Introduction
Ischemic stroke is a major cause of mortality and
disability worldwide. Transplantation methods using stem cells,
such as bone marrow mesenchymal stem cells (BMSCs), are being
developed as potential regenerative therapies for ischemic attacks
in the brain. BMSCs are multipotent cells that are able to
differentiate into not only mesodermal lineage cells (such as
osteoblasts, chondrocytes, adipocytes and muscle cells), but also
into neurons and astrocytes (1,2). In
addition, basic and clinical studies have suggested that human
BMSCs are not antigen-presenting cells and so will not activate the
immune system of the host (3,4).
However, this has been questioned in recent years because very few
transplanted BMSCs are found to home to and survive in the ischemic
region of the brain. Several factors may affect cell survival in
the acute phase of cerebral infarction, including limited blood
supply, hypoxia, trophic factor deficiency, oxidative stress and
inflammatory response (5).
Improvements in the survival, migration, homing and engraftment
rate of transplanted BMSCs are urgently required.
Hypoxic preconditioning (HP) has been shown to be
neuroprotective against ischemic brain injury (6). It is reported that HP promotes the
survival of embryonic stem cells and provides functional benefits
following transplantation into the ischemic rat brain (7). However, little is known of the effect
of hypoxic conditioning on BMSC neural differentiation. In the
present study, the effects of HP on the proliferation and migration
of canine BMSCs as well as the effects on neural differentiation
were investigated.
The therapeutic effects of granulocyte-colony
stimulating factor (G-CSF) are pleiotropic. G-CSF has been used for
bone marrow reconstruction and stem cell mobilization (8). A study has shown that G-CSF may
contribute to improving the outcome of BMSC transplantation therapy
for central nervous system disorders (9). However, the effect of G-CSF on
hypoxically preconditioned BMSCs is not well characterized. In the
present study, it was hypothesized that HP treatment could improve
the proliferation of BMSCs, protect them from necrotic and
apoptotic insults and promote their survival in vitro, and
that culturing with G-CSF may further promote this efficiency.
Materials and methods
Cell culture and characterization
In a previous study (10), we successfully cultured BMSCs
obtained from 16 healthy adult beagle dogs of either gender and
13.96±1.0 kg body weight (Laboratory Animal Centre, Anhui, China).
In brief, BMSCs were isolated from bone marrow obtained from the
humerus of beagle dogs by density-gradient separation, using
lymphocyte separation medium (TBD Science, Tianjin, China). In the
present study, BMSCs were isolated by the previously described
method (10). Confluent cells at
passage 3 (P3) were used for all experiments. These cells were
propagated as a monolayer culture in Dulbecco's modified Eagle's
medium (DMEM) containing 10% fetal bovine serum (FBS), 4.5 g/l
D-glucose, 100 U/ml penicillin and 100 µg/ml streptomycin. The
DMEM, penicillin and streptomycin were from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). For cell characterization,
CD90, CD44 and CD34 expression were identified by flow cytometry
(BD FACSCalibur, BD Biosciences, CA, USA). In brief, passage 2
BMSCs were collected in FACS tubes (BD Biosciences) at
2×105 cells/tube and washed with FACS buffer. The cells
were incubated with antibodies at room temperature for 1 h.
Antibodies used were as follows: CD90-PE (561970; BD Pharmingen,
San Jose, CA, USA), CD45-PE (555480; BD Pharmingen) and
CD34-fluorescein isothiocyanate (FITC; 8011-0349; eBioscience Inc.,
San Diego, CA, USA). The cells were then washed twice and
resuspended in 500 µl FACS buffer. The cells incubated with CD90,
CD44 or CD34 were incubated with anti-human IgG secondary
antibodies labeled with PE or FITC for 1 h. Cells were washed twice
and resuspended in 500 µl FACS buffer. Cell fluorescence was then
evaluated and data were analyzed using FlowJo software (Tree Star,
Ashland, OR, USA).
Hypoxic and normoxic conditions
Culture using 1% O2 was defined as
hypoxic and 21% O2 as normoxic conditions. Hypoxic
treatment of cells comprised culturing them in a hypoxic incubator,
where the O2 concentration in the chamber was maintained
at 1%. Cells of the normoxic control were subjected to the same
procedures, with the exception that they were maintained in an
atmosphere comprising 21% O2. To evaluate the effects of
hypoxia, BMSCs were divided into the following four groups: Groups
HP-1, HP-2 and HP-3, in which cells were cultured under hypoxic
conditions for 6,12 and 24 h, respectively, before being
transferred to normoxic conditions, and the normoxic group (group
N), in which cells were exposed to 21% O2 throughout the
culture.
G-CSF treatment
In order to evaluate the effect of G-CSF on
hypoxia-treated BMSCs, the BMSCs were co-cultured with 2 u/cell
G-CSF immediately after the hypoxic treatment.
Cell apoptosis
For assessment of cell apoptosis, flow cytometry (BD
FACSCalibur) was used to determine the percentages of dead cells
and cells undergoing apoptosis as described previously (10). In brief, Annexin
V+/propidium iodide (PI−) cells were
considered as early apoptotic while Annexin
V+/PI+ cells were counted as late apoptotic
or dead.
Cell viability assay
To detect the viability of the BMSCs, cells were
cultured in 96-well plates with 1,000 cells per well. After
different treatments, the cell viability was assayed at three
different time points: 24, 48 and 72 h. A Cell Counting Kit 8
(CCK8) assay (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) was used according to the manufacturer's protocol. The
absorbance (A) at 450 nm was measured using a microplate reader.
All experimental conditions were repeatedly tested as least three
times.
Cell migration
The migration ability of BMSCs was detected using a
24-well Transwell cell culture chamber (polycarbonate membrane;
Corning Incorporated, Corning, NY, USA). Briefly, an aliquot of
1×104 cells was placed into the upper chamber with 100
µl serum-free medium. The lower chamber was filled with 600 µl
medium containing 10% FBS. After 36 h incubation, the cells on the
upper surface of the filters were removed. The filters were fixed
in 4% paraformaldehyde and the cells that had migrated to the lower
side of the filter were stained with crystal violet. Next, the
cells were counted under a CKX31 microscope (Olympus Corporation,
Tokyo, Japan). At least three chambers from three different
experiments were analyzed for each condition.
Cell neuronal differentiation
Neuronal differentiation was induced following
previously reported procedures (11,12).
Briefly, sub-confluenced BMSCs were preinduced for 24 h with DMEM,
20% FBS and 1 mM β-mercaptoethanol, and then were induced for 6 h
with DMEM, 100 mM butylated hydroxyanisole (BHA) and 2%
dimethylsulfoxide (DMSO). Finally, cells were cultured in
maintenance media containing DMEM, 100 mM BHA, 2% DMSO, 25 mM KCl,
2 mM valproic acid, 10 µM forskolin and N-2 supplement for 1–3
days. At the beginning of preinduction, cells were randomly
transferred to normoxic and hypoxic conditions, respectively. BMSCs
cultured under normoxic conditions with DMEM and 20% FBS were used
as control. The percentages of neuron-like cells were measured by
two experienced unrelated individuals blindly via the random
selection of 10 non-overlapping visual fields per well.
Additionally, the mRNA expression of the neuronal markers nestin
(NSE), oligodendrocyte transcription factor 1 (Olig1), glial
fibrillary acidic protein (GFAP), microtubule-associated protein 2
(MAP-2) and neurofilament light chain (NF-L), and C-X-C chemokine
receptor type 4 (CXCR-4) were analyzed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
RT-qPCR analysis
Expression of neuronal markers (Nestin, Olig1, GFAP,
MAP-2 and NF-L) and CXCR-4 was analyzed by RT-qPCR. Total RNA was
extracted from cultured cells using TRIzol reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. RNAs
were reverse transcribed using a PrimeScript RT Reagent kit and
Oligo dT primer (Takara Bio, Inc., Otsu, Japan). qPCR was performed
at 95°C for 5 sec and 60°C for 34 sec in 20 µl buffer containing
SYBR premix ExTaq II and ROX Reference Dye (Takara Bio, Inc.) and
0.2 µM of each primer using SYBR premix DimerEraser (Takara Bio,
Inc.) on a 7900HT Fast Real-Time PCR system. GUSB was used as an
internal control for ∆∆Cq analysis (13). The primers are listed in Table I.
 | Table I.Primers for canine-specific
markers. |
Table I.
Primers for canine-specific
markers.
| Gene | Description | Primer sequence | Marker |
|---|
| GUSB | Glucuronidase β | F:
ACATCGACGACATCACCGTCA | Reference |
|
|
| R:
GGAAGTGTTCACTGCCCTGGA |
|
| NSE | Nestin | F:
GGACGGGCTTGGTGTCAATAG | Neural progenitor
cell |
|
|
| R:
AGACTGCTGCAGCCCATTCA |
|
| GFAP | Glial fibrillary
acidic protein | F:
CTAGCTTGGATACAGAGAGG | Astrocyte |
|
|
| R:
CCAAGTGTATCTGGTTGCCC |
|
| Olig1 | Oligodendrocyte
transcription factor 1 | F:
GTCAATGGCTACATGACTGC | Oligodendrocyte |
|
|
| R:
GTCATCAATCCACATCGTCC |
|
| MAP-2 |
Microtubule-associated protein 2 | F:
AAGCATCAACCTGCTCGAATCC | Neuron |
|
|
| R:
GCTTAGCGAGTGCAGCAGTGAC |
|
| NF-L | Neurofilament light
chain | F:
TGAATATCATGGGCAGAAGTGGAA | Neuron |
|
|
| R:
GGTCAGGATTGCAGGCAACA |
|
| CXCR-4 | C-X-C chemokine
receptor type 4 | F:
GACTCCATGAAGGAACCCTG | Cell migration |
|
|
| R:
GCCAGTCAAGAAGATGATGG |
|
Statistical analysis
Data are presented as mean ± standard deviation.
Student's unpaired t-test and one-way analysis of variance were
used to compare two or three independent groups, respectively.
P<0.05 was considered to indicate a statistically significant
difference. All analyses were conducted using SPSS statistical
analysis software (version 13.0; SPSS, Inc., Chicago, IL, USA).
Results
HP facilitated the proliferation and
migration of BMSCs
BMSCs were harvested from healthy beagles as
previously described (10). When
observed under a microscope, typical BMSCs were elongated and
spindle-shaped cells with branching extensions under either
normoxic (21% O2) or hypoxic (1% O2)
conditions (Fig. 1A). BMSCs at P3
were characterized by flow cytometry for the phenotypic expression
of cell surface markers for mesenchymal stem cells. BMSCs were
positive for the cell surface marker CD44 and negative for CD34 and
CD90 (Fig. 1B).
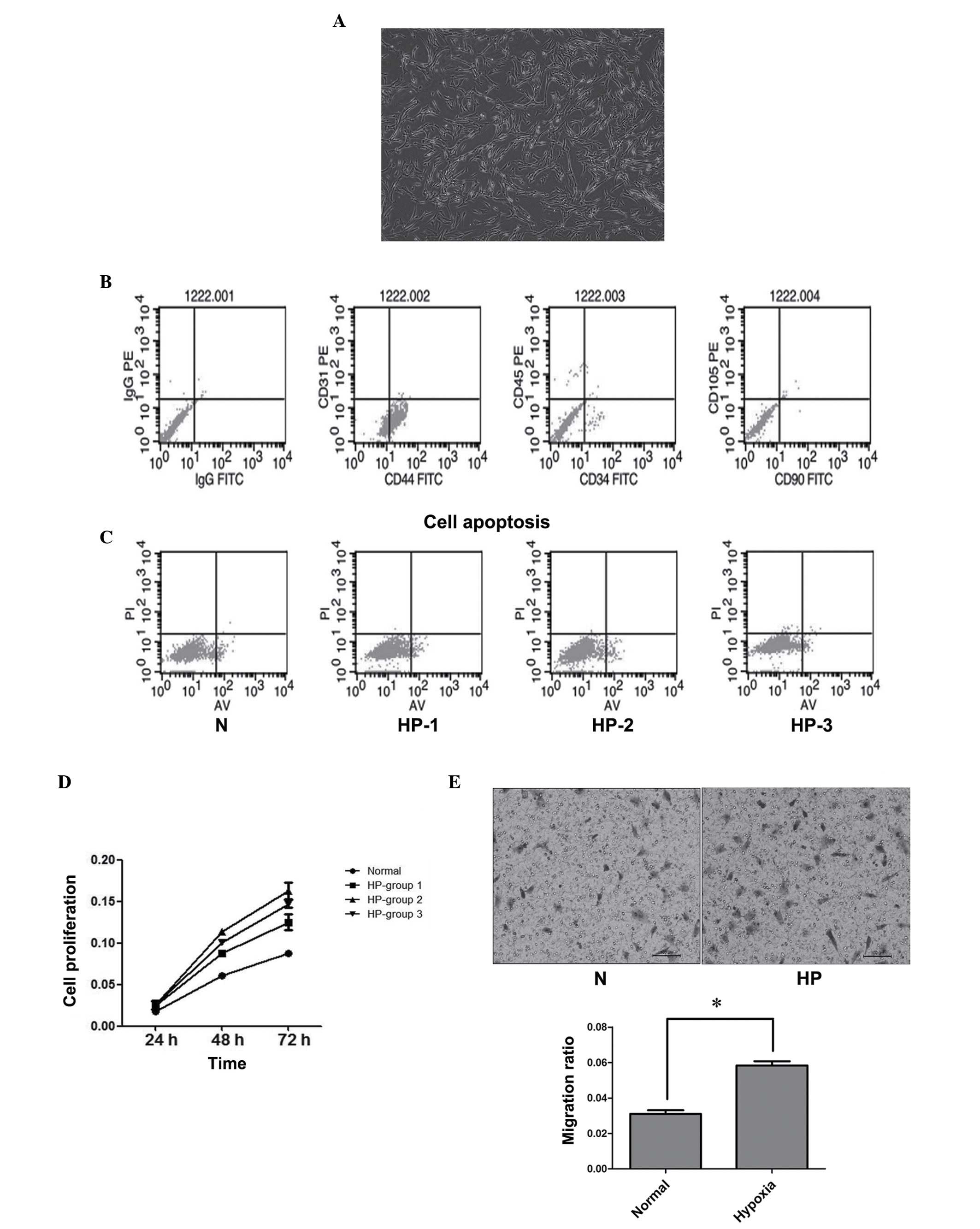 | Figure 1.Hypoxic preconditioning (HP)
facilitated BMSC proliferation and migration. (A) Phase contrast
photo shows typical isolated cells in culture dishes exhibiting
spindle or triangular shapes, consistent with the morphology of
BMSCs. (B) Characterization of BMSCs isolated from canines. BMSCs
were identified by fluorescence-activated cell sorting and the
positive expression of CD44 and negative expression of CD34 and
CD90 was confirmed. (C) Noticeable apoptosis was not detected in
any of the three HP groups or the normoxia (N) group. Lower left
quadrant, viable cells (Annexin V-FITC−/PI−);
lower right quadrant, early apoptotic cells (Annexin
V-FITC+/PI−); upper right quadrant, late
apoptotic or necrotic cells (Annexin
V-FITC+/PI+). (D) HP promoted BMSC
proliferation. (E) HP treatment facilitated the migration ability
of BMSCs. The graph presents the summarized data from the Transwell
assay. *P<0.05. BMSC, bone marrow mesenchymal stem cell; CD,
cluster of differentiation; PE, phycoerythrin; FITC, fluorescein
isothiocyanate; PI, propidium iodide; AV, Annexin V. |
To investigate the influence of hypoxia on BMSCs,
cells were cultured under hypoxic conditions (1% O2)
prior to being transferred to normoxic conditions (21%
O2). Flow cytometry was used for the assessment of cell
apoptosis. No significant apoptosis was observed in the three HP
groups or the normoxic group (Fig.
1C).
To detect cell proliferation, cells were moved to
normoxic conditions for 24 h following hypoxia treatment.
Subsequent to this, cell proliferation in each group was detected
using the CCK8 assay. HP promoted BMSC proliferation in comparison
with that in the normoxia group (Fig.
1D). Also, the 12-h hypoxic treatment group exhibited the
greatest increase in cell proliferation.
Cell migration to the region of injury is a critical
step in stroke therapy. To understand whether HP could affect the
migration activity of BMSCs, Transwell assays were performed. The
results confirmed that HP treatment facilitated the migration
ability of BMSCs, significantly increasing the migration rate of
the cells (P<0.05; Fig. 1E).
HP facilitated the neural
differentiation of BMSCs
Neuronal differentiation under hypoxic conditions
resulted in a significantly higher percentage of neuron-like cells
in contrast to normoxic conditions (68.5±5.3 vs. 54.3±7.4%,
P<0.05; Fig. 2A). Furthermore,
MAP-2 and NF-L, which are mature neuronal markers, as well as
CXCR-4, which contributes to cell migration capacity, were
expressed at markedly higher levels in the neuronal induction
groups (P<0.05). Additionally, in the neuronal induction group,
these markers were expressed at significantly higher levels under
hypoxia than normoxia. However, the expression of Nestin and Olig1
revealed no significant difference between the hypoxic neuronal
induction group and the normoxic group (Fig. 2B). The expression of GFAP was higher
in the hypoxic neuronal induction group compared with the normoxic
group.
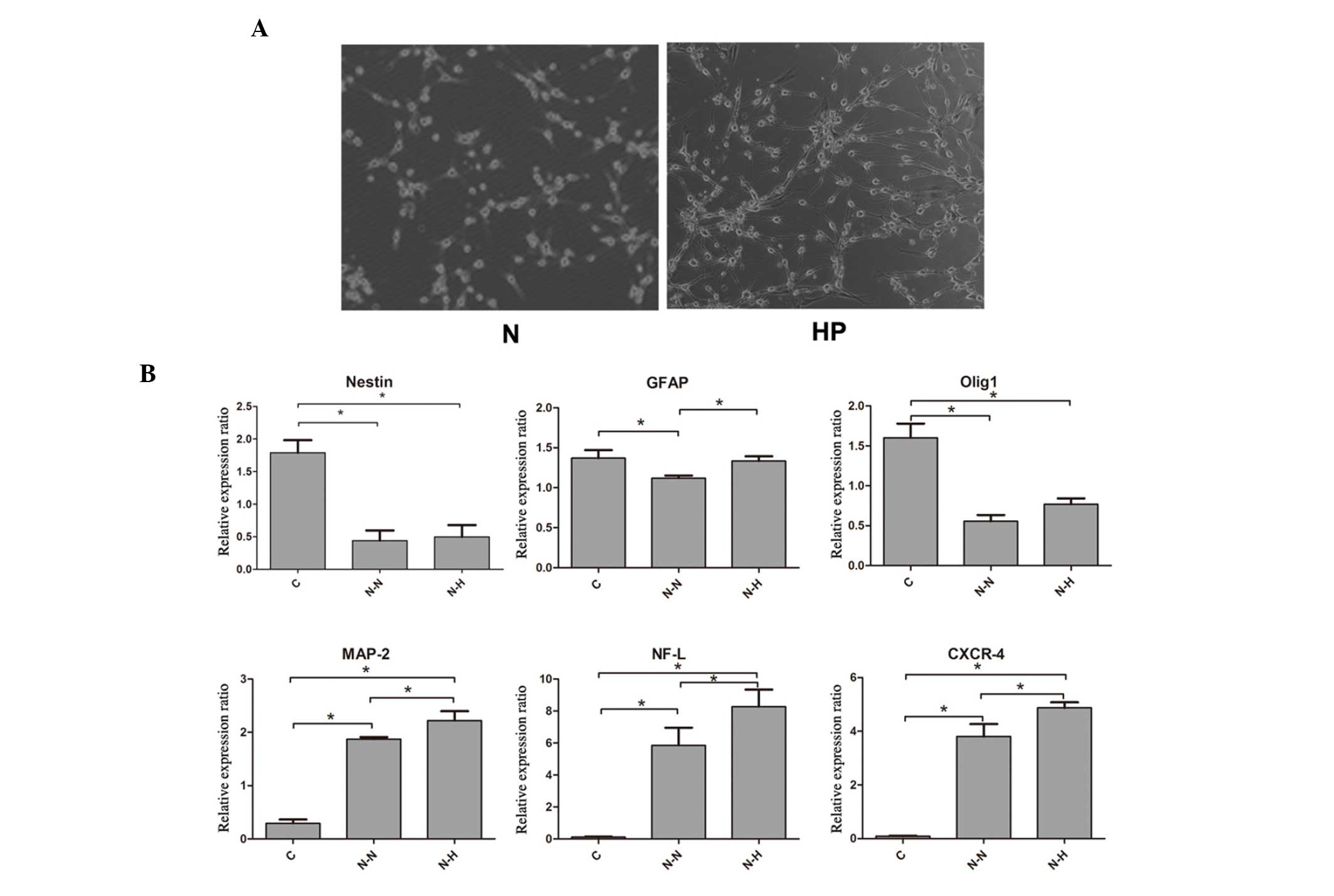 | Figure 2.Hypoxic preconditioning (HP) promoted
neural differentiation compared with that under normoxic (N)
conditions. (A) Neuronal differentiation under hypoxic conditions
showed a higher percentage of neuron-like cells in contrast with
that under normoxia. (B) Reverse transcription-quantitative
polymerase chain reaction assay showed significantly increased
MAP-2, NF-L and CXCR-4 mRNA levels in the neuronal induction groups
compared with the C group (P<0.05). Additionally, these markers
were expressed at significantly higher levels under hypoxic
conditions (P<0.05). Except for GFAP, the expression of Nestin
and Olig1 showed no significant difference between the hypoxic and
normoxic neuronal induction groups. *P<0.05. N-N, neuronal
induction, normoxic; N-H, neuronal induction, hypoxic; GFAP, glial
fibrillary acidic protein; Olig1, oligodendrocyte transcription
factor 1; MAP-2, microtubule-associated protein 2; NF-L,
neurofilament light chain; CXCR4, C-X-C chemokine receptor type 4;
C, control. |
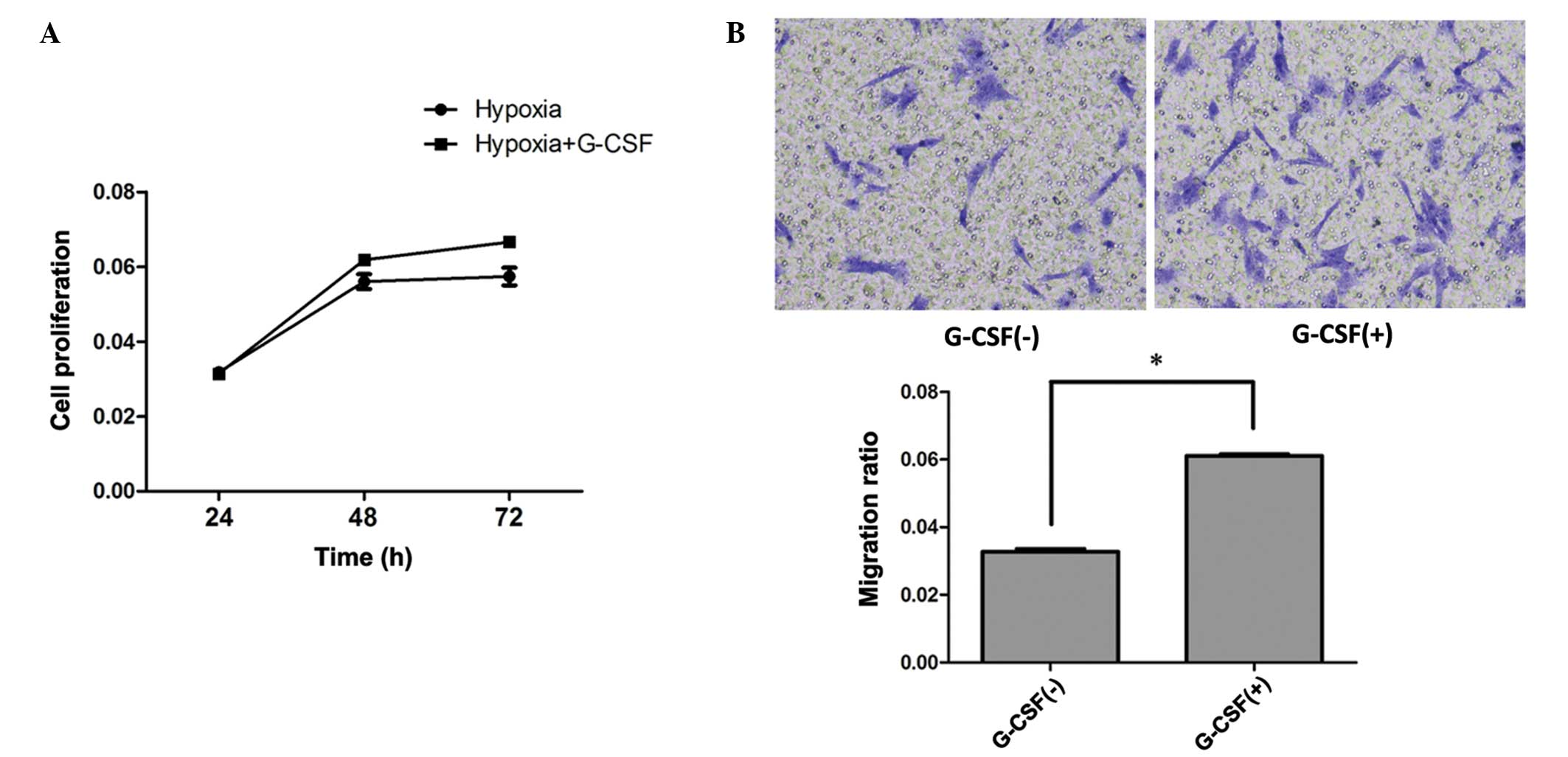
G-CSF treatment promoted the
efficiency of HP on BMSCs
To further evaluate the effect of G-CSF on
HP-treated BMSCs, cells were co-cultured with 2 u/cell G-CSF after
HP treatment; 2 u/cell G-CSF shows a protective effect on BMSCs
(data not shown). The proliferating ability of BMSCs was increased
in the G-CSF culture group compared with that of the group treated
with hypoxia alone (P<0.05; Fig.
3A).
To further evaluate the cell migration ability,
Transwell assays were used. BMSCs were plated in the upper chamber
with G-CSF. A greater number of cells migrated following G-CSF
culture than with HP treatment only (P<0.05; Fig. 3B).
Discussion
BMSCs have shown great promise in ischemic tissue
repair and have the following advantages: i) No ethical issues, as
they are a self-derived bone marrow derivative ii) high
proliferation rate, and so can be amplified in a short period of
time; iii) no transplant rejection occurs so no immune-suppressants
are required. It has been reported that the transplantation of
BMSCs promotes the repair and regeneration of nerve tissue within
the central and peripheral nervous systems (14). A study of experimental stroke in
animals have shown that the transplantation of BMSCs has promising
benefits on functional recovery following ischemic stroke or
traumatic injury (15). However, the
therapeutic effect of BMSC transplantation is limited due to the
poor survival and migration of BMSCs. The present in vitro
study provided direct evidence that HP preserves BMSCs by
increasing their proliferation ability and facilitating their
migration rate. In brief, HP is favorable to BMSC culture. This
finding is also supported by the ‘Adaptive cytoprotection’ theory:
pretreatment of cells with a moderate stimulus that does not cause
cell damage improves the cell tolerance (16,17).
Similar to the results of the present study, in
vitro and in vivo studies have shown the benefits of
hypoxic pre-conditioned stem cells on neural differentiation
(18,19). However, in these studies, neural
differentiation and hypoxic conditioning were not conducted
simultaneously. In the present study, neural differentiation
induced under hypoxic conditions exhibited significantly higher
neural differentiation and relative marker expression than that
observed under normoxic conditions. This may better mimic the in
vivo environment of transplanted mesenchymal stem cells (MSCs),
and indicates that transplanted MSCs may act better in ischemic
regions than in normal tissues. The stromal cell-derived factor
1/CXCR-4 axis plays an important role in stem cell recruitment
(20). The results of the present
study showed that CXCR-4 was expressed with neural differentiation
concurrently, and hypoxic conditioning could markedly improve this
effect. Nichols et al induced CD133+
ABCG2+CXCR4+ MSCs with human peripheral
blood-derived MSCs by priming with β-mercaptoethanol combined with
trans-retinoic acid and culturing in neural basal media
(21). These induced MSCs
consistently expressed markers of neural lineage and could be
recruited predominantly to the site of nervous injury to reduce
apoptosis (21). Therefore, we
speculate that hypoxia co-induced BMSCs may be more suitable and
beneficial in ischemic stroke, although additional in vivo
evidence is required.
Cell death following cerebral ischemia is
acknowledged to be mediated by a complex pathophysiologic
interaction of different mechanisms. Therefore, the present study
focused on identifying new and effective strategies that can
improve the survival and migration ability of BMSCs. The results
demonstrated that G-CSF improved the efficiency of HP in the
promotion of canine BMSC survival and migration in vitro.
This is consistent with the previously reported finding that G-CSF
treatment following Trypanosoma cruzi infection enhanced the
migration and homing of BMSCs (11),
and that G-CSF has an influence on recovery following neuronal
injury (12). Thus, the present
study not only explored a simple and effective strategy to improve
the proliferation and migration ability of BMSCs, but also
suggested that HP and co-culture with G-CSF may represent a
clinically effective and feasible method of manipulation of cell
preparations for a better transplantation therapy outcome.
The use of BMSCs from beagle dogs is rarely reported
and this type of experimental cell has advantages and superiority
as in comparison with small animals without gyri in the brain, the
beagle dog brain is structurally and functionally more similar to
the human brain. The present study focused on beagle dog BMSCs,
which should provide more useful information for clinical
transplantation therapy. We plan to detect BMSC viability and
migration in vivo in the future. BMSCs are known to secrete
numerous biological factors, and under strenuous hypoxia and serum
starvation, the expression levels of some of these factors are
upregulated. Kinnaird et al reported that these included the
following cytokines: Vascular endothelial growth factor, fibroblast
growth factor 2, interleukin-6, placental growth factor and
monocyte chemoattractant protein-1 (22). The intramyocardial injection of
MSC-conditioned media improved collateral blood flow recovery and
limb function, and reduced muscle atrophy (22). The changes of these cytokines in our
canine model were not tested in the present study. In future
studies, we plan to detect these cytokines and signaling that may
improve the biological characteristics of BMSCs and their effects
on bone marrow cell therapy in ischemic injury.
In conclusion, the present study showed that HP and
G-CSF culture can improve the proliferation and migration ability
of canine BMSCs in vitro. This study provides a potential
novel strategy for BMSCs culture that might be useful in ischemic
stroke therapy.
Acknowledgements
This study was supported by China Postdoctoral
Science Foundation (2014M56038 to S. Liu) and National Natural
Science Foundation of China (81471764 to S. Liu, 81401497 to X-Q Xu
and 81401383 to S-S Lu).
Glossary
Abbreviation
Abbreviations:
|
BMSCs
|
bone marrow mesenchymal stem cells
|
|
HP
|
hypoxic preconditioning
|
|
G-CSF
|
granulocyte-colony stimulating
factor
|
References
|
1
|
Chen Y, Teng FY and Tang BL: Coaxing bone
marrow stromal mesenchymal stem cells towards neuronal
differentiation: Progress and uncertainties. Cell Mol Life Sci.
63:1649–1657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Phinney DG and Isakova I: Plasticity and
therapeutic potential of mesenchymal stem cells in the nervous
system. Curr Pharm Des. 11:1255–1265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tse WT, Pendleton JD, Beyer WM, Egalka MC
and Guinan EC: Suppression of allogeneic T-cell proliferation by
human marrow stromal cells: Implications in transplantation.
Transplantation. 75:389–397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nemeth K and Mezey E: Bone marrow stromal
cells as immunomodulators. A primer for dermatologists. J Dermatol
Sci. 77:11–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
White BC, Sullivan JM, DeGracia DJ, O'Neil
BJ, Neumar RW, Grossman LI, Rafols JA and Krause GS: Brain ischemia
and reperfusion: Molecular mechanisms of neuronal injury. J Neurol
Sci. 179:1–33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharp FR, Ran R, Lu A, Tang Y, Strauss KI,
Glass T, Ardizzone T and Bernaudin M: Hypoxic preconditioning
protects against ischemic brain injury. NeuroRx. 1:26–35. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Theus MH, Wei L, Cui L, Francis K, Hu X,
Keogh C and Yu SP: In vitro hypoxic preconditioning of embryonic
stem cells as a strategy of promoting cell survival and functional
benefits after transplantation into the ischemic rat brain. Exp
Neurol. 210:656–670. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weaver CH, Buckner CD, Longin K, Appelbaum
FR, Rowley S, Lilleby K, Miser J, Storb R, Hansen JA and Bensinger
W: Syngeneic transplantation with peripheral blood mononuclear
cells collected after the administration of recombinant human
granulocyte colony-stimulating factor. Blood. 82:1981–1984.
1993.PubMed/NCBI
|
|
9
|
Chiba Y, Kuroda S, Osanai T, Shichinohe H,
Houkin K and Iwasaki Y: Impact of ageing on biological features of
bone marrow stromal cells (BMSC) in cell transplantation therapy
for CNS disorders: Functional enhancement by granulocyte-colony
stimulating factor (G-CSF). Neuropathology. 32:139–148. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu SS, Liu S, Zu QQ, Xu XQ, Yu J, Wang JW,
Zhang Y and Shi HB: In vivo MR imaging of intraarterially delivered
magnetically labeled mesenchymal stem cells in a canine stroke
model. PLoS One. 8:e549632013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
González MN, Dey N, Garg NJ and Postan M:
Granulocyte colony-stimulating factor partially repairs the damage
provoked by Trypanosoma cruzi in murine myocardium. Int J
Cardiol. 168:2567–2574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schäbitz WR, Kollmar R, Schwaninger M,
Juettler E, Bardutzky J, Schölzke MN, Sommer C and Schwab S:
Neuroprotective effect of granulocyte colony-stimulating factor
after focal cerebral ischemia. Stroke. 34:745–751. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nandoe TR, Hurtado A, Levi AD, Grotenhuis
JA and Oudega M: Bone marrow stromal cells for repair of the spinal
cord: Towards clinical application. Cell Transplant. 15:563–577.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y and Chopp M: Marrow stromal cell
transplantation in stroke and traumatic brain injury. Neurosci
Lett. 456:120–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kacimi R, Chentoufi J, Honbo N, Long CS
and Karliner JS: Hypoxia differentially regulates stress proteins
in cultured cardiomyocytes: Role of the p38 stress-activated kinase
signaling cascade, and relation to cytoprotection. Cardiovasc Res.
46:139–150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Corbucci GG, Marchi A, Lettieri B and
Luongo C: Mechanisms of cell protection by adaptation to chronic
and acute hypoxia: Molecular biology and clinical practice. Minerva
Anestesiol. 71:727–740. 2005.PubMed/NCBI
|
|
18
|
Wei L, Fraser JL, Lu ZY, Hu X and Yu SP:
Transplantation of hypoxia preconditioned bone marrow mesenchymal
stem cells enhances angiogenesis and neurogenesis after cerebral
ischemia in rats. Neurobiol Dis. 46:635–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung DJ, Wong A, Hayashi K and Yellowley
CE: Effect of hypoxia on generation of neurospheres from adipose
tissue-derived canine mesenchymal stromal cells. Vet J.
199:123–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Fu W, Zhang S, He X, Liu Z, Gao D
and Xu T: CXCR-7 receptor promotes SDF-1α-induced migration of bone
marrow mesenchymal stem cells in the transient cerebral
ischemia/reperfusion rat hippocampus. Brain Res. 1575:78–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nichols JE, Niles JA, DeWitt D, Prough D,
Parsley M, Vega S, Cantu A, Lee E and Cortiella J: Neurogenic and
neuro-protective potential of a novel subpopulation of peripheral
blood-derived CD133+ ABCG2+ CXCR4+ mesenchymal stem cells:
Development of autologous cell-based therapeutics for traumatic
brain injury. Stem Cell Res Ther. 4:32013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kinnaird T, Stabile E, Burnett MS, Shou M,
Lee CW, Barr S, Fuchs S and Epstein SE: Local delivery of
marrow-derived stromal cells augments collateral perfusion through
paracrine mechanisms. Circulation. 109:1543–1549. 2004. View Article : Google Scholar : PubMed/NCBI
|