |
Table I.
Clinicopathological factors of oral
cancer patients.
|
|
| Oral care group, n
(%) | Non-oral care group,
n (%) |
|---|
|
|
|
|
|
|---|
| Clinicopathological
factors | No. of patients | MIS | SIS | MIS | SIS |
|---|
| Age (years) |
|
|
<60 | 14 | 3
(21.4) | 1 (7.1) | 9
(64.3) | 1 (7.1) |
| ≥60 | 56 | 12 (21.4) | 6
(10.7) | 27 (48.2) | 11 (19.6) |
| Histological
type |
|
| SCC | 60 | 14 (23.3) | 7
(11.7) | 28 (46.7) | 11 (18.3) |
| Salivary
gland cancers | 9 | 1 (0.0) | 0 (0.0) | 7
(77.8) | 1
(11.1) |
|
Sarcoma | 1 | 0 (0.0) | 0 (0.0) | 1
(100) | 0 (0.0) |
| Tumor size |
|
|
T1/T2 | 55 | 13 (23.2) | 0 (17.9) | 34 (62.5) | 8 (14.3) |
|
T3/T4 | 15 | 2
(13.3) | 7 (46.7) | 2 (13.3) | 4 (26.7) |
| Clinical stage |
|
| Stage
I/II | 48 | 13 (12.8) | 0 (0.0) | 34 (70.8) | 1 (2.1) |
| Stage
III/IV | 22 | 2 (9.1) | 7 (31.8) | 2 (9.1) | 11 (50.0) |
| Diabetes |
|
| No | 60 | 12 (20.0) | 6 (10.0) | 31 (51.7) | 11 (18.3) |
|
Yes | 10 | 3
(30.0) | 1 (10.0) | 5
(50.0) | 1
(10.0) |
| Cardiovascular
disease |
|
| No | 66 | 13 (19.7) | 7 (10.6) | 35 (53.0) | 11 (16.7) |
|
Yes | 4 | 2
(50.0) | 0 (0.0) | 1
(25.0) | 1
(25.0) |
| Respiratory
disease |
|
| No | 68 | 14 (20.6) | 7 (10.3) | 35 (51.5) | 12 (17.6) |
|
Yes | 2 | 1
(50.0) | 0 (0.0) | 1
(50.0) | 0
(0.0) |
Oral care
With regard to oral health care, patients in the
oral care group received professional teeth cleaning or scaling by
a dental hygienist within 3 days prior to surgery. In addition,
self care instructions, including tooth brushing and tongue
cleaning with a sponge brush, were performed for the patients in
the oral care group by dental hygienists. Patients in the non-oral
care group did not receive special care from a dental hygienist
prior to the surgery. Following surgery, regular oral care was
performed for patients in both groups by a doctor at least once a
day.
Evaluation of inflammatory response
and complications
Markers, including body temperature (BT), white
blood cell (WBC) count and C-reactive protein (CRP) levels were
examined in order to evaluate the inflammatory response following
surgery. BT was examined on the previous day of the surgery and 1,
3, 5, 7 and 9 days after surgery, whereas the WBC count and CRP
level were examined on the day before and 1, 3–5, 7–9 and 14–16
days after surgery. The occurrence of complications such as
anastomotic leak, surgical site infection and aspiration pneumonia
within the 14 days after surgery was investigated. Surgical site
infection was determined according to the method of Johnson et
al (9).
Statistical analysis
Welch's t-test and Fisher's exact test were used for
statistical analysis, in addition to Mann-Whitney U tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of inflammatory response
between oral care and non-oral care groups
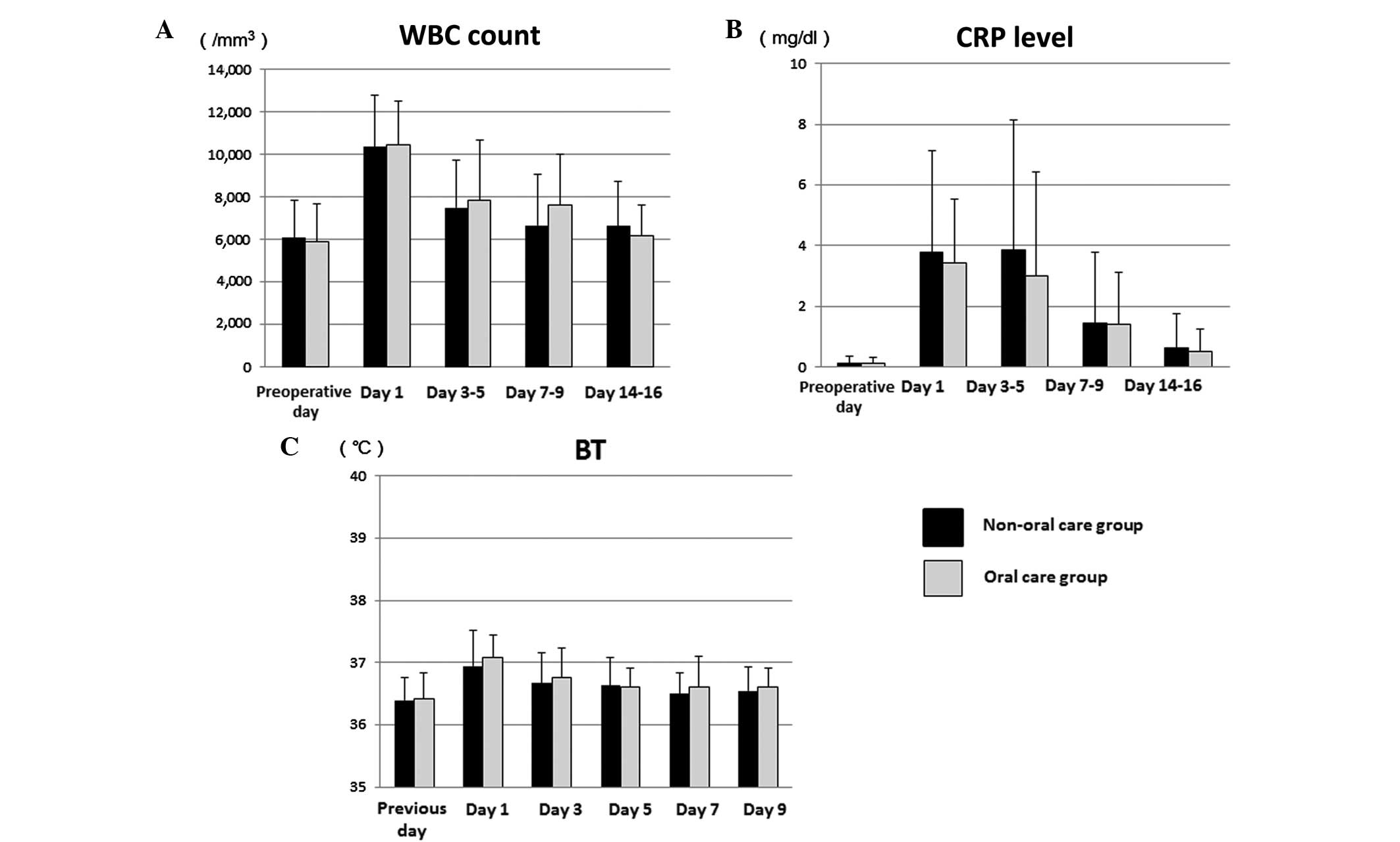
In the oral care and non-oral care groups, the
preoperative WBC counts were 5,901 and 6,130/mm3,
respectively, which rose to maximum values of 10,448 and
10,646/mm3 on day 1, decreased to 7,843 and
7,331/mm3 on days 3–5 and further reduced to 6,152 and
6,077/mm3 on days 14–16, respectively (Fig. 1A). Furthermore, there was no
significant difference in mean WBC count between the two groups at
1, 3–5, 7–9 and 14–16 days after surgery.
The preoperative CRP level was within a normal range
(<0.2 mg/dl) in all patients in both groups. The mean
postoperative CRP then rose to a maximum of 3.84 mg/dl on days 3–5,
and decreased to 0.63 mg/dl on days 14–16 in the non-oral care
group (Fig. 1B). In the oral care
group, the mean post-operative CRP rose to a maximum of 3.42 mg/dl
on day 1, then decreased to 3.01 mg/dl on days 3–5 and returned to
0.53 mg/dl on days 14–16 (Fig. 1B).
The mean CRP in the oral care group was lower compared with that of
the non-oral care group at each time point after surgery, although
the differences were not significant.
The mean BT in the oral care and non-oral care
groups was 36.4°C for both prior to surgery, then 37.1 and 37.0°C
on day 1, 36.8 and 36.6°C on day 3, 36.6 and 36.6°C on day 5, 36.6
and 36.6°C on day 7, and 36.6 and 36.6°C on day 9, respectively
(Fig. 1C). There were no
statistically significant differences in BT between the two
groups.
Comparison of inflammatory response
between groups for patients who underwent minimally invasive
surgery
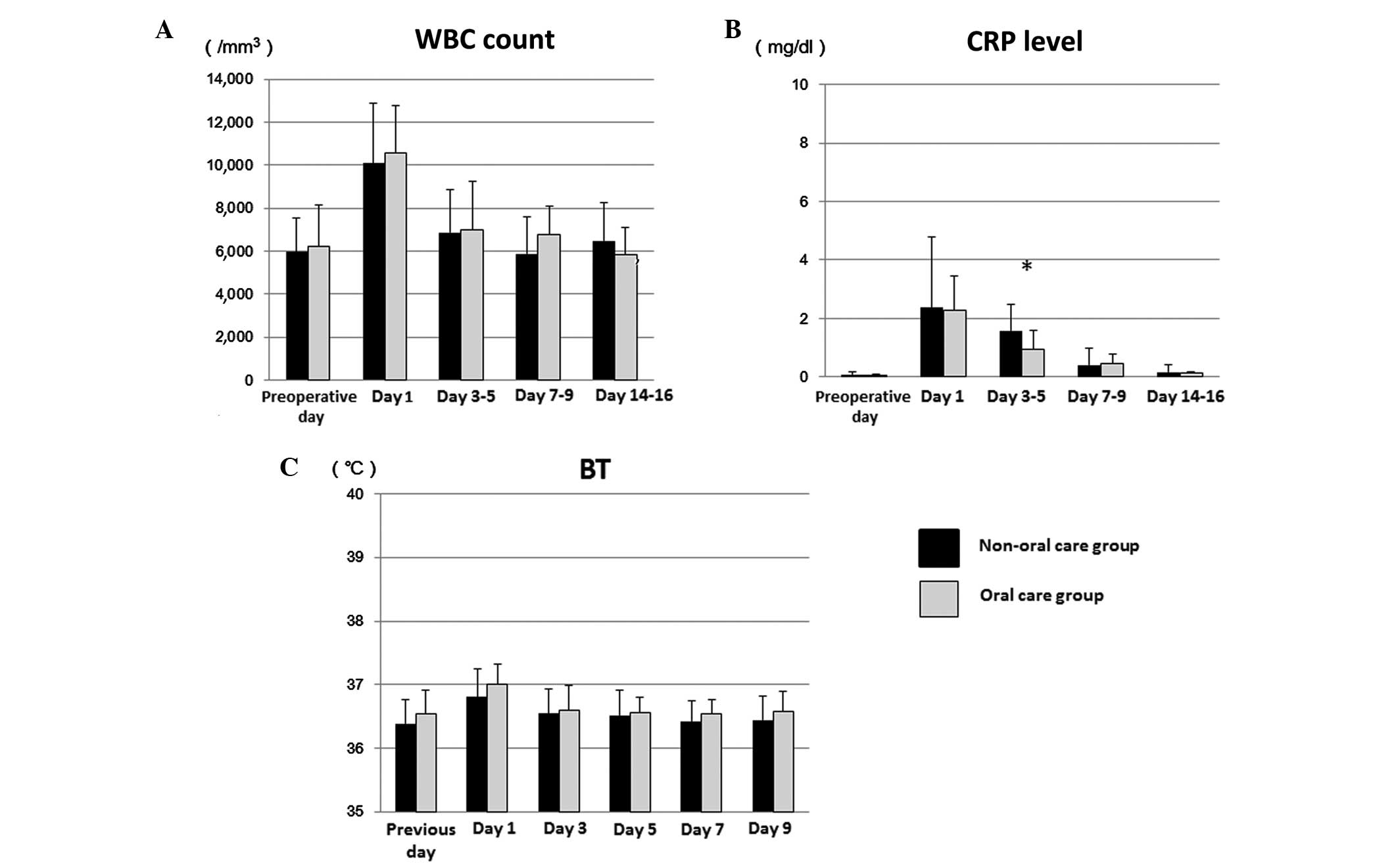
The 70 patients were divided into those who received
minimally invasive surgery and severely invasive surgery in order
to assess the effects of surgical damage on postoperative clinical
examination observations. In the 51 patients with minimally
invasive surgery, there was no significant difference with regard
to the WBC count and BT between the oral care and non-oral care
groups at 1, 3–5, 7–9 and 14–16 days after surgery (Fig. 2A and C). However, the mean CRP on
days 3–5 was significantly lower in the oral care group (0.92
mg/dl) compared with the non-oral care group (1.58 mg/dl) patients
who underwent a minimally invasive procedure by Welch's t-test
(P=0.033; Fig. 2B). The differences
in CRP levels were relatively small, likely because all patients
underwent minimally invasive surgery.
Comparison of inflammatory response
between groups for patients who underwent severely invasive
surgery
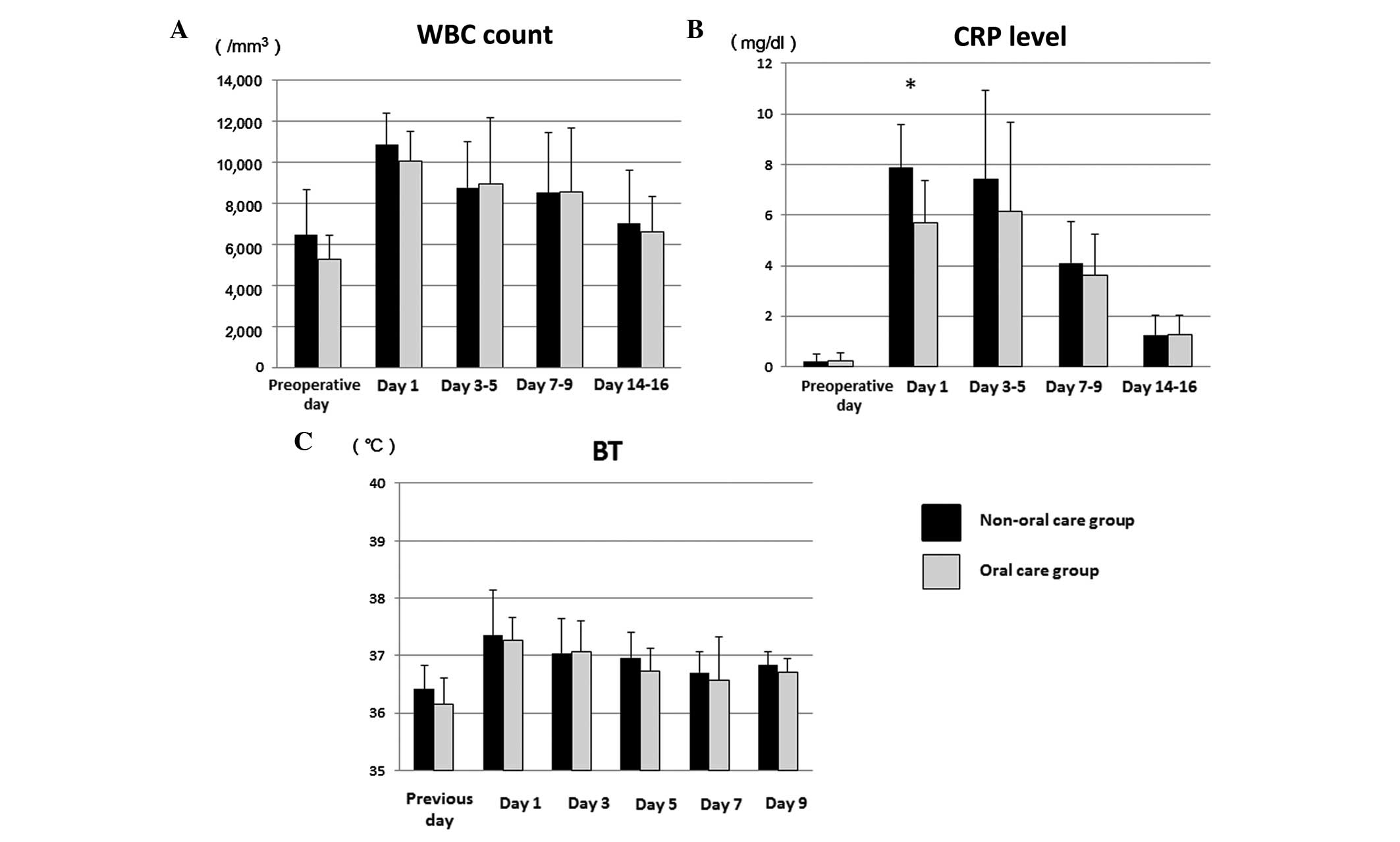
In the patients who received severely invasive
surgery (for example, neck dissection or reconstructive surgery),
no significant difference was identified with regard to the WBC
count and BT between the oral care and non-oral care groups
(Fig. 3A and C). By contrast, the
CRP level was greater in those who underwent severely invasive as
compared with minimally invasive surgery, indicating that extensive
surgical damage induced an increase of CRP (Figs. 2B and 3B). The mean postoperative CRP rose to a
maximum of 7.90 mg/dl in the non-oral care group and 5.69 mg/dl in
the oral care group on day 1, then changed to 6.97 mg/dl and 6.15
mg/dl on days 3–5, and decreased to 3.80 and 3.61 mg/dl on days
7–9, and 1.27 and 1.26 mg/dl on days 14–16, respectively (Fig. 3B). On postoperative day 1, the level
of CRP was significantly decreased in the oral care group as
compared with the non-oral care group by Welch's t-test (P=0.030;
Fig. 3B). Furthermore, the level of
CRP was lower in the oral care group as compared with the non-oral
care group on days 3–5, although not significantly. Severe damage
may have caused the distinct difference in the CRP levels between
the two groups. These results suggest that preoperative oral care
reduces early postoperative CRP levels in oral cancer patients who
undergo severely invasive surgery. CRP levels on days 3–5 were
increased compared to those on day 1 in patients who received oral
health care. One possible reason for this is that a number of
patients demonstrated a peak level of CRP on days 3–5, though that
peak was also identified in patients who did not receive oral care
on days 3–5. These results indicate that severe surgical damage may
have an influence on the prolonged inflammatory response.
Correlation between postoperative
complications and clinicopathological features
The correlations between preoperative oral care and
postoperative infectious complications (for example, surgical site
infection, aspiration pneumonia and anastomotic leak) were
investigated. One case of surgical site infection and one of
aspiration pneumonia were revealed among the non-oral care group
patients who received reconstructive surgery. Furthermore, an
anastomotic leak was identified in 9 of 41 (22.0%) patients in the
non-oral care group and 3 of 19 (15.8%) in the oral care group. The
correlations between postoperative complications and
clinicopathological features, including age, tumor size, clinical
stage, diabetes, respiratory disorder and cardiovascular disease,
surgical treatment, performance of blood transfusion and
tracheostomy (Fisher's exact test) were analyzed (Table II). Mann-Whitney U tests for serum
albumin levels and blood loss volume were also performed. The
results of these are summarized in Table III. Although no statistical
correlation was identified between postoperative complications and
those clinicopathological factors, the percentage of complications
was greater in patients ≥60 years old than in younger patients and
in patients who underwent severely invasive surgery as compared
with minimally invasive surgery. The frequency of complications was
also increased in patients with diabetes, cardiovascular disease
and respiratory disease compared with those without those
respective conditions. Notably, the preoperative serum albumin
level was lower in the patients with postoperative complications
compared with those without (Table
III). However, the percentage of complications was decreased in
the oral care group (13.6%) compared with the non-oral care group
(20.8%) (Table II), although the
difference was not statistically significant.
 | Table II.Correlation between post-operative
complications and clinicopathological factors in oral cancer
patients. |
Table II.
Correlation between post-operative
complications and clinicopathological factors in oral cancer
patients.
|
|
| Postoperative
complications, n (%) |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factors | No. of
patients | No | Yes | P-value |
|---|
| Age (years) |
|
|
|
|
|
<60 | 14 | 12 (85.7) | 2
(14.3) |
0.65 |
|
≥60 | 56 | 45 (80.4) | 11 (19.6) |
|
| Tumor size |
|
|
|
|
|
T1/T2 | 55 | 44 (80.0) | 11 (20.0) |
0.72 |
|
T3/T4 | 15 | 13 (86.7) | 2
(13.3) |
|
| Clinical stage |
|
|
|
|
| Stage
I/II | 48 | 38 (79.2) | 10 (20.8) |
0.74 |
| Stage
III/IV | 22 | 19 (86.4) | 3
(13.6) |
|
| Oral care |
|
|
|
|
| No | 48 | 38 (79.2) | 10 (20.8) |
0.74 |
|
Yes | 22 | 19 (86.4) | 3
(13.6) |
| Diabetes |
|
|
|
|
| No | 60 | 49 (81.7) | 11 (18.3) | 1.0 |
|
Yes | 10 | 8
(80.0) | 2
(20.0) |
|
| Cardiovascular
disease |
|
|
|
|
| No | 66 | 54 (81.8) | 12 (18.2) | 1.0 |
|
Yes | 4 | 3
(75.0) | 1
(25.0) |
|
| Respiratory
disease |
|
|
|
|
| No | 68 | 56 (82.4) | 12 (17.6) |
0.57 |
|
Yes | 2 | 1
(50.0) | 1
(50.0) |
|
| Surgical
treatment |
|
|
|
|
|
Minimally invasive | 51 | 42 (80.4) | 9
(17.6) |
0.34 |
|
Severely invasive | 19 | 15 (78.9) | 4
(21.1) |
|
| Blood
transfusion |
|
|
|
|
| No | 56 | 45 (80.4) | 11 (19.6) | 1.0 |
|
Yes | 14 | 12 (85.7) | 2
(14.3) |
|
| Tracheostomy |
|
|
|
|
| No | 53 | 44 (83.0) | 9
(17.0) |
0.72 |
|
Yes | 17 | 13 (76.5) | 4
(23.5) |
|
 | Table III.Correlation between post-operative
complications and blood loss volume or serum albumin levels in oral
cancer patients. |
Table III.
Correlation between post-operative
complications and blood loss volume or serum albumin levels in oral
cancer patients.
|
| Postoperative
complications |
|
|---|
|
|
|
|
|---|
| Clinicopathological
factors | No | Yes | P-value |
|---|
| Blood loss volume
(ml) | 277.3±371.8 | 417.0±842.9 | 0.56 |
| Preoperative serum
albumin (g/dl) | 4.42±0.44 | 4.18±0.47 | 0.59 |
| Postoperative serum
albumin (g/dl) | 3.55±0.59 | 3.55±0.44 | 0.95 |
Discussion
The bulk of dental plaque such as dental biofilm is
composed of microcolonies of oral bacteria. Without regular
removal, this biofilm undergoes a process of maturation that
results in the formation of a pathogenic bacterial complex. Oral
cancer patients have difficulties with removing such pathogens
because of the oral tumor in addition to cancer-related bleeding
and pain. In addition, mucosal damage caused by oral cancer
increases the risk of infectious complications. Therefore,
professional oral hygiene procedures such as the mechanical removal
of plaque from teeth, gingival and mucosal surfaces, and self care
instructions are required for oral cancer patients to prevent
infection. A previous study demonstrated that oral care is
important in reducing the risk of pneumonia in elderly individuals
(10), thus it is associated not
only with oral health but also general well-being. In addition,
oral care has been reported to be necessary to reduce postoperative
complications in oral cancer patients (6). However, there is a lack of research
into the effects of preoperative oral health care on inflammation
in oral cancer patients following surgery. Therefore, the
correlations between preoperative oral care and inflammatory
response parameters obtained from blood test results were
investigated in the present study. CRP levels, WBC count and BT
were examined in order to assess the inflammatory response
following surgery. Although there was no significant difference
with regard to the WBC count and BT, CRP was reduced in the oral
care group regardless of whether the surgical procedure was
minimally or severely invasive. In addition, differences in CRP
levels between the two groups on day 1 and days 3–5 were more
evident in patients who underwent severely invasive surgery. BT and
WBC are considered to be markers of inflammation, though they are
not specific for evaluation of infectious disease (11). CRP was initially described as a
protein in serum samples obtained from acute pneumonia patients,
and its plasma concentration has been reported to be associated
with the clinical courses of inflammation and infection (12,13). The
CRP concentration can significantly increase during the acute phase
of inflammation and peaks at 24–48 h after inflammatory stimulation
(14). Furthermore, several studies
have noted that CRP is a sensitive inflammatory marker and useful
for detection of infection following surgery (15–17).
Thus, preoperative oral health care was hypothesized to inhibit the
inflammatory response following surgery and to potentially reduce
the recovery time. In addition, low CRP may have indicated an
inactive infection status in patients in the oral care group. There
was a significant difference for CRP between the oral care and
non-oral care groups in the early postoperative period, but not in
the late period. One possible reason for this is that an
inflammatory condition in the early postoperative period may be
affected by a preoperative oral health condition, whereas that in
the late postoperative period may be influenced by both pre- and
postoperative oral health conditions. Therefore, it may be
concluded that preoperative oral care is important in inhibiting
the inflammatory response during the early postoperative stage,
particularly in patients who have undergone severely invasive
surgery. The timing of preoperative oral care is another important
issue. In the present patients, this was performed within 3 days
prior to surgery. For reducing oral pathogens as much as possible,
oral care should be performed just prior to surgical treatment and
additional examinations are necessary to evaluate those effects on
the inflammatory response.
With regard to the correlation between postoperative
complications and clinicopathological factors, an age of ≥60 years
and severely invasive surgery were associated with a high frequency
of complications, indicating that they were risk factors for
postoperative infectious complications in oral cancer patients. In
addition, preoperative oral health care by dental hygienists was
demonstrated to reduce the frequency of postoperative complications
in the present cohort. Furthermore, professional oral health care
appears to be important in managing infectious complications by
reducing oral pathogens present at the time of surgical treatment.
As for wound infection in oral cancer patients, the tumor size,
degree of surgical resection and patient history are risk factors,
with diabetes also reported to be a factor for local infection
(6,18). In the present study, no significant
correlation was identified between diabetes and wound infection.
However, all of the diabetic patients were well controlled by
insulin or antidiabetic drug administrations during recovery, thus
they may not have suffered from delayed wound healing or increased
susceptibility to infections. With regard to the risk factors for
surgical site infection, preoperative hypoalbuminemia has been
revealed to be the greatest risk factor in patients undergoing head
and neck reconstructive surgery (19). However, in the present study, no
significant correlation was identified between the preoperative
serum albumin level and postoperative complications, whereas the
preoperative serum albumin level was distinctly decreased in
patients with postoperative complications as compared with those
without complications. Thus, the results of the present study
support previous observations that preoperative serum albumin is an
important risk factor for postoperative infectious disease.
Postoperative oral hygiene is also required for patients who have
difficulty with cleaning their own oral cavity due to surgical
damage such as reconstructive surgery. At Hiroshima University
Hospital, nurses are regularly involved in postoperative oral care
(for example removing dry expectoration and wiping off tooth,
tongue, oral mucosa and gingiva surfaces) until the patient can
perform this on their own. Even when the patient is using a
mechanical ventilator after reconstructive surgery, oral care is
regularly performed by a doctor or nurse. Such continuous oral
health care may have contributed to the low number of aspiration
pneumonia and local infection cases that occurred during the
hospital stay in the present study.
In summary, the present observations indicate that
preoperative oral health care may be involved in reducing early
postoperative inflammation in patients with oral cancer who have
undergone severely invasive surgery. Thus, appropriate oral health
management is critical to ensure better outcomes in oral cancer
patients undergoing surgical treatment.
Acknowledgements
This study was supported by a Grant-in-aid (grant
no. 23592963) from the Japanese Ministry of Education, Culture,
Sports and Technology.
Glossary
Abbreviations
Abbreviations:
|
SCC
|
squamous cell carcinoma
|
|
BT
|
body temperature
|
|
WBC
|
white blood cell
|
|
CRP
|
C-reactive protein
|
References
|
1
|
Petersen PE: Oral cancer prevention and
control - the approach of the World Health Organization. Oral
Oncol. 45:454–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Institutes of Health consensus
development conference on oral complications of cancer therapies:
Diagnosis, prevention and treatment. Bethesda, Maryland, April
17–19, 1989. NCI Monogr. 9:1–184. 1990.PubMed/NCBI
|
|
3
|
Genden EM, Rinaldo A, Suárez C, Wei WI,
Bradley PJ and Ferlito A: Complications of free flap transfers for
head and neck reconstruction following cancer resection. Oral
Oncol. 40:979–984. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rubenstein EB, Peterson DE, Schubert M,
Keefe D, McGuire D, Epstein J, Elting LS, Fox PC, Cooksley C, Sonis
ST, et al: Mucositis Study Section of the Multinational Association
for Supportive Care in Cancer; International Society for Oral
Oncology. Clinical practice guidelines for the prevention and
treatment of cancer therapy-induced oral and gastrointestinal
mucositis. Cancer. 100(Suppl 9): 2026–2046. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamada C: Effects of oral health care for
lung cancer patients with surgery - improvement of cough reflex.
Kokubyo Gakkai Zasshi. 79:95–99. 2012.(In Japanese). PubMed/NCBI
|
|
6
|
Sato J, Goto J, Harahashi A, Murata T,
Hata H, Yamazaki Y, Satoh A, Notani K and Kitagawa Y: Oral health
care reduces the risk of postoperative surgical site infection in
inpatients with oral squamous cell carcinoma. Support Care Cancer.
19:409–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoneda S, Imai S, Hanada N, Yamazaki T,
Senpuku H, Ota Y and Uematsu H: Effects of oral care on development
of oral mucositis and microorganisms in patients with esophageal
cancer. Jpn J Infect Dis. 60:23–28. 2007.PubMed/NCBI
|
|
8
|
Japan Society for Head and Neck Cancer.
General Rules for Clinical Studies on Head and Neck Cancer (5th).
Kanehara & Co., Ltd. (Tokyo). 2012.(In Japanese).
|
|
9
|
Johnson JT, Myers EN, Thearle PB, Sigler
BA and Schramm VL Jr: Antimicrobial prophylaxis for contaminated
head and neck surgery. Laryngoscope. 125:275–280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoneyama T, Yoshida M, Matsui T and Sasaki
H: Oral Care Working Group: Oral care and pneumonia. Lancet.
354:5151999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Castelli GP, Pognani C, Cita M and
Paladini R: Procalcitonin as a prognostic and diagnostic tool for
septic complications after major trauma. Crit Care Med.
37:1845–1849. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tillet WS and Francis T: Serological
reactions in pneumonia with a non-protein somatic fraction of the
Pneumococcus. J Exp Med. 52:561–571. 1930. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ansar W and Ghosh S: C-reactive protein
and the biology of disease. Immunol Res. 56:131–142. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pepys MB: C-reactive protein: A critical
update. J Clin Invest. 111:1805–1812. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iizuka T and Lindqvist C: Changes in
C-reactive protein associated with surgical treatment of mandibular
fractures. J Oral Maxillofac Surg. 49:464–467. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mustard RA Jr, Bohnen JM, Haseeb S and
Kasina R: C-reactive protein levels predict postoperative septic
complications. Arch Surg. 122:69–73. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aono H, Ohwada T, Kaneko N, Fuji T and
Iwasaki M: The post-operative changes in the level of inflammatory
markers after posterior lumbar interbody fusion. J Bone Joint Surg
Br. 89:1478–1481. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee DH, Kim SY, Nam SY, Choi SH, Choi JW
and Roh JL: Risk factors of surgical site infection in patients
undergoing major oncological surgery for head and neck cancer. Oral
Oncol. 47:528–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kamizono K, Sakuraba M, Nagamatsu S,
Miyamoto S and Hayashi R: Statistical analysis of surgical site
infection after head and neck reconstructive surgery. Ann Surg
Oncol. 21:1700–1705. 2014. View Article : Google Scholar : PubMed/NCBI
|