Introduction
Decorin (DCN) is one of the important members of the
small leucine-rich proteoglycan family, which is mainly composed of
the 44-kD core proteins and the dermatan sulfate side chains
(1,2). DCN is the main component of the
extracellular matrix (ECM), serving an important role in
maintaining the biological activity of the ECM protein in general,
regulating the cell proliferation and differentiation, and
preventing tissue fibrosis (3–6).
Furthermore, DCN has been found to have significant antitumor
effects (7). Since DCN is widely
expressed in the microenvironment of normal and tumor tissues, it
may influence the biological activity of the tumor cells by
affecting the matrix structure and regulating various receptors
associated with cell proliferation and survival, in order to
further exert an antitumor effect (7,8).
Currently, the expression of DCN in various tumor types and its
inhibitory effects on tumor cell proliferation have been
intensively investigated (9).
However, the role of DCN in hepatic carcinoma has not yet been
fully established.
The signaling pathways involved in the action of DCN
include the following: The regulation of the epidermal growth
factor receptor (EGFR) and other ErbB family members, and the
subsequent activation of the MAPK signaling pathway (10–12); the
modulation of insulin-like growth factor receptor (IGFR) and
low-density lipoprotein receptor-related protein 1, and the further
activation of the phosphoinositide-3 kinase/protein kinase
B/mammalian target of rapamycin pathway (13–16); and
the regulation of the transforming growth factor-β (TGF-β) pathway,
including TGF-β1, TGF-β2, and TGF-β3 (17,18). In
particular, the TGF-β signaling pathway serves different roles at
different stages in the development of hepatic carcinoma. At the
early stage, TGF-β acts as a tumor suppressor, inhibiting cell
proliferation and enhancing cellular differentiation or apoptosis
(19,20). However, at the later stage, TGF-β
gradually loses its antiproliferative effects, and subsequently
stimulates angiogenesis, inhibits immune responses and promotes ECM
formation, which facilitates the cell proliferation and tumor
metastasis (19,20). In the activation of the TGF-β
signaling pathway, TGF-β binds with the extracellular end of the
TGF-β receptor II (TGF-βRII) to form a complex, which is able to
phosphorylate TGF-βRI, further regulating the cell proliferation
and differentiation via the Smad signaling pathway (21–23). It
has been reported that DCN may interact with TGF-β and modulate the
signaling pathway (24,25). However, it has not yet been
established whether there is direct interaction between DCN and
TGF-βRs.
In the present study, the effects of DCN on the
proliferation of human hepatoma HepG2 cells and the involvement of
the TGF-β signaling pathway were investigated. The results
demonstrated that DCN may elevate the expression of TGF-βRII,
enhance the phosphorylation of TGF-βRI and then induce the
overexpression of p15 protein, thus inhibiting the proliferation of
HepG2 cells. Furthermore, knockdown of TGF-βRII would result in the
attenuation of the inhibiting effect of DCN on HepG2 cell
proliferation.
Materials and methods
Cell line and culture
Human hepatoma HepG2 cells were obtained from Thermo
Fisher Scientific, Inc. (Gaithersburg, MD, USA). These cells were
cultured in Dulbecco's modified Eagle's medium supplemented with
10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.,
Carlsbad, CA, USA). The cells were divided into the four following
groups: i) Control group, in which the cells were untreated; ii)
DCN group, in which the cells were transfected with DCN; iii) siRNA
group, in which the cells were transfected with mismatch-siRNA; and
iv) DCN+siRNA, in which the cells were transfected with
DCN+siRNA.
Cell transfection
The pcDNA5/FRT vector (Invitrogen; Thermo Fisher
Scientific, Inc.), harboring an FRT site and a cDNA fragment
containing DCN, was transfected into the HepG2 cells using
Lipofectamine 2000 transfection reagent (cat. no. 11668072;
Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. Enhanced green fluorescent protein was
used as the positive control. In order to detect the transfection
efficiency, the cells were transfected with an enhanced green
fluorescent protein-containing plasmid, and then stained with DAPI
for 5 min. Fluorescence was detected with the IX83 microscope
(Olympus Corp., Tokyo, Japan). The percentage of cells with green
fluorescence was calculated to express the transfection
efficiency.
Small interfering RNA (siRNA)
silencing
siRNAs targeting DCN were purchased from Guangzhou
RiboBio Co., Ltd. (Guangzhou, China) with the following sequences:
i) 5′-GAGGGCAUGUAGACGGUUA d TdT-3′, and 3′-dTdT
CUCCCGUACAUCUGCCAAU-5′; ii) 5′-ACAAUAUGCUACCUCCAAA dTdT-3′, and
3′-dTdT UGUUAUACGAUGGAGGUUU-5′; and iii) 5′-GACUGGAUCCAUACAAUAU
dTdT-3′, and 3′-dTdT CUGACCUAGGUAUGUUAUA-5′. siRNA (30 nM) was used
to silence DCN, and the mismatch-siRNA, i.e. the non-sense RNS
sequence, was used as the control. The transfection was performed
with Lipofectamine 2000, according to the manufacturer's
instructions. After 48 h, the cells were collected and subjected to
analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted with the SYBR Premix Ex Taq
(Takara Bio Inc., Kyoto, Japan), and then reverse transcribed into
cDNA with the M-MLV reverse transcription kit (Takara Bio Inc.),
according to the manufacturer's instructions. The primer sequences
used in qPCR were as follows: DCN forward,
5′-TCGCTCGAGATTTTTTTTTATCAAGAGGG-3′, and reverse,
5′-TCGGCGGCCGCGACAAGAATGAGACTTTAATC-3′; TGF-βRII forward,
5′-GTGTGGAGCAACATGTGGAACTCTA-3′, and reverse,
5′-TTGGTTCAGCCACTGCCGTA-3′; β-actin forward,
5′-CATGTACGTTGCTATCCAGGC-3′, and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′. The 25 ml PCR reaction system
contained 12.5 µl SYBR Premix Ex Taq (Takara Bio Inc.), 1 µl of
each primer, 2 µl template and 8.5 µl double-distilled
H2O. The reaction conditions were as follows:
Denaturation at 95°C for 30 sec; then 95°C for 5 sec and 60°C for
20 sec, for a total of 40 cycles. The relative expression of the
target gene was calculated with the 2−ΔΔCq method
(26). Experiments were performed in
triplicate.
MTT assay
Cellular proliferation was assessed by the MTT
assay. Briefly, HepG2 cells were seeded onto the 96-well plates at
the density of 5,000 cells/well. After 48 h, the culture medium was
discarded, and 200 µl MCDB 131 (cat. no. L-1202-500; Thermo Fisher
Scientific, Inc., Gaithersburg, MD, USA) and 20 µl MTT (Cell
Proliferation kit I; cat. no. 11465007001; Roche, Indianapolis, IN,
USA) were added into each well. After 3.5-h incubation, the
solution was discarded and 100 µl dimethyl sulfoxide (DMSO; cat.
no. D2650; Sigma-Aldrich, St. Louis, MO, USA) was added. After 10
min, the DMSO solution was transferred into a new 96-well plate,
and the absorbance at 490 nm was read on a microplate reader
(iMark; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
The protein expression levels of TGF-βRI,
phosphorylated (p)TGF-βRI, TGF-βRII and p15 were detected by
western blot analysis. Briefly, cells were cultured on 6-well
plates at the density of 20,000 cells/well. After transfection,
cells from all the control and transfection groups were collected
and lysed on ice with the lysis buffer (cat. no. 74255;
Sigma-Aldrich). A total of 30 µg protein sample was subjected to
10% SDS-PAGE, and electronically transferred onto a polyvinylidene
difluoride membrane. The membrane was blocked with 50 g/l skimmed
milk at room temperature for 1 h, and then incubated at 4°C
overnight with the following polyclonal rabbit anti-human primary
antibodies: Anti-TGF-βRI antibody (dilution, 1:5,000; cat. no.
ab31013), anti-pTGF-βRI antibody (dilution, 1:5,000; cat. no.
ab112095), anti-p15 antibody (dilution, 1:5,000; cat. no. ab53034),
and anti-TGF-βRII (dilution, 1:2,000; cat. no. ab61213; all from
Abcam, Cambridge, MA, USA). Next, the membrane was incubated with
goat anti-rabbit horseradish peroxidase-conjugated IgG secondary
antibody (dilution, 1:1,000; cat. no. sc-2030; Santa Cruz
Biotechnology, Inc.) at room temperature for 1 h. The protein bands
were visualized using an enhanced chemiluminescence detection kit
(Sigma-Aldrich). Image Lab software (version 3; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was used to acquire and
analyze the images. GAPDH was used as the control.
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA) software was used
for the statistical analysis. The Student's t-test was used for
pairwise comparison, while analysis of variance was performed for
multiple comparison. P<0.05 was considered to indicate
statistically significant differences.
Results
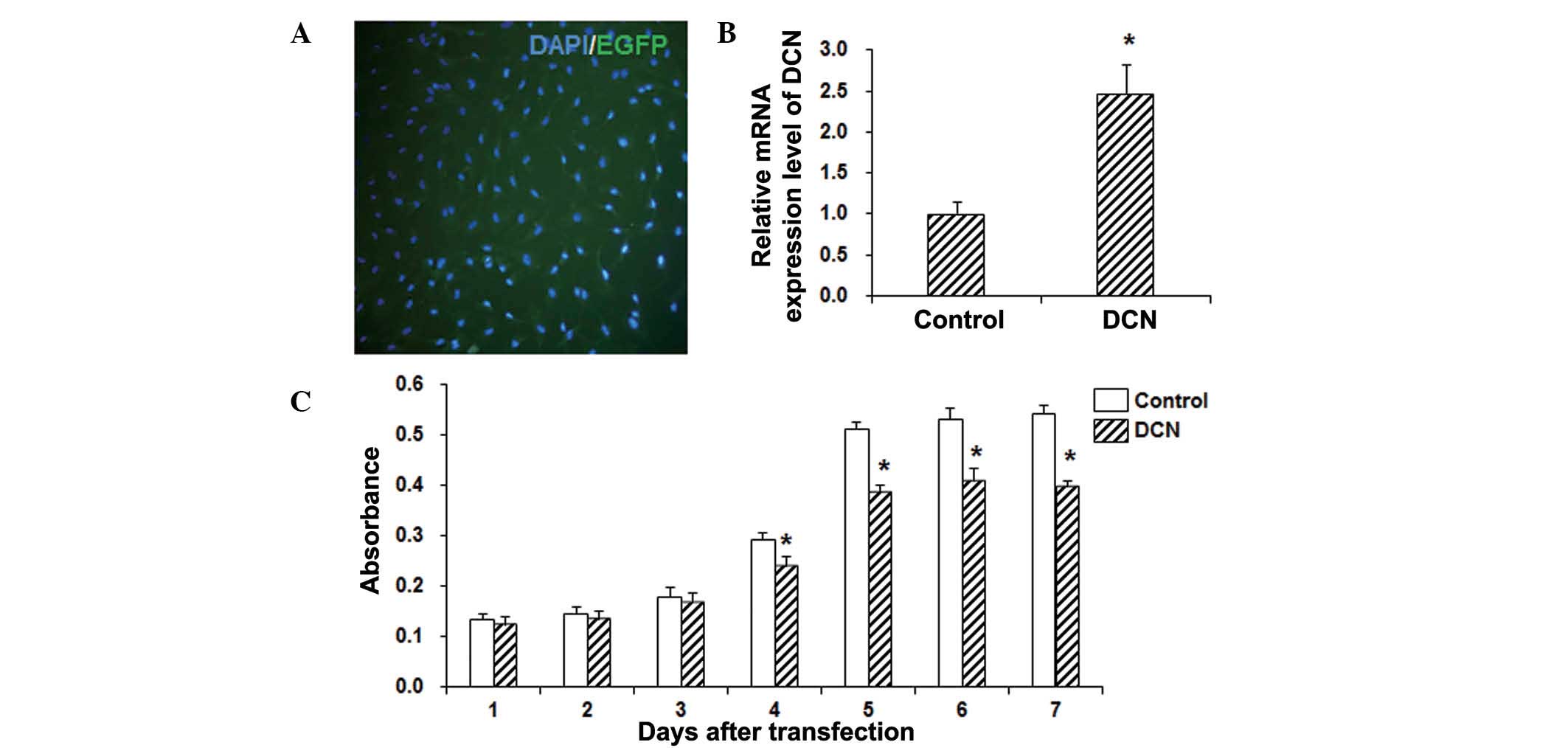
DCN transfection inhibits the
proliferation of HepG2 cells
The vector containing a DCN fragment was transfected
into the HepG2 cells using Lipofectamine 2000, and the mRNA
expression of DCN was detected with RT-qPCR. As shown in Fig. 1A, the transfection efficiency was
>90%. The qPCR results demonstrated that DCN was stably
overexpressed in these HepG2 cells following transfection, and its
expression significantly higher when compared with that in the
control group (P<0.05; Fig. 1B).
In order to investigate the effects of DCN on the proliferation of
HepG2 cells, an MTT assay was performed. The results revealed that,
compared with the control group, the cell viability was
significantly decreased in HepG2 cells transfected with DCN
(P<0.05; Fig. 1C). These results
suggest that DCN is able to significantly inhibit the proliferation
of HepG2 cells.
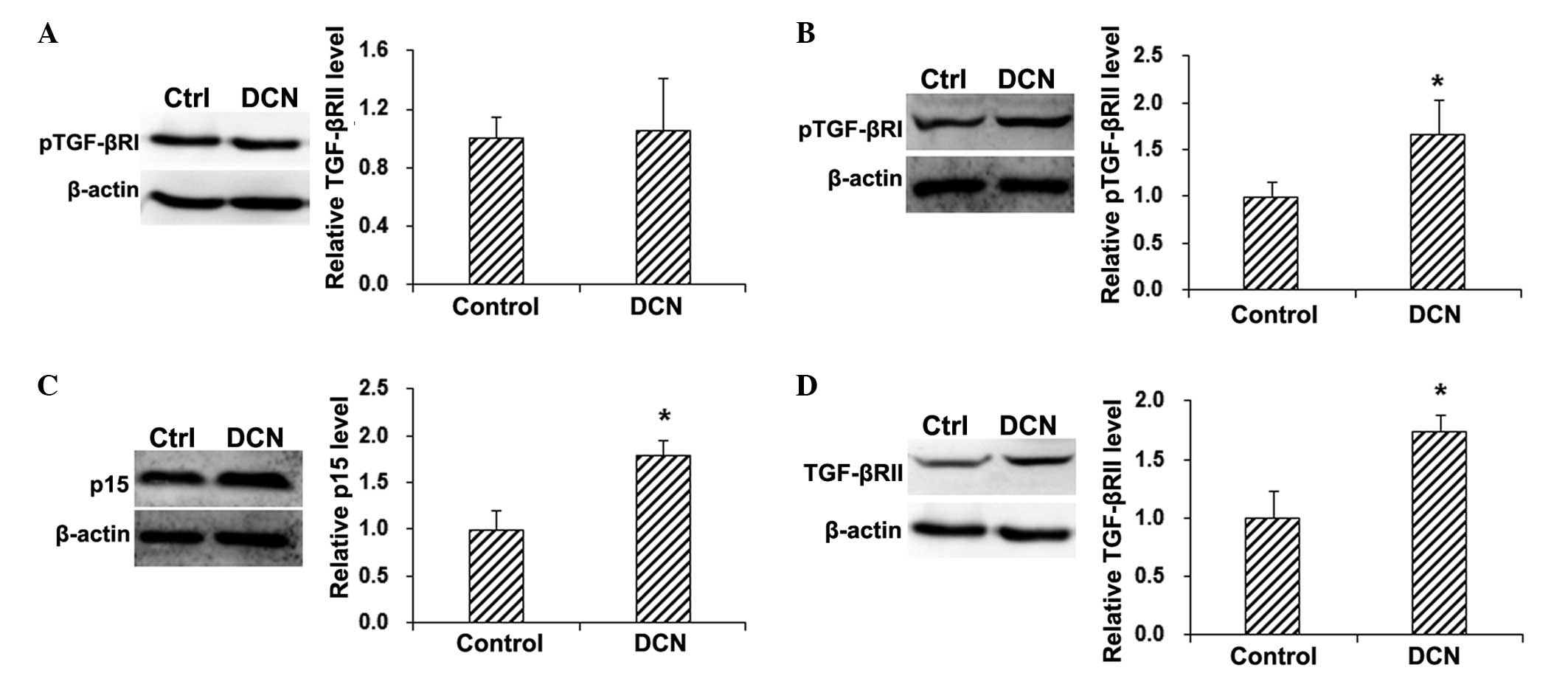
DCN induces TGF-βRI phosphorylation
and elevates p15 expression
To investigate the underlying mechanisms through
which DCN inhibited the proliferation of HepG2 cells, the
expression of cell proliferation-associated signaling pathways was
detected by the western blot analysis. The results showed that,
compared with the control group, no statistically significant
differences were observed in the total TGF-βRI protein expression
level in the HepG2 cells transfected with DCN (P>0.05; Fig. 2A). By contrast, the phosphorylation
of TGF-βRI was significantly increased in HepG2 cells following DCN
transfection (P<0.05; Fig. 2B).
In addition, compared with the control group, the expression of the
downstream factor p15 was significantly elevated in the
DCN-transfected HepG2 cells (P<0.05; Fig. 2C). These results suggest that DCN
transfection is able to activate TGF-βRI and elevate the expression
of p15 in HepG2 cells, implying the involvement of the TGF-β
signaling pathway in the inhibitory effects of DCN on HepG2 cell
proliferation.
DCN increases the expression of
TGF-βRII in HepG2 cells
In the TGF-β signaling pathway, TGF-β binds with the
extracellular end of TGF-βRII, and the complex formed can induce
the phosphorylation of TGF-βRI and regulate the downstream cell
proliferation-associated signaling pathways via Smad (20–22).
Therefore, the expression levels of TGF-βRII in DCN-transfected and
control HepG2 cells were detected by the western blot analysis. The
results demonstrated that, compared with the control group, DCN
transfection significantly elevated the protein expression of
TGF-βRII in HepG2 cells (P<0.05; Fig.
2D). These results suggest that TGF-βRII may be involved in the
inducing effect of DCN on TGF-βRI phosphorylation.
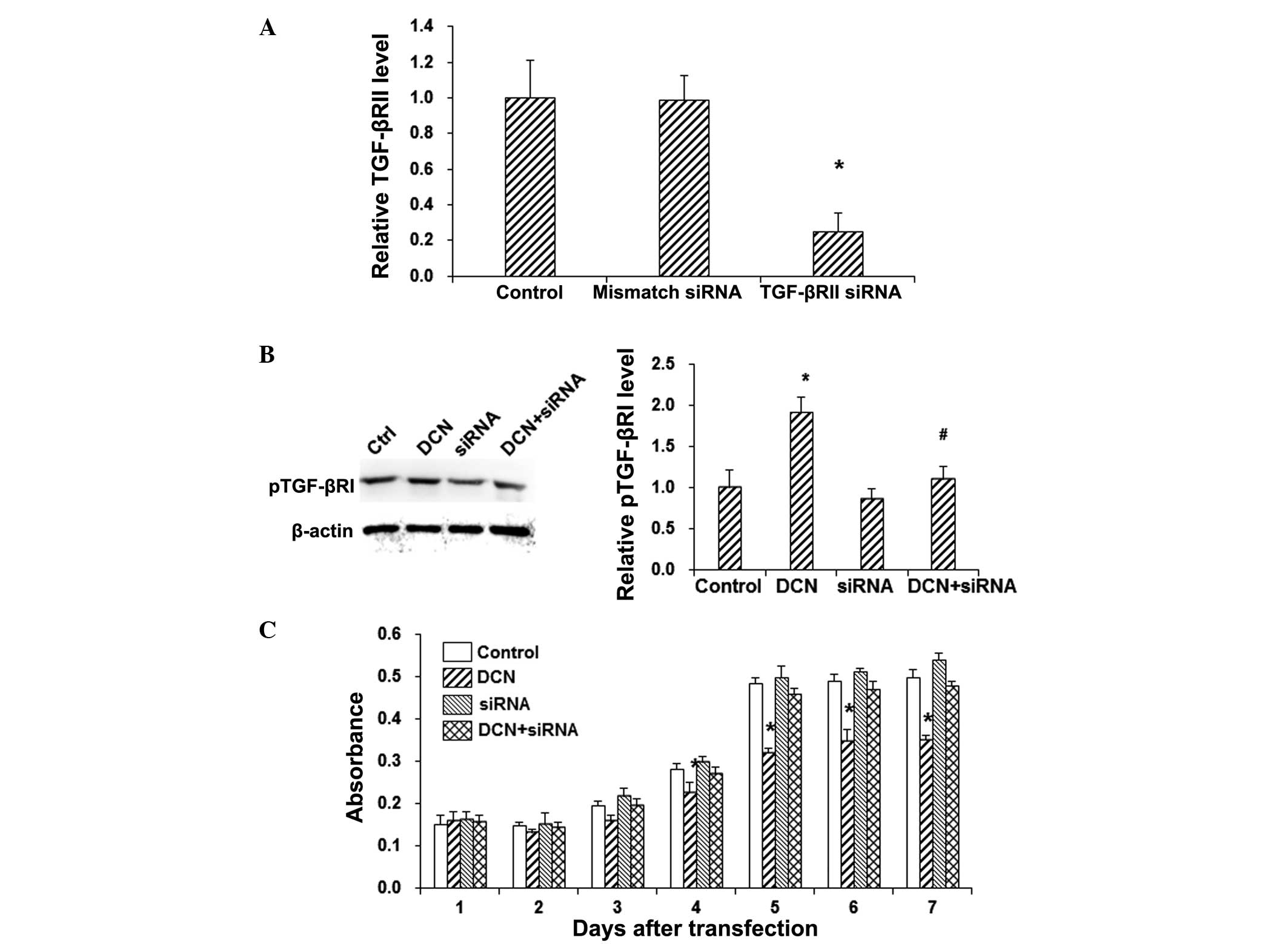
TGF-βRII silencing abolishes the
effects of DCN on TGF-β signaling and HepG2 cell proliferation
In order to further investigate the role of TGF-βRII
in DCN-induced phosphorylation of TGF-βRI in HepG2 cells, TGF-βRII
was knocked down with siRNA silencing. RT-qPCR demonstrated that
the mRNA expression level of TGF-βRII was not significantly altered
by the mismatch-siRNA treatment, while the TGF-βRII mRNA expression
level was significantly decreased in the siRNA silencing group
(P<0.05; Fig. 3A). In addition,
the results from western blot analysis showed that the siRNA
silencing of TGF-βRII alone did not induce significant alteration
in TGF-βRI phosphorylation in the HepG2 cells (P>0.05; Fig. 3B). However, TGF-βRII silencing
significantly decreased the phosphorylation of TGF-βRI in
DCN+siRNA-transfected HepG2 cells, when compared with the DCN group
(P<0.05; Fig. 3B). Furthermore,
the MTT assay revealed that TGF-βRII silencing abolished the
inhibitory effects of DCN on the proliferation of HepG2 cells
(P<0.05; Fig. 3C). These results
suggest that TGF-βRII is a key player in the DCN-induced pTGF-βRI
enhancement and HepG2 cell proliferation inhibition.
Discussion
In the present study, the results demonstrated that
DCN transfection significantly inhibited the proliferation of HepG2
cells, in which the TGF-β signaling pathway was found to serve an
important role. The DCN transfection elevated the phosphorylation
level of TGF-βRI in HepG2 cells, without affecting the total
TGF-βRI expression. The p15 protein is one of the key downstream
factors of TGF-βRI, whose activation may influence cell
cycle-associated proteins, further inhibiting the cell
proliferation (20–22). The current results showed that DCN
transfection was able to significantly elevate the expression level
of p15. Within cells, TGF-β binds with TGF-βRII to further
phosphorylate TGF-βRI (18). The
present study results showed that DCN transfection increased the
expression level of TGF-βRII. In addition, when TGF-βRII was
silenced with siRNA, the phosphorylated TGF-βRI in DCN-transfected
HepG2 cells was significantly decreased, and the cell proliferation
was also significantly inhibited.
Previous studies have shown that DCN inhibited the
TGF-β signaling pathway via binding with TGF-β (16,23). The
current results indicated that DCN transfection elevated the
expression level of TGF-βRII, which then activated the TGF-β
signaling pathway and inhibited the proliferation of HepG2 cells.
Although HepG2 cells may not be an ideal model of the pathogenesis
and development of hepatic carcinoma, the present study revealed
the significance of the TGF-β signaling pathway in the disease
pathology. The TGF-β signaling pathway is able to inhibit the cell
proliferation and differentiation at the early disease stage.
However, at the later stage, TGF-β gradually loses its inhibitory
effects, and thus promotes tumor metastasis (18). The present study results revealed
that DCN enhanced TGF-βRII expression, promoting the TGF-β
signaling pathway, which may represent the early event in the
disease pathogenesis. However, further studies are still required
to investigate the detailed mechanisms through which DCN functions
on the TGF-β signaling pathway.
In conclusion, the results of the present study
showed that DCN transfection significantly elevated the expression
of TGF-βRII, increased the phosphorylation of TGF-βRI, enhanced the
expression of p15, and finally inhibited the proliferation of HepG2
cells. Upon silencing TGF-βRII with siRNA, the effects of DCN on
the TGF-β signaling pathway and the HepG2 cell proliferation were
abolished. The current findings may contribute to the understanding
of the role of DCN in the pathogenesis of hepatic carcinoma and the
disease treatment.
Acknowledgements
This study was supported by a grant from the Twelfth
Five-Year Science and Technology Research Project of the Education
Department of Jilin Province [no. (2012) 127].
References
|
1
|
Goldoni S, Owens RT, McQuillan DJ, Shriver
Z, Sasisekharan R, Birk DE, Campbell S and Iozzo RV: Biologically
active decorin is a monomer in solution. J Biol Chem.
279:6606–6612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scott PG, Dodd CM, Bergmann EM, Sheehan JK
and Bishop PN: Crystal structure of the biglycan dimer and evidence
that dimerization is essential for folding and stability of class I
small leucine-rich repeat proteoglycans. J Biol Chem.
281:13324–13332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kresse H and Schönherr E: Proteoglycans of
the extracellular matrix and growth control. J Cell Physiol.
189:266–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geng Y, McQuillan D and Roughley PJ: SLRP
interaction can protect collagen fibrils from cleavage by
collagenases. Matrix Biol. 25:484–491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rühland C, Schönherr E, Robenek H, Hansen
U, Iozzo RV, Bruckner P and Seidler DG: The glycosaminoglycan chain
of decorin plays an important role in collagen fibril formation at
the early stages of fibrillogenesis. FEBS J. 274:4246–4255. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reed CC and Iozzo RV: The role of decorin
in collagen fibrillogenesis and skin homeostasis. Glycoconj J.
19:249–255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iozzo RV: Tumor stroma as a regulator of
neoplastic behavior. Agonistic and antagonistic elements embedded
in the same connective tissue. Lab Invest. 73:157–160.
1995.PubMed/NCBI
|
|
8
|
Buraschi S, Neill T, Owens RT, Iniguez LA,
Purkins G, Vadigepalli R, Evans B, Schaefer L, Peiper SC, Wang ZX
and Iozzo RV: Decorin protein core affects the global gene
expression profile of the tumor microenvironment in a
triple-negative orthotopic breast carcinoma xenograft model. PLoS
One. 7:e455592012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nyman MC, Sainio AO, Pennanen MM, Lund RJ,
Vuorikoski S, Sundström JT and Järveläinen HT: Decorin in human
colon cancer: Localization in vivo and effect on cancer cell
behavior in vitro. J Histochem Cytochem. 63:710–720. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Santra M, Reed CC and Iozzo RV: Decorin
binds to a narrow region of the epidermal growth factor (EGF)
receptor, partially overlapping with but distinct from the
EGF-binding epitope. J Biol Chem. 277:35671–35681. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santra M, Eichstetter I and Iozzo RV: An
anti-oncogenic role for decorin: Down-regulation of ErbB2 leads to
growth suppression and cytodifferentiation of mammary carcinoma
cells. J Biol Chem. 275:35153–35161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Csordás G, Santra M, Reed CC, Eichstetter
I, McQuillan DJ, Gross D, Nugent MA, Hajnóczky G and Iozzo RV:
Sustained down-regulation of the epidermal growth factor receptor
by decorin. A mechanism for controlling tumor growth in vivo. J
Biol Chem. 275:32879–32887. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schönherr E, Sunderkötter C, Iozzo RV and
Schaefer L: Decorin, a novel player in the insulin-like growth
factor system. J Biol Chem. 280:15767–15772. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schaefer L, Tsalastra W, Babelova A,
Baliova M, Minnerup J, Sorokin L, Gröne HJ, Reinhardt DP,
Pfeilschifter J, Iozzo RV and Schaefer RM: Decorin-mediated
regulation of fibrillin-1 in the kidney involves the insulin-like
growth factor-1 receptor and mammalian target of rapamycin. Am J
Pathol. 170:301–315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schönherr E, Sunderkötter C, Schaefer L,
Thanos S, Grässel S, Oldberg A, Iozzo RV, Young MF and Kresse H:
Decorin deficiency leads to impaired angiogenesis in injured mouse
cornea. J Vasc Res. 41:499–508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schönherr E, Levkau B, Schaefer L, Kresse
H and Walsh K: Decorin affects endothelial cells by akt-dependent
and -independent pathways. Ann NY Acad Sci. 973:149–152. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Imai K, Hiramatsu A, Fukushima D,
Pierschbacher MD and Okada Y: Degradation of decorin by matrix
metalloproteinases: Identification of the cleavage sites, kinetic
analyses and transforming growth factor-beta 1 release. Biochem J.
322:809–814. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brown S, Melrose J, Caterson B, Roughley
P, Eisenstein SM and Roberts S: A comparative evaluation of the
small leucine-rich proteoglycans of pathological human
intervertebral discs. Eur Spine J. 21(Suppl 2): S154–S159. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
BarcellosHoff MH: Latency and activation
in the control of TGF-beta. J Mammary Gland Biol Neoplasia.
1:353–363. 1996. View Article : Google Scholar
|
|
20
|
Halper J: Proteoglycans and diseases of
soft tissues. Adv Exp Med Biol. 802:49–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park C, Kim WS, Choi Y, Kim H and Park K:
Effects of transforming growth factor beta (TGF-beta) receptor on
lung carcinogenesis. Lung Cancer. 38:143–147. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saji H, Nakamura H, Awut I, Kawasaki N,
Hagiwara M, Ogata A, Hosaka M, Saijo T, Kato Y and Kato H:
Significance of expression of TGF-beta in pulmonary metastasis in
non-small cell lung cancer tissues. Ann Thorac Cardiovasc Surg.
9:295–300. 2003.PubMed/NCBI
|
|
23
|
Paik SY, Park YN, Kim H and Park C:
Expression of transforming growth factor-beta 1 and transforming
growth factor-beta receptors in hepatocellular carcinoma and
dysplastic nodules. Mod Pathol. 16:86–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schönherr E, Broszat M, Brandan E,
Bruckner P and Kresse H: Decorin core protein fragment
Leu155-Val260 interacts with TGF-beta but does not compete for
decorin binding to type I collagen. Arch Biochem Biophys.
355:241–248. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harper J: Proteoglycans and diseases of
soft tissuesProgress in Heritable Soft Connective Tissue Diseases.
Springer; Dordrecht: pp. 49–58. 2014
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔ Ct method. Methods. 254:402–408.
2001. View Article : Google Scholar
|