Introduction
The vascular endothelium, which lines the inner
surface of the entire cardiovascular system (1,2),
performs vital functions in maintaining permeability, vessel wall
integrity and homeostasis, and preventing thrombosis (1–4).
Caveolae, transendothelial channels (TECs), and fenestrae are
subcellular organelles that are present in a subset of endothelial
cells (ECs) that regulate microvascular permeability (1–3).
Caveolae are plasma membrane invaginations displaying a thin
stomatal diaphragm (SD) in their necks (5,6).
Fenestrae, which are 60–80 nm transcellular pores spanned by
fenestral diaphragms (FDs), consist of radial fibrils on their
lumenal side (7–9). TECs are spanned by two stomatal
diaphragms, and arise interspersed with fenestrae in discontinued
areas of ECs (9–11).
Plasmalemma vesicle-associated protein (PLVAP) is
commonly considered to be endothelium-specific as it is an antigen
for two classic antibodies of the endothelium: Mouse endothelial
cell antigen (MECA)-32 and pathologische anatomie
Leiden-endothelium (PAL-E) (4,10,12,13).
PLVAP was the first molecular component of fenestrae to be
identified, and is essential for the development of FDs and SDs in
ECs (14,15). Previous studies have reported roles
for PLVAP in the regulation of basal permeability, leukocyte
migration and angiogenesis (16–20). In
addition, upregulation of PLVAP has previously been associated with
cancer, traumatic spinal cord injury, transplant glomerulopathy
(TG), ischemic brain disease and ocular disease (16,17,21–26)
(Table I). Furthermore, PLVAP has
been investigated as a novel target in cancer therapy (27). The present review provides a detailed
description of the current understanding of the biological
properties and functions of PLVAP.
 | Table I.Functions of plasmalemma
vesicle-associated protein in diseases. |
Table I.
Functions of plasmalemma
vesicle-associated protein in diseases.
| Disease | Function | Refs. |
|---|
| Cancer | Angiogenesis↑ | (16–18,43) |
|
| Permeability↑ | (32,43) |
| Traumatic spinal
cord injury | Angiogenesis↑ | (25) |
| Transplant
glomerulopathy | Permeability↑ | (26,47) |
| Acute ischemic
brain disease | Permeability↑,
angiogenesis↑ | (17,43) |
| Norrie disease | Permeability↑,
angiogenesis↑ | (21) |
| Diabetic
retinopathy | Permeability↑,
angiogenesis↑ | (22–24) |
PLVAP protein structure
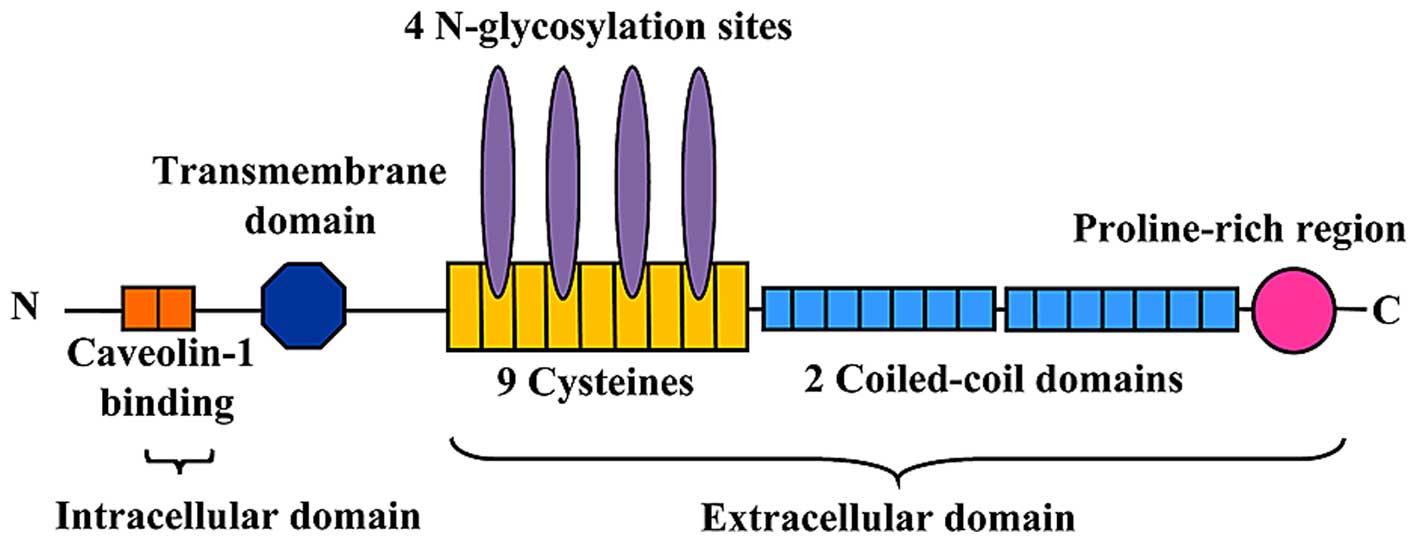
PLVAP, a type II integral membrane glycoprotein with
a molecular weight of ~60 kDa, forms dimers in situ and
binds to heparin at physiological pH (3,5,28,29).
PLVAP consists of three sections: A short (27-amino acid)
intracellular tail, a transmembrane domain and a long (358-amino
acid) extracellular C-terminal domain (6,30)
(Fig. 1). The intracellular domain
of PLVAP consists of two short identical stretches of amino acids:
One is adjacent to the transmembrane region (8 amino acids) and
contains a putative caveolin-1 binding domain, whereas the other is
at the extreme N-terminus (7 amino acids) of the protein (6). The extracellular domain consists of
four N-glycosylation sites, a proline-rich region near the
C-terminus and two large coiled-coil domains (31) (Fig.
1). Every seventh amino acid of the α-helix of the coiled-coil
domain is hydrophobic to facilitate the formation of an
intermolecular superhelix (4).
PLVAP protein expression pattern
The PLVAP protein is restricted to the membrane of a
subset of ECs in the normal microvasculature (3). The highest levels of PLVAP were
detected in the lungs, kidneys, spleen, endocrine glands and
digestive tract (28). Notably,
PLVAP is not expressed in the ECs of large vessels, with the
exception of the endocardial lining of the heart chambers (6,32).
Regulation of PLVAP
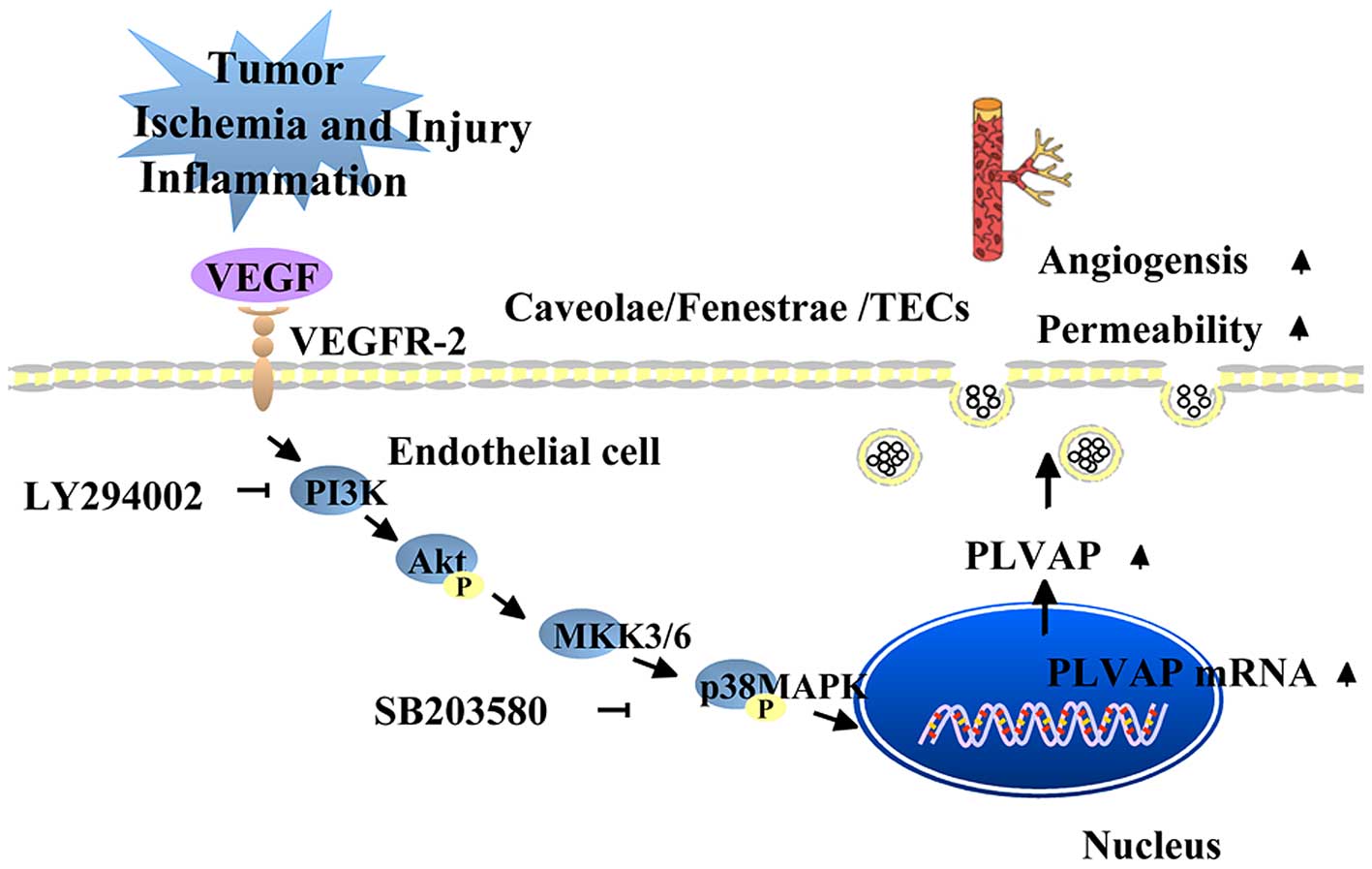
Vascular endothelial growth factor (VEGF), which
stimulates increased vascular permeability and angiogenesis, is the
primary regulator of PLVAP (33).
However, the reports of the effects of VEGF on PLVAP expression
have been conflicting. Hofman et al (34) suggested that PLVAP was directly or
indirectly induced by VEGF, as VEGF and PLVAP (the then PAL-E) were
revealed to simultaneously be present on the retina of diabetic
patients with retinal vascular leakage. Consistent with this,
Strickland et al (33)
demonstrated that treatment of human umbilical vein ECs (HUVECs)
with VEGF increased the mRNA and protein expression levels of PLVAP
via activation of the VEGF receptor 2 (33). Furthermore, this effect was
attenuated by an anti-VEGF monoclonal antibody, and was reported to
be mediated via the phosphatidylinositol 3-kinase (PI3K) and p38
mitogen-activated protein kinase (p38MAPK) signaling pathways
(33) (Fig. 2). In addition, treatment with the
PI3K inhibitor LY294002 or the p38MAPK inhibitor SB203580 induced a
dose-dependent decrease in the mRNA and protein expression levels
of PLVAP (33). However, experiments
using caveolin-1−null mice suggested that PLVAP
expression in the lungs was negatively regulated by VEGF (35). Notably, the PLVAP expression level
remained unchanged in caveolin-2-null mice under identical
experimental conditions (35). These
seemingly contradictory results suggested that other endothelial
proteins, such as caveolin-1, may affect VEGF-mediated regulation
of PLVAP expression. In addition, the effects of increased VEGF
expression on PLVAP expression may vary across different organs
and/or species (33,35). PLVAP expression has also been shown
to be regulated by phorbol myristate acetate (PMA), an activator of
protein kinase C (14). The
treatment of EC cultures with PMA resulted in the upregulation of
PLVAP expression in a dose-dependent and time-dependent manner
(14). Furthermore, PMA-induced
upregulation of PLVAP expression was hypothesized to be dependent
on the activation of the extracellular signal-regulated protein
kinase 1/2-MAPK signaling pathway (14).
Roles of PLVAP in physiological
processes
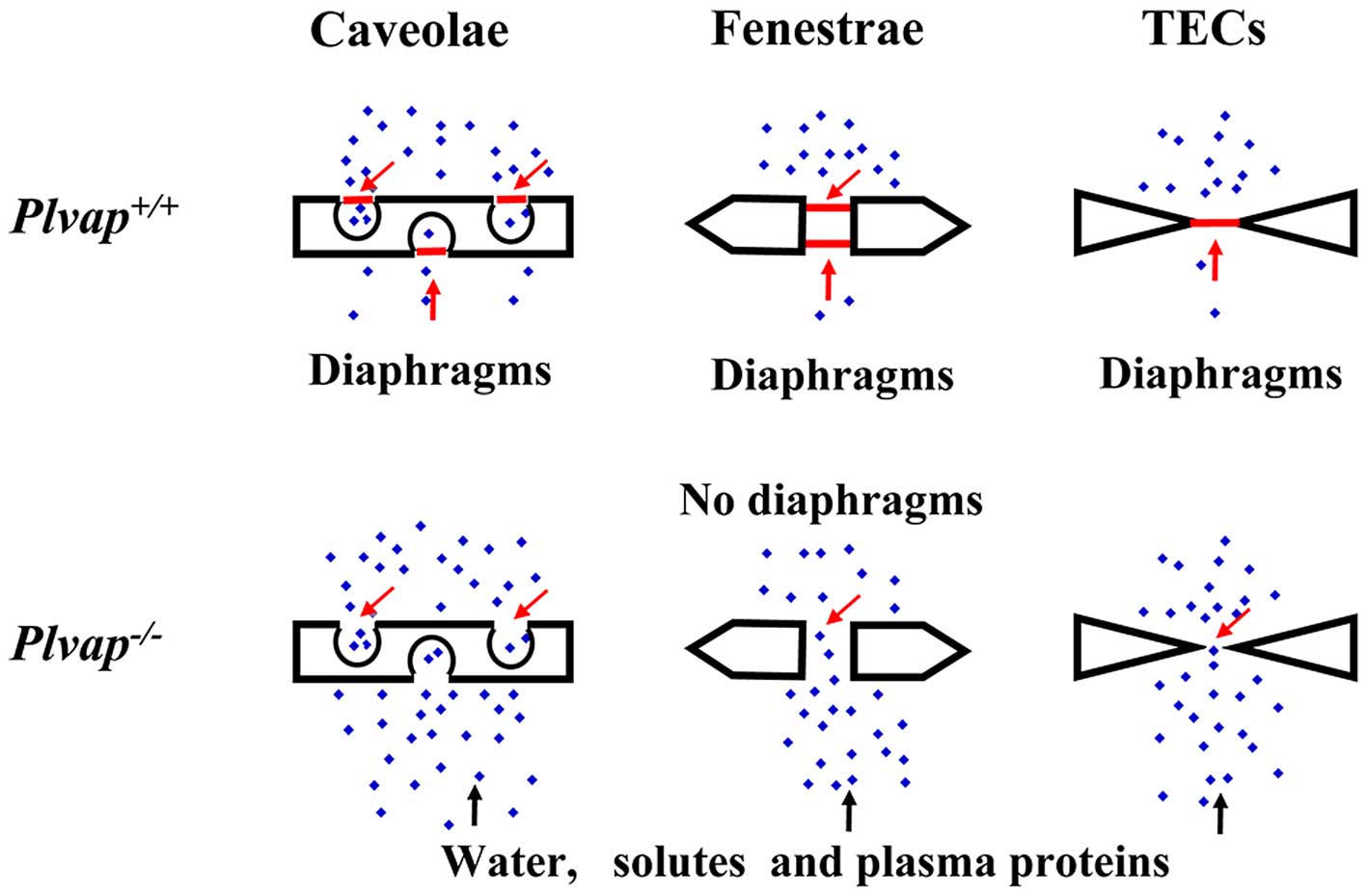
PLVAP forms SDs and FDs
PLVAP, which is the only known molecular component
of SDs and FDs (14,28), forms homodimers that are cross-linked
in situ (5,14,28). The
upregulation of PLVAP expression upon treatment of EC cultures with
PMA was associated with the de novo formation of SDs and FDs
that were demonstrated to contain PLVAP (14). In addition, silencing of PLVAP mRNA
expression inhibited de novo diaphragm formation in
caveolae, TECs and fenestrate (14,15), and
knockout of the PLVAP gene in mice resulted in the complete absence
of SDs and FDs (10). These findings
suggested that PLVAP is required for the formation of SDs and FDs
in ECs.
It has been hypothesized that PLVAP is responsible
for the formation of the radial fibrils that constitute both SDs
and FDs (31). To elaborate, these
diaphragms consist of thin fibrils originating at the inner
surfaces that then intertwine into a knot at the lumenal center of
caveolae, fenestrae or TECs (4). At
present, it is unclear whether PLVAP is the only protein present in
the diaphragms (31). Various
functional groups at the C-terminus of PLVAP potentially offer
binding sites for other PLVAPs, causing the fibrils to interweave
in the observed manner (31). In
addition, the polysaccharides occupying ~15% of the molecular
weight of PLVAP near the membrane may support the membrane and
maintain the fibrils in their correct position (4). Furthermore, interactions between PLVAP
C-termini and the existence of a rigid structure attaching PLVAP to
the cytoskeleton may contribute to the stabilization of the
diaphragms (4).
PLVAP regulates basal permeability and
maintains the integrity of blood vessels
PLVAP is restricted to a subset of capillary
endothelial linings, as demonstrated by immunofluorescence using
anti-PLVAP antibodies (5,28). On a C57BL/6N genetic background,
PLVAP-null embryos died before birth and suffered from edema,
hemorrhages, and defects to the vascular wall of subcutaneous
capillaries (36). Furthermore,
diaphragms were missing from caveolae of the subcutaneous
capillaries and endocardium ECs of PLVAP-null mice (36). In addition, capillaries of the
pancreas and kidney peritubular interstitium of PLVAP-null mice
demonstrated a complete absence of diaphragms in fenestrae,
caveolae, and TECs (36).
Transmission electron microscopy confirmed the lack of endothelial
integrity, detecting extensive defects in the endothelial lining of
capillaries (36).
Similarly, Stan et al (10) demonstrated that the deletion of the
PLVAP gene in mice of a mixed BALB/c-C57Bl/6J-129Sv/J
background resulted in decreased survival due to the absence of
diaphragms. In particular, the loss of diaphragms disrupted the
barrier function of fenestrated capillaries, causing a major leak
of plasma proteins, and leading to hypoproteinemia,
hypertriglyceridemia, and an increased plasma concentration of
chylomicron remnants (10). As a
result, PLVAP-null mice suffered from premature death due to
severe enteropathy and edema of the intestine, kidneys, and
pancreas (10). In addition, the
results of an Evans Blue dye extravasation assay indicated that the
intestinal capillaries displayed the highest rate of protein
leakage (10). Conversely,
endothelial reconstitution of PLVAP by breeding with
PLVAP transgenic mice rescued the PLVAP-null
phenotype and restored the diaphragms of fenestrae and caveolae
(10). As human influenza
hemagglutinin (HA)-tagging of PLVAP did not alter its behavior in
cultured cells, VEC-PV1HA-HA transgenic mice were generated
that expressed PV1-HA under the control of the vascular
endothelial-cadherin promoter and a 5′-intronic enhancer element
(10,37). These mice demonstrated ~30–50%
reconstitution of PLVAP expression, in addition to the
restoration of FDs/SDs in the lungs, adrenal glands, kidneys,
pancreas, thyroid and intestine, which led to improved survival
(10). Therefore, PLVAP is crucial
for the regulation of vascular permeability (Fig. 3).
PLVAP facilitates leukocyte
trafficking
The migration of leukocytes from the bloodstream
into tissues via a transcellular pathway through the endothelium is
one of the central paradigms of inflammation and immunity (19,38).
In vitro transmigration experiments have demonstrated that
rings containing PLVAP and caveolin-1 surround lymphocytes during
their migration (19). PLVAP was
also reported to redistribute in the presence of and partially
colocalize with vimentin and caveolin-1 (19). In capillary flow assays, tumor
necrosis factor-α activated HUVECs grown in glass capillaries and
incubated with anti-PLVAP antibody (19). As a result, the transmigration of
peripheral blood mononuclear cells through the HUVEC layer was
significantly inhibited, although the rolling and adhesion
functions were unaffected (19).
Blockage of PLVAP using a MECA-32 antibody in an acute peritonitis
model decreased leukocyte migration by ~85% (19). Therefore, PLVAP potentially
aggravates inflammation by increasing leukocyte trafficking.
PLVAP controls the entry of
lymphocytes into lymph nodes
Lymph nodes are specialized for efficient
interaction of peripheral antigens with lymphocytes (39,40).
Caveolae, TECs and vesiculo-vacuolar organelles (VVOs) with
diaphragms were identified in the subcapsular sinus lymphatic ECs
(LECs) of lymph nodes (41). PLVAP
is synthesized in LECs, which line the sinuses and cover the distal
lymphatic vessels (41). Diaphragms
consisting of PLVAP fibrils in TECs act as physical sieves that are
responsible for regulating the entry of soluble antigens and
lymphocytes into the parenchyma (41). PLVAP-null mice demonstrated an
increase in lymphocyte transmigration through the sinus floor, in
addition to non-selective antigen entry into the lymph system
(41). Similarly, in the absence of
PLVAP diaphragms on the sinusoidal floor LECs, the migration of
lymph-generated lymphocytes to the parenchyma of peripheral lymph
nodes (PLNs) is increased (41).
When PLVAP-containing diaphragms interacted with antibodies, the
entry of lymphocytes into the PLNs was prohibited at the
sinus-cortex interface (41). These
observations suggested that the selective entry of lymphocytes and
antigens into the lymphatic nodes requires PLVAP (41).
In a previous study, migrating lymphocytes were
demonstrated to produce F-actin-rich podosomes that probe the
lymphatic sinus floor (41). The
large PLVAP-positive patches observed on the LEC floor at points of
transendothelial diapedesis in the sinus were colocalized with the
areas populated by F-actin-rich podosomes (41). Electron microscopy revealed that,
lymphocytes typically made contact with the wild-type LEC membrane
in regions containing caveolae, VVOs, TECs and diaphragms (41). Therefore, PLVAP-positive diaphragms
guarding the pores, tubular structures and channels may offer the
path of least resistance through the sinusoidal floor LECs
(41).
PLVAP and diseases
Cancer
With regard to cancerous tissues, PLVAP was first
observed in malignant glioma microvasculature associated with
increased fenestration, malperfusion and hyperpermeability
(16). Subsequently, PLVAP was shown
to be upregulated in ECs in tumors of the brain, lungs, breasts,
stomach, liver, pancreas, colon, small intestine, kidneys, ovaries,
prostate, uterus, skin and lymph nodes (16,33,42).
Furthermore, upregulation of PLVAP was reported to be similar in
primary and metastatic tumors (18).
Increased expression levels of PLVAP in tumors have been associated
with angiogenesis (17). PLVAP is
typically induced in large, well-vascularized tumors, in which
PLVAP colocalizes with the vascular endothelial markers, cluster of
differentiation 31 (CD31) and von Willebrand factor (16,18,43).
Furthermore, PLVAP was reported to be induced in vitro
following exposure to VEGF-enriched medium and tumor cell lines
(17).
As the presence of PLVAP facilitates vascular growth
in cancer, it is considered a novel target for cancer therapy
(16,17). Downregulation of PLVAP using
small hairpin RNA prevented the development of pancreatic
adenocarcinoma in xenografts (32).
In a previous study, infusion of recombinant monoclonal anti-PLVAP
antigen-binding fragment co-expressed with the extracellular domain
of human tissue factor into the feeding artery of hepatocellular
carcinoma (HCC) led to vascular thrombosis and extensive necrosis
of HCC (27). Furthermore, the
suppression of tumor growth and minimal systemic toxicity indicated
that PLVAP is a novel therapeutic target for the treatment of HCC
(27).
Traumatic spinal cord injury
Spinal cord injury (SCI) is a potentially
life-threatening condition (44,45).
Following SCI, both ECs and neurons are rapidly lost (45,46).
Expeditious and extensive cell death leads to inflammation followed
by adaptive angiogenic responses at and around the epicenters of
primary injury (45,46). In contusive SCI mice, PLVAP was
detected in several microvascular beds at 12 h post-SCI, with the
level of PLVAP peaking at around 3–7 days post-injury (25). The majority of newly generated
vessels at the injury sites were PLVAP-positive on day 3
post-injury, thus suggesting that PLVAP is expressed in
neovasculature post-SCI (25).
Furthermore, microvessels expressing PLVAP appeared to be
spatially correlated with tissues containing actively extravasated
neutrophils (25). On day 3
post-SCI, the expression of intracellular junction component zonula
occludens-1 (ZO-1) and occludin was negligible in PLVAP-positive
microvessels, which was indicative of a significant disruption of
neurovascular integrity (25).
Therefore, the upregulation of PLVAP may lead to secondary
injury post-SCI via the induction of inflammation and deterioration
of neurovascular function (25).
TG
Normal mature glomerular endothelium contains
fenestrae without FDs for trafficking of macromolecules (26,47)
(Fig. 3). One of the
histopathological features of TG is the upregulation of
PLVAP expression and the increased numbers of caveolae in
glomerular ECs (26). Transcytotic
pathways that deliver albumin and immunoglobulins are largely
dependent on vesicles originating from caveolae (20). As glomerular ECs in TG patients
typically demonstrate reduced fenestration under the transmission
electron microscope (26), Yamamoto
et al (26) hypothesized that
the elevation of PLVAP expression and caveolae formation may
be a compensatory mechanism to increase the permeability of ECs to
macromolecules. TG with double contouring of the glomerular
capillaries is a characteristic manifestation of chronic rejection
in an allograft recipient (48). The
double contouring in TG is caused by endothelial injury and
subsequent formation of a basement membrane-like structure beneath
the endothelium; hence, the upregulation of PLVAP expression
in glomerular capillaries may reflect vascular remodeling
post-injury (26,47,48).
Acute ischemic brain disease
In ischemic brain disease, damage to the blood-brain
barrier results in an increased permeability of the
microvasculature and fluid accumulation in the extracellular space,
leading to brain ischemia, hypoxia and eventually mortality
(43). PLVAP was suspected to be
induced during hypoxia, as PLVAP upregulation was detected in the
brain tissues of patients with acute ischemia (17). In C57/B6 mouse models with focal
cerebral ischemia, negligible PLVAP staining was observed at 48 h
following disease onset, and marked upregulation was detected 5
days later (43). Furthermore, all
PLVAP-positive cells were shown to be located around the area of
ischemic damage (43). Within the
tissues, ~17% of CD31-positive vessels were expressing
PLVAP by 48 h (43). As a
result of PLVAP upregulation, no statistical difference was
observed between the number of PLVAP-positive vessels and
CD31 expressing vessels on day 7 following disease onset
(43).
Norrie disease
Norrie disease, which is characterized by abnormal
angiogenesis and exudative vitreoretinopathy, is caused by
mutations in the Norrie disease pseudoglioma (NDP) gene
(49–51). One-week-old NDP-null mice had
reduced retinal capillarization (52), and increased endothelial fenestration
and disrupted vascular integrity were observed in the retinas of
NDP-null mice a week later (21). Unlike the wild-type mice, ectopic
expression of PLVAP protein was demonstrated in the developing
retinal vasculature of NDP-null mice, providing evidence for
a potential role of PLVAP in the pathogenesis of Norrie disease
(21).
Diabetic retinopathy (DR)
Loss of blood-retinal barrier (BRB) integrity is an
important feature underlying the pathogenesis of diabetic macular
edema (DME) (22). Increased retinal
VEGF levels are associated with BRB breakdown in diabetic rodents,
primates and humans (22,23,34).
VEGF is considered to induce transcellular transport via caveolae
(22). In previous studies, exposure
to exogenous VEGF led to an increase in the number of pinocytotic
vesicles at the lumenal side of retinal capillaries, and to
increased levels of PLVAP in caveolae (23,53). To
mimic the in vivo pathophysiology of DME, bovine retinal ECs
(BRECs) were stimulated with VEGF, which led to an increase in the
mRNA expression levels of caveolin-1 and PLVAP (22). In addition, a cell-based
enzyme-linked immunosorbent assay detected a significant increase
in the PLVAP content of BRECs at 72 h following VEGF treatment
(23).
Mating the diabetic Ins2Akita (Akita)
mice with the human VEGF photoreceptor-overexpressing trVEGF029
(Kimba) mice resulted in the Akimba
(Ins2AkitaVEGF+/−)
mouse model that displayed retinal neovascularization and
hyperglycemia, which are characteristics of advanced clinical DR
(24). BRB loss, which was
characterized by fluorescein leakage, was observed in both Kimba
and Akimba mice (24). Among these
mice, fluorescein leakage was associated with focal angiogenesis
and PLVAP gene expression, suggesting a vital role for PLVAP
in regulating the permeability of the BRB (24).
Conclusion
PLVAP is an endothelial cell-specific protein that
is crucial for the development of SDs and FDs in subcellular
structures, including caveolae, fenestrae and TECs (1–4). These
diaphragms act as physical sieves that size-dependently control the
exchange of soluble molecules between the blood plasma and
interstitial fluid (10). In a
previous study, PLVAP-deficient mice demonstrated increased
premature mortality due to non-inflammatory protein severe
enteropathy (10). Furthermore,
PLVAP facilitates lymphocyte migration, presumably by sustaining
the selective entry of lymphocytes into the parenchyma, in addition
to offering the path of least resistance through cell bodies
(39–41). Previous studies have also suggested
that PLVAP is upregulated in various pathophysiological processes
associated with angiogenesis, including tumorigenesis or the
secondary injury of neurons following SCI (16,25). In
a previous study, PLVAP was shown to be a preferable therapeutic
target for cancer therapy, since administration of PLVAP antibodies
effectively suppressed tumor growth and had minimal systemic
toxicity (27). Therefore, PLVAP may
have a vital role in maintaining vascular integrity and
homeostasis, under both normal and pathological conditions.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no. 81370269).
We are grateful for the support from the Shandong Taishan
Scholarship, which was awarded to Dr Ju Liu.
Glossary
Abbreviations
Abbreviations:
|
BRB
|
blood-retinal barrier
|
|
BRECs
|
bovine retinal endothelial cells
|
|
DR
|
diabetic retinopathy
|
|
ECs
|
endothelial cells
|
|
FD
|
fenestral diaphragms
|
|
HCC
|
hepatocellular carcinoma
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
LECs
|
lymphatic endothelial cells
|
|
NDP
|
Norrie disease pseudoglioma
|
|
PLVAP
|
plasmalemma vesicle-associated
protein-1
|
|
PLNs
|
peripheral lymph nodes
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
p38MAPK
|
p38 mitogen-activated protein
kinase
|
|
PMA
|
phorbol myristate acetate
|
|
SCI
|
spinal cord injury
|
|
SD
|
stomatal diaphragm
|
|
TECs
|
transendothelial channels
|
|
TG
|
transplant glomerulopathy
|
|
VEGF
|
vascular endothelial growth
factor
|
|
VVOs
|
vesiculo-vacuolar organelles
|
|
ZO-1
|
zonula occludens-1
|
References
|
1
|
Aird WC: Phenotypic heterogeneity of the
endothelium: I. Structure, function and mechanisms. Circ Res.
100:158–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aird WC: Phenotypic heterogeneity of the
endothelium: II. Representative vascular beds. Circ Res.
100:174–190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tse D and Stan RV: Morphological
heterogeneity of endothelium. Semin Thromb Hemost. 36:236–245.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stan RV: Endothelial stomatal and
fenestral diaphragms in normal vessels and angiogenesis. J Cell Mol
Med. 11:621–643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stan RV, Ghitescu L, Jacobson BS and
Palade GE: Isolation, cloning, and localization of rat PV-1, a
novel endothelial caveolar protein. J Cell Biol. 145:1189–1198.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stan RV: Structure of caveolae. Biochim
Biophys Acta. 1746:334–348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bearer EL and Orci L: Endothelial
fenestral diaphragms: A quick-freeze, deep-etch study. J Cell Biol.
100:418–428. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simionescu M, Simionescu N, Silbert JE and
Palade GE: Differentiated microdomains on the luminal surface of
the capillary endothelium. II. Partial characterization of their
anionic sites. J Cell Biol. 90:614–621. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rostgaard J and Qvortrup K: Electron
microscopic demonstrations of filamentous molecular sieve plugs in
capillary fenestrae. Microvasc Res. 53:1–13. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stan RV, Tse D, Deharvengt SJ, Smits NC,
Xu Y, Luciano MR, McGarry CL, Buitendijk M, Nemani KV, Elgueta R,
et al: The diaphragms of fenestrated endothelia: Gatekeepers of
vascular permeability and blood composition. Dev Cell.
23:1203–1218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Milici AJ, L'Hernault N and Palade GE:
Surface densities of diaphragmed fenestrae and transendothelial
channels in different murine capillary beds. Circ Res. 56:709–717.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hallmann R, Mayer DN, Berg EL, Broermann R
and Butcher EC: Novel mouse endothelial cell surface marker is
suppressed during differentiation of the blood brain barrier. Dev
Dyn. 202:325–332. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niemela H, Elima K, Henttinen T, Irjala H,
Salmi M and Jalkanen S: Molecular identification of PAL-E, a widely
used endothelial-cell marker. Blood. 106:3405–3409. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stan RV, Tkachenko E and Niesman IR: PV1
is a key structural component for the formation of the stomatal and
fenestral diaphragms. Mol Biol Cell. 15:3615–3630. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ioannidou S, Deinhardt K, Miotla J,
Bradley J, Cheung E, Samuelsson S, Ng YS and Shima DT: An in vitro
assay reveals a role for the diaphragm protein PV-1 in endothelial
fenestra morphogenesis. Proc Natl Acad Sci USA. 103:16770–16775.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Madden SL, Cook BP, Nacht M, Weber WD,
Callahan MR, Jiang Y, Dufault MR, Zhang X, Zhang W, WalterYohrling
J, et al: Vascular gene expression in nonneoplastic and malignant
brain. Am J Pathol. 165:601–608. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
CarsonWalter EB, Hampton J, Shue E,
Geynisman DM, Pillai PK, Sathanoori R, Madden SL, Hamilton RL and
Walter KA: Plasmalemmal vesicle associated protein-1 is a novel
marker implicated in brain tumor angiogenesis. Clin Cancer Res.
11:7643–7650. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, CarsonWalter EB, Cooper A, Winans
BN, Johnson MD and Walter KA: Vascular gene expression patterns are
conserved in primary and metastatic brain tumors. J Neurooncol.
99:13–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keuschnigg J, Henttinen T, Auvinen K,
Karikoski M, Salmi M and Jalkanen S: The prototype endothelial
marker PAL-E is a leukocyte trafficking molecule. Blood.
114:478–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Minshall RD and Malik AB: Transport across
the endothelium: Regulation of endothelial permeability. Handb Exp
Pharmacol. 107–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schafer NF, Luhmann UF, Feil S and Berger
W: Differential gene expression in Ndph-knockout mice in retinal
development. Invest Ophthalmol Vis Sci. 50:906–916. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klaassen I, Hughes JM, Vogels IM,
Schalkwijk CG, Van Noorden CJ and Schlingemann RO: Altered
expression of genes related to blood-retina barrier disruption in
streptozotocin-induced diabetes. Exp Eye Res. 89:4–15. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
WisniewskaKruk J, Hoeben KA, Vogels IM,
Gaillard PJ, Van Noorden CJ, Schlingemann RO and Klaassen I: A
novel co-culture model of the blood-retinal barrier based on
primary retinal endothelial cells, pericytes and astrocytes. Exp
Eye Res. 96:181–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
WisniewskaKruk J, Klaassen I, Vogels IM,
Magno AL, Lai CM, Van Noorden CJ, Schlingemann RO and Rakoczy EP:
Molecular analysis of blood-retinal barrier loss in the Akimba
mouse, a model of advanced diabetic retinopathy. Exp Eye Res.
122:123–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mozer AB, Whittemore SR and Benton RL:
Spinal microvascular expression of PV-1 is associated with
inflammation, perivascular astrocyte loss, and diminished EC
glucose transport potential in acute SCI. Curr Neurovasc Res.
7:238–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto I, Horita S, Takahashi T, Tanabe
K, Fuchinoue S, Teraoka S, Hattori M and Yamaguchi Y: Glomerular
expression of plasmalemmal vesicle-associated protein-1 in patients
with transplant glomerulopathy. Am J Transplant. 7:1954–1960. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang YH, Cheng TY, Chen TY, Chang KM,
Chuang VP and Kao KJ: Plasmalemmal vesicle associated protein
(PLVAP) as a therapeutic target for treatment of hepatocellular
carcinoma. BMC Cancer. 14:8152014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stan RV, Kubitza M and Palade GE: PV-1 is
a component of the fenestral and stomatal diaphragms in fenestrated
endothelia. Proc Natl Acad Sci USA. 96:13203–13207. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hnasko R, McFarland M and Ben-Jonathan N:
Distribution and characterization of plasmalemma vesicle protein-1
in rat endocrine glands. J Endocrinol. 175:649–661. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stan RV, Arden KC and Palade GE: cDNA and
protein sequence, genomic organization and analysis of cis
regulatory elements of mouse and human PLVAP genes. Genomics.
72:304–313. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stan RV: Multiple PV1 dimers reside in the
same stomatal or fenestral diaphragm. Am J Physiol Heart Circ
Physiol. 286:H1347–H1353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deharvengt SJ, Tse D, Sideleva O, McGarry
C, Gunn JR, Longnecker DS, Carriere C and Stan RV: PV1
down-regulation via shRNA inhibits the growth of pancreatic
adenocarcinoma xenografts. J Cell Mol Med. 16:2690–2700. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Strickland LA, Jubb AM, Hongo JA, Zhong F,
Burwick J, Fu L, Frantz GD and Koeppen H: Plasmalemmal
vesicle-associated protein (PLVAP) is expressed by tumour
endothelium and is upregulated by vascular endothelial growth
factor-A (VEGF). J Pathol. 206:466–475. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hofman P, Blaauwgeers HG, Vrensen GF and
Schlingemann RO: Role of VEGF-A in endothelial phenotypic shift in
human diabetic retinopathy and VEGF-A-induced retinopathy in
monkeys. Ophthalmic Res. 33:156–162. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hnasko R, Frank PG, BenJonathan N and
Lisanti MP: PV-1 is negatively regulated by VEGF in the lung of
caveolin-1, but not caveolin-2, null mice. Cell Cycle. 5:2012–2020.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Herrnberger L, Seitz R, Kuespert S, Bösl
MR, Fuchshofer R and Tamm ER: Lack of endothelial diaphragms in
fenestrae and caveolae of mutant Plvap-deficient mice. Histochem
Cell Biol. 138:709–724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hisatsune H, Matsumura K, Ogawa M, Uemura
A, Kondo N, Yamashita JK, Katsuta H and Nishikawa S, Chiba T and
Nishikawa S: High level of endothelial cell-specific gene
expression by a combination of the 5′ flanking region and the 5′
half of the first intron of the VE-cadherin gene. Blood.
105:4657–4663. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Williamson JR and Grisham JW: Electron
microscopy of leukocytic margination and emigration in acute
inflammation in dog pancreas. Am J Pathol. 39:239–256.
1961.PubMed/NCBI
|
|
39
|
Girard JP, Moussion C and Förster R: HEVs,
lymphatics and homeostatic immune cell trafficking in lymph nodes.
Nat Rev Immunol. 12:762–773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Germain RN, Robey EA and Cahalan MD: A
decade of imaging cellular motility and interaction dynamics in the
immune system. Science. 336:1676–1681. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rantakari P, Auvinen K, Jäppinen N,
Kapraali M, Valtonen J, Karikoski M, Gerke H, Iftakhar-E-Khuda I,
Keuschnigg J, Umemoto E, et al: The endothelial protein PLVAP in
lymphatics controls the entry of lymphocytes and antigens into
lymph nodes. Nat Immunol. 16:386–396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tichauer KM, Deharvengt SJ, Samkoe KS,
Gunn JR, Bosenberg MW, Turk MJ, Hasan T, Stan RV and Pogue BW:
Tumor endothelial marker imaging in melanomas using dual-tracer
fluorescence molecular imaging. Mol Imaging Biol. 16:372–382. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shue EH, CarsonWalter EB, Liu Y, Winans
BN, Ali ZS, Chen J and Walter KA: Plasmalemmal vesicle associated
protein-1 (PV-1) is a marker of blood-brain barrier disruption in
rodent models. BMC Neurosci. 9:292008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Whetstone WD, Hsu JY, Eisenberg M, Werb Z
and Noble-Haeusslein LJ: Blood-spinal cord barrier after spinal
cord injury: Relation to revascularization and wound healing. J
Neurosci Res. 74:227–239. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Benton RL, Maddie MA, Minnillo DR, Hagg T
and Whittemore SR: Griffonia simplicifolia isolectin B4 identifies
a specific subpopulation of angiogenic blood vessels following
contusive spinal cord injury in the adult mouse. J Comp Neurol.
507:1031–1052. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Casella GT, Bunge MB and Wood PM:
Endothelial cell loss is not a major cause of neuronal and glial
cell death following contusion injury of the spinal cord. Exp
Neurol. 202:8–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ichimura K, Stan RV, Kurihara H and Sakai
T: Glomerular endothelial cells form diaphragms during development
and pathologic conditions. J Am Soc Nephrol. 19:1463–1471. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Joosten SA, Sijpkens YW, van Kooten C and
Paul LC: Chronic renal allograft rejection: Pathophysiologic
considerations. Kidney Int. 68:1–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shastry BS, Hejtmancik JF and Trese MT:
Identification of novel missense mutations in the Norrie disease
gene associated with one X-linked and four sporadic cases of
familial exudative vitreoretinopathy. Hum Mutat. 9:396–401. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Black GC, Perveen R, Bonshek R, Cahill M,
ClaytonSmith J, Lloyd IC and McLeod D: Coats' disease of the retina
(unilateral retinal telangiectasis) caused by somatic mutation in
the NDP gene: A role for norrin in retinal angiogenesis. Hum Mol
Genet. 8:2031–2035. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen ZY, Battinelli EM, Fielder A, Bundey
S, Sims K, Breakefield XO and Craig IW: A mutation in the Norrie
disease gene (NDP) associated with X-linked familial exudative
vitreoretinopathy. Nat Genet. 5:180–183. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Luhmann UF, Lin J, Acar N, Lammel S, Feil
S, Grimm C, Seeliger MW, Hammes HP and Berger W: Role of the Norrie
disease pseudoglioma gene in sprouting angiogenesis during
development of the retinal vasculature. Invest Ophthalmol Vis Sci.
46:3372–3382. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hofman P, Blaauwgeers HG, Tolentino MJ,
Adamis AP, Nunes Cardozo BJ, Vrensen GF and Schlingemann RO: VEGF-A
induced hyperpermeability of blood-retinal barrier endothelium in
vivo is predominantly associated with pinocytotic vesicular
transport and not with formation of fenestrations. Vascular
endothelial growth factor-A. Curr Eye Res. 21:637–645. 2000.
View Article : Google Scholar : PubMed/NCBI
|