Introduction
Microglial cells, which comprise 12% of the cell
population in the central nervous system (CNS), are macrophage-like
cells (1). Although they have been
recognized mainly for their scavenging functions in the brain,
certain studies have suggested that inflammation mediated by
microglia contributes to the development of neurodegenerative
diseases, such as Alzheimer's disease, multiple sclerosis and
neuropathic pain (2–4). Microglial cells, once activated during
brain pathologies, release a variety of neurotoxic substances
including nitric oxide, cytotoxic cytokines, tumor necrosis
factor-α (TNF-α) and interleukin (IL)-1β. As a result of their
remarkable ability to respond rapidly to a changing external
environment and participate in the regulation of the innate and
adaptive immune system in the CNS, microglia have become a prime
target for therapeutic intervention in a variety of CNS insults
(5,6).
Previous studies have demonstrated that a rise in
the intracellular 3′,5′-cyclic adenosine monophosphate (cAMP) level
leads to the suppression of the activities and proliferation of
microglial cells (7,8). Among the various cAMP-elevating agents,
inhibitors of phosphodiesterase type 4 (PDE4) deserve increased
attention because they increase the cAMP level in mononuclear cells
or macrophages more effectively than other types of PDE inhibitors
(9). Recent studies in mammals have
revealed that PDE4 is a family of cAMP-specific PDEs, with >20
isozymes that are encoded by 4 genes (PDE4A-D) (10,11). The
four subfamilies share a conserved catalytic domain and upstream
conserved region, which are upstream conserved region (UCR)1 and
UCR2 motifs of the regulatory domain. Therefore, the developed
inhibitors often are not able to target a subfamily specifically.
Clinical evaluations in various inflammatory conditions have
revealed that the anti-inflammatory effect of PDE4 inhibitors has a
very broad spectrum, affecting almost all inflammatory cells
(12–14). Some adverse effects that are
associated with this lack of specificity, such as nausea and
emesis, limit the dosage level of those inhibitors and prevent them
from achieving an ideal level of immunomodulatory activity
(15).
RNA interference (RNAi) is a method that is able to
directly eliminate the function of a targeted gene. Studies
performed on mammals have demonstrated the superiority of silencing
induced by small interfering RNAs (siRNAs) to the traditional
antisense molecules (16,17). Therefore, in recent years, RNAi has
been established as a preferred technology for investigating and
validating protein function. More importantly, the high specificity
of RNAi provides the potential to selectively silence a subtype
from a family of closely related genes (18).
PDE4 inhibitors have already been shown to increase
the cAMP level in microglial cells (19), but it is not clear which particular
subtype is responsible for such increase. The present study was
designed to first study whether microglial cells actually express
the four PDE4 subtypes. The RNAi technique was then applied to
silence different subtypes of the PDE4 gene, and the specific
effects of each subtype on the production of cytokines in
endotoxin-stimulated primary cultured microglia were observed.
Materials and methods
Materials
Lipopolysaccharide (LPS) from Escherichia
coli 011:B4 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
was suspended in phosphate-buffered saline (PBS) at a concentration
of 20 mg/ml and stored at −20°C. Primers for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) were
synthesized by Invitrogen (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Antibodies for western blot analysis were: i) Anti-PDE4A
(ab14607; 1:1,000), anti-PDE4B (ab14611; 1:1,000), anti-PDE4D
(ab14613; 1:500) and anti-CD11b (ab75476; 1:1,000) antibodies (all
purchased from Abcam, Cambridge, MA, USA); ii) anti-PDE4C antibody
(1:300; PD4C-301AP; Fabgennix International, Inc., Frisco, TX,
USA); iii) anti-p44/42 mitogen-activated protein kinase [4695;
1:1,000; MAPK; extracellular signal-regulated kinase (ERK)1/2; Cell
Signalling Technology, Inc., Danvers, MA, USA], anti-phosphorylated
(P)-p44/42 MAPK (T202/Y204; 14474; 1:1,000; Cell Signalling
Technology, Inc.), and anti-rabbit IgG horseradish peroxidase
(HRP)-linked antibody (ab6721; 1:1,000; Cell Signaling Technology,
Inc.). Except where indicated, all other materials were from
Invitrogen (Thermo Fisher Scientific, Inc.).
Micoglial culture
Microglia were isolated from primary cultures of rat
brains by the method previously described with slight modifications
(20). Briefly, two whole brains of
Sprague-Dawley rats (Experimental Animal Center of Jinling
Hospital, Nanjing, China) at postnatal days 1–3 were used for each
flask (Delta-treated flask, 75 cm2; Nunc™). Following
the dissection of the brains and careful stripping of the meninges,
brains were triturated with a Pasture pipette with 3 ml Dulbecco's
modified Eagle's medium (DMEM) containing 10% fetal calf serum, 2
mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin. The
solution was centrifuged at 800xg for 10 min. The precipitates were
suspended completely with 2 ml DMEM and were plated in the
aforementioned flask. These mother cultures were maintained in a 5%
CO2 humidified incubator at 37°C and the medium was
exchanged every 3 days after the initial seeding. After 9–10 days,
many round and phase-bright microglia were isolated by shaking for
4 h at 40xg. Floating cells in the supernatant were collected,
centrifuged and disseminated onto 24-well plates (Corning
Incorporated, Corning, NY, USA; 5×105 cells/well) or
96-well plates (Corning Incorporated; 5×104 cells/well).
After 20 min, the medium was changed and the cells were washed
twice with PBS to remove non-adherent cells. The remaining
microglia were allowed to stabilize for 1 day in DMEM containing
10% fetal bovine serum (FBS) prior to use. In this culture, the
purity of the microglia was 95–98% as determined using an
anti-CD11b antibody (21).
Western blot analysis
Western blotting was performed for the analysis of
the expression of PDE4 subtypes A-D at the protein level. Briefly,
protein samples (cell extracts, 50 mg protein) were separated by a
10% sodium dodecyl sulfate-polyacrylamide gel and transferred to
0.45-µm polyvinylidene difluoride membranes. The membranes were
blocked with 5% fat-free dry milk dissolved in Tris-buffered saline
(NaCl, 0.138 M; KCl, 0.0027 M, pH 8.0) plus 0.1% Tween-20 (TBST)
and incubated overnight with specific antibodies against PDE4
subtypes A-D (1:1,000). After extensive washing with TBST, the
membranes were incubated with anti-rabbit IgG HRP-linked antibody
solution (1:1,000) for 1 h at room temperature. The proteins were
visualized using chemiluminescence reagents provided with an ECL
kit (Amersham; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) and
exposure to film. Scanning densitometry using Image J 2X (Rawak
Software Inc., Stuttgart, Germany) was used for semi-quantitative
analysis of the data.
siRNA preparation
The siRNAs were designed based on the rat PDE4A
(Rattus norvegicus, PDE4A, NM_013101), PDE4B (Rattus
norvegicus, PDE4B, NM_017031), PDE4C (Rattus norvegicus,
PDE4C, XM_001070301) and PDE4D (Rattus norvegicus, PDE4D,
NM_017032) using BLOCK-iT RNAi Designer (Invitrogen; Thermo Fisher
Scientific, Inc.). An siRNA target sequence was selected for each
of the subtypes. One non-specific siRNA (mismatch RNA) was also
selected. Sequences of the siRNAs used in the present study are
summarized in Table I.
 | Table I.Sequences of the siRNAs used in the
present study. |
Table I.
Sequences of the siRNAs used in the
present study.
| siRNA | Strand | Sequence (5′ to
3′) |
|---|
| PDE4A | Sense |
AAGAGUGAGAAGUUGCUUCGAACGC |
|
| Antisense |
GCGUUCGAAGCAACUUCUCACUCUU |
| PDE4B | Sense |
UCUUCUUGCAGGCUGACUCUGGUGA |
|
| Antisense |
UCACCAGAGUCAGCCUGCAAGAAGA |
| PDE4C | Sense |
AGGAAAGUCUGCGAGAUGUACUCUG |
|
| Antisense |
CAGAGUACAUCUCGCAGACUUUCCU |
| PDE4D | Sense |
AUGGAUGGUUGGUUGCACAUGGGUG |
|
| Antisense |
CACCCAUGUGCAACCAACCAUCCAU |
Transfection of siRNAs into
microglia
Primary microglial cells were randomly divided into
eight groups: Control group (no LPS, no transfection), LPS group
(no transfection), and transfection groups, including vehicle group
(transfected with Lipofectamine™ RNAiMAX reagent; Thermo Fisher
Scientific, Inc.), mismatch group (transfected with mismatch siRNA)
and PDE4-siRNA groups A-D (transfected with siRNA targeting PDE4
subtypes A-D, respectively).
To transfect the vehicle (Lipofectamine RNAiMAX
reagent), mismatch RNA, PDE4A, PDE4B, PDE4C or PDE4D stealth siRNAs
into microglia, respectively, 1 day prior to transfection,
microglia were cultured in DMEM without antibiotics and FBS. For
each transfection in 24-well plates, 20 nM mismatch RNA, PDE4A,
PDE4B, PDE4C or PDE4D stealth siRNA was diluted in 100 µl DMEM
without antibiotics and FBS and gently mixed with 1 µl
Lipofectamine RNAiMAX reagent according to the manufacturer's
protocol. Following incubation for 15 min at room temperature,
transfection mixture was added to each well and applied to each
well in a total volume of 500 µl DMEM. Transfected cells were
further incubated at 37°C for 48 h before assays were carried
out.
RT-qPCR analysis
RT-qPCR was performed to detect the expression of
PDE4A, B, C and D genes. Total RNA was isolated from cells using
TRIzol reagent and a spectrophotometer was used to determine the
quality and quantity of the RNA. TRIzol reagent was used according
to the manufacturer's instructions. In brief, a total of 250 µl
TRIzol reagent was added into the well (5×105
cells/well) to lyse the cells by pipetting them up and down several
times after removing the growth media from the well. Next, 50 µl
chloroform was added after incubating the homogenized sample for 5
min in the tube at room temperature. Following vigorous shaking for
15 sec and incubation at room temperature for 3 min, the sample was
centrifuged at 12,000 × g for 15 min at 4°C. The aqueous phase of
the sample was removed into a new tube, then incubated with 125 µl
isopropanol at room temperature for 10 min. The total RNA was
precipitated by centrifugation at 12,000xg for 15 min at 4°C. The
RNA pellet was washed with 250 µl 75% ethanol, then centrifuged at
7,500xg for 5 min at 4°C. After discarding the wash, the RNA pellet
was air dried for 8 min. Finally, the RNA pellet was suspended in
RNase-free water (20 µl). The isolated RNA was stored at −70°C
before RT-qPCR. The quality and quantity of RNA were determined
using a spectrophotometer by measuring the OD levels at 260 nm and
280 nm, respectively. The ratios of OD 260/OD 280 for the samples
were between 1.8–2.0, which indicated the purity of the samples was
quite good. Thus we did not treat the isolated RNA before
RT-qPCR.
cDNA was synthesized from the RNA using reverse
transcription. The reverse transcription (RT) reaction was
performed in a 20 µl total reaction volume containing 4 µl of 5X RT
buffer, 4 µl of 2.5 mM dNTPs, 1 µl of Multiscribe reverse
transcriptase (50 U/µl) (Promega, Madison, WI, USA), 1 µl of RNase
inhibitor, 5 µl of RNase-free water and 3 µg of isolated total RNA
in a 5 µl volume. The RT reaction was performed at 25°C for 10 min,
37°C for 120 min and 95°C for 5 min. qPCR analysis was performed
using a 7500 Fast Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.). qPCR was performed in a final volume of
20 µl, containing 10 µM each primer, 1 µl SYBR green (20X) and 1 µl
template. The program profile was as follows: 95°C for 2 min, 40
cycles of denaturation for 10 sec at 95°C, annealing for 30 sec at
60°C and extension for 30 sec at 70°C. The PCR primers used in this
study are summarized in Table
II.
 | Table II.PCR primers used in the present
study. |
Table II.
PCR primers used in the present
study.
| Primer | Direction | Sequence (5′ to
3′) |
|---|
| PDE4A | Forward |
GAAGACAACCGGGACTCCT |
|
| Reverse |
CCTCAGTGGTAGGCAATCC |
| PDE4B | Forward |
CCTCCGACACCTTCGTAAC |
|
| Reverse |
CCAGGTCTGTGAAGACAGC |
| PDE4C | Forward |
GAAGGGCACTACCACTCCA |
|
| Reverse |
GTGTATAGCGCACGCAAAGA |
| PDE4D | Forward |
CCCTCTTGACTGTTATCATGCACACC |
|
| Reverse |
GATCCTACATCATGTATTGCACTGGC |
| GAPDH | Forward |
CCATGTTCGTCATGGGTGTGAACCA |
|
| Reverse |
GCCAGTAGAGGCAGGGATGATGTTC |
The ΔΔCq method was used to normalize the qPCR data,
and the level of GADPH mRNA was utilized as a reference. The
relative expression level of target mRNA was calculated according
to the difference between the reference and target Cq values for
each sample. Next, the level of target mRNA in groups with
different treatment was normalized by setting the level in the
control group as 1.
Analysis of cytokines and cAMP
For measuring the production of TNF-α, IL-1β, IL-10
and cAMP, primary microglial cells were randomly divided into the
eight aforementioned groups. Following transfection and 48 h of
incubation, the culture medium was discarded and LPS was added at
final concentration of 100 ng/ml. The supernatants of the cultured
microglia were collected after 2, 4, 6, 12 and 24 h of stimulation
by LPS.
The intracellular cAMP level in the microglia was
detected following 30 min of stimulation by LPS. Briefly, cells
were washed three times in cold PBS and resuspended in cell lysis
buffer. Cells were frozen and thawed with gentle mixing twice. Cell
lysates were then centrifuged at 600 × g for 10 min to remove
cellular debris. The above supernatants and cell lysates were
stored at −80°C before assaying.
ELISA kits for TNF-α, IL-1β, IL-10 and cAMP
determination were purchased from R&D Systems, Inc.
(Minneapolis, MN, USA) and used to determine the levels of these
proteins according to the manufacturer's protocol. Each experiment
was performed in triplicate.
Detection of ERK protein expression
using western blot analysis
At 24 h after stimulation by LPS, cells from the
control, LPS, vehicle, mismatch RNA and PDE4B groups were collected
and the expression levels of total ERK and p-ERK protein were
assessed by western blotting as described above. The primary
antibodies were p44/42 MAPK (ERK1/2) (1:1,000) and P-p44/42 MAPK
(T202/Y204) (1:1,000).
Statistical analysis
Results are expressed as the mean ± standard
deviation of at least three independent determinations. SPSS
statistical software version 13.0 was used for data analysis. Data
from the RT-qPCR and western blot experiments were accomplished
using one-way analysis of variance (ANOVA). Data from the ELISA
were analyzed using repeated-measures ANOVA. Least-significance
difference post-hoc comparison was used to detect significant
differences for the ANOVA analyses. P<0.05 was considered to
indicate a statistically significant result.
Results
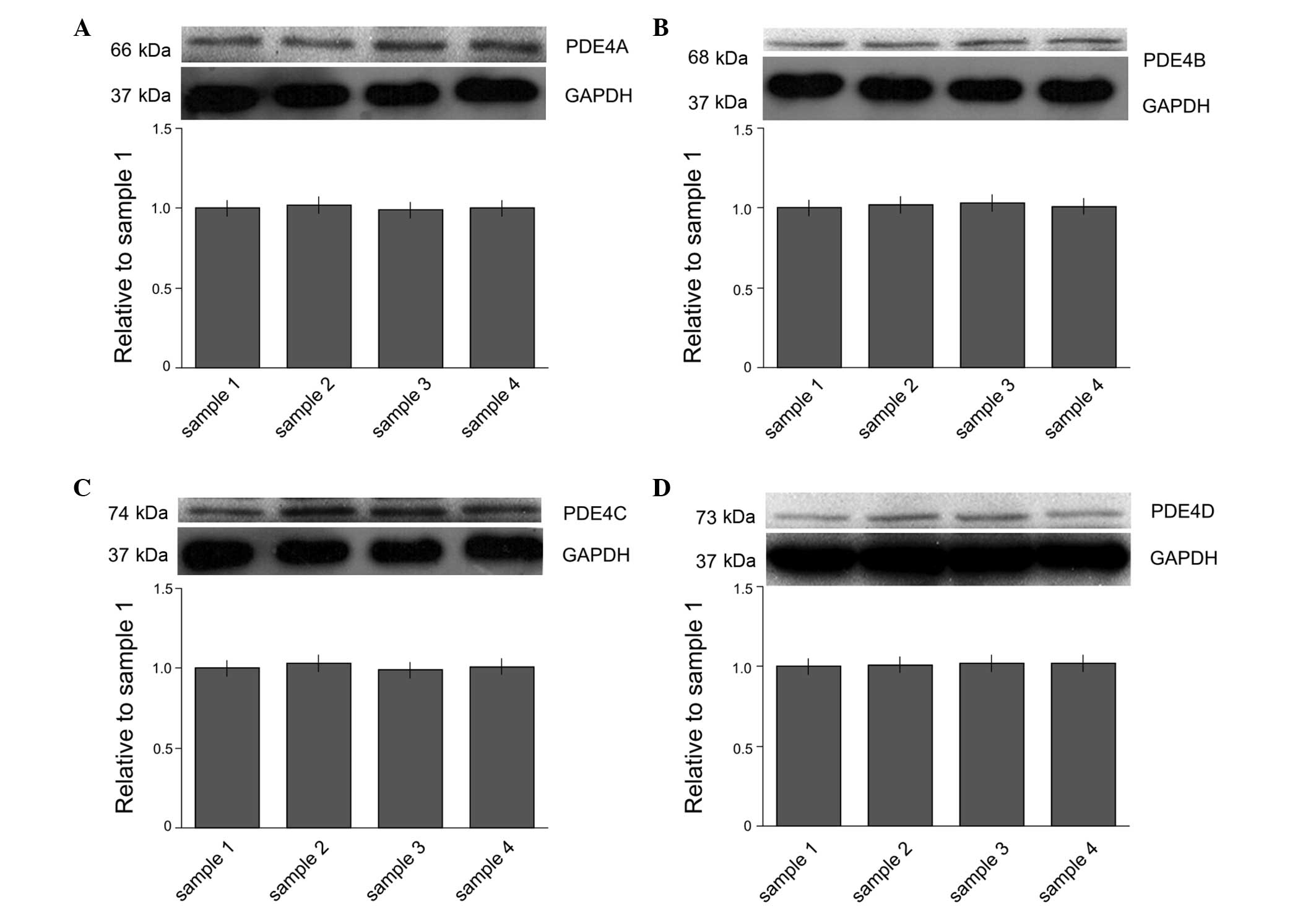
Expression levels of PDE4 subtypes A-D
in microglia
The expression of PDE4 subtypes A-D in microglia
were studied at the protein level. The protein expression levels of
PDE4 subtypes A-D were determined by western blot analysis
(Fig. 1). The results demonstrated
that the microglia expressed all four subtypes of PDE4.
Expression levels of PDE4 subtypes A-D
are reduced by siRNAs
To investigate the potential function of the four
PDE4 subtypes in microglia, siNRAs were used to knock down
endogenous PDE4A-D gene expression. A siRNA sequence was selected
for each of the subtypes A-D. The expression of PDE4A at the mRNA
level was reduced to 36.6±5.2% of that in the control group
(P<0.05; Fig. 2A). The expression
of PDE4B, PDE4C and PDE4D at the mRNA level was reduced to
34.7±6.4, 33.2±4.8 and 27.5±7.0% of that in the control group,
respectively (P<0.05; Fig. 2B-D).
However, no significant differences were observed among the LPS,
mismatch RNA and vehicle groups (P>0.05).
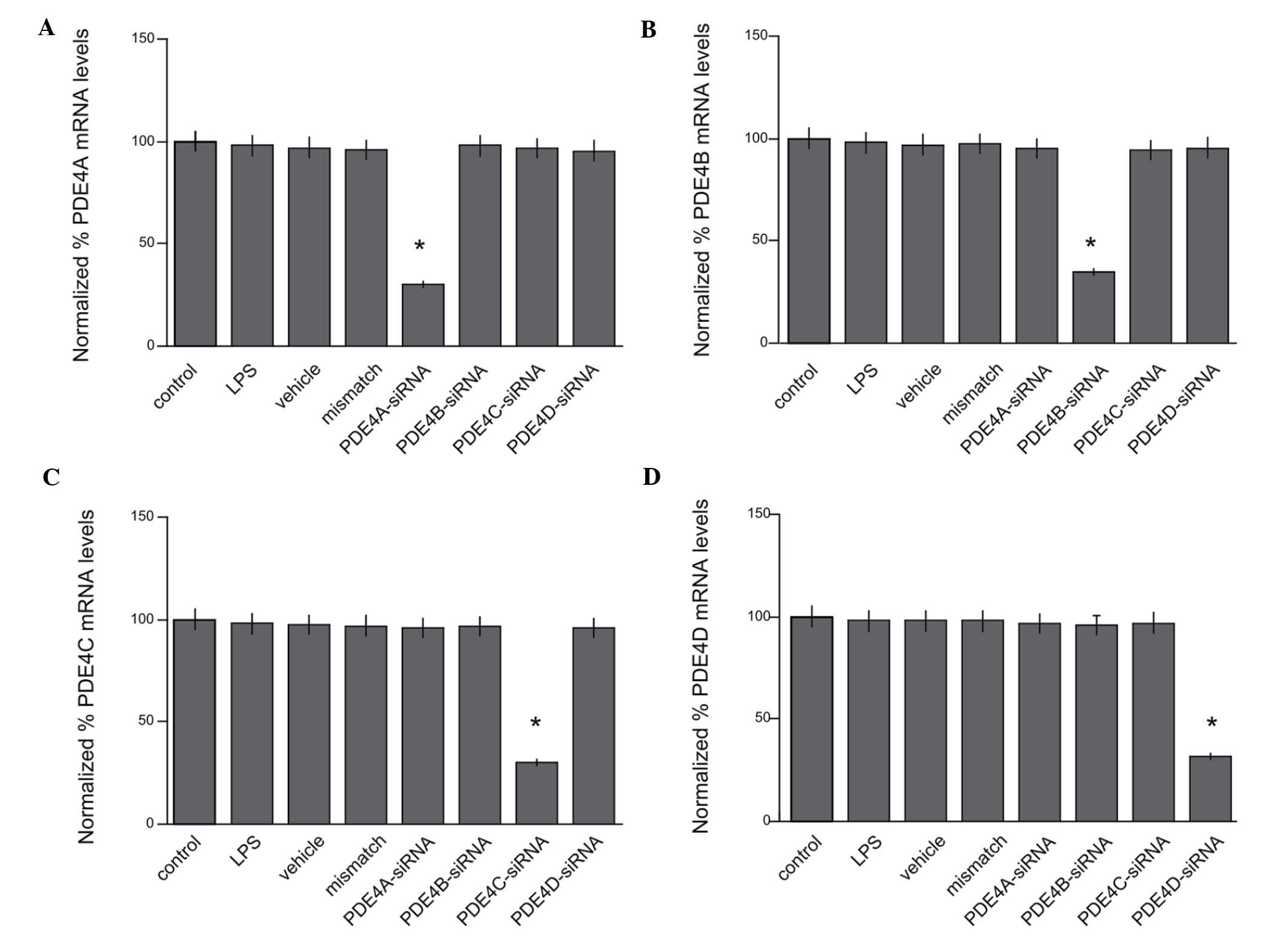 | Figure 2.Reduced expression levels of PDE4A,
PDE4B, PDE4C and PDE4D mRNA by siRNAs. (A) The expression of PDE4A
at the mRNA level was reduced to 36.6±5.2% of that in the control
group (*P<0.05). The expression of (B) PDE4B, (C) PDE4C and (D)
PDE4D at the mRNA level was reduced to 34.7±6.4, 33.2±4.8 and
27.5±7.0% of that in the control group, respectively (*P<0.05).
However, no significant differences were observed among the LPS,
mismatch RNA and vehicle groups (P>0.05). PDE,
phosphodiesterase; siRNA, small interfering RNA; LPS,
lipopolysaccharide. |
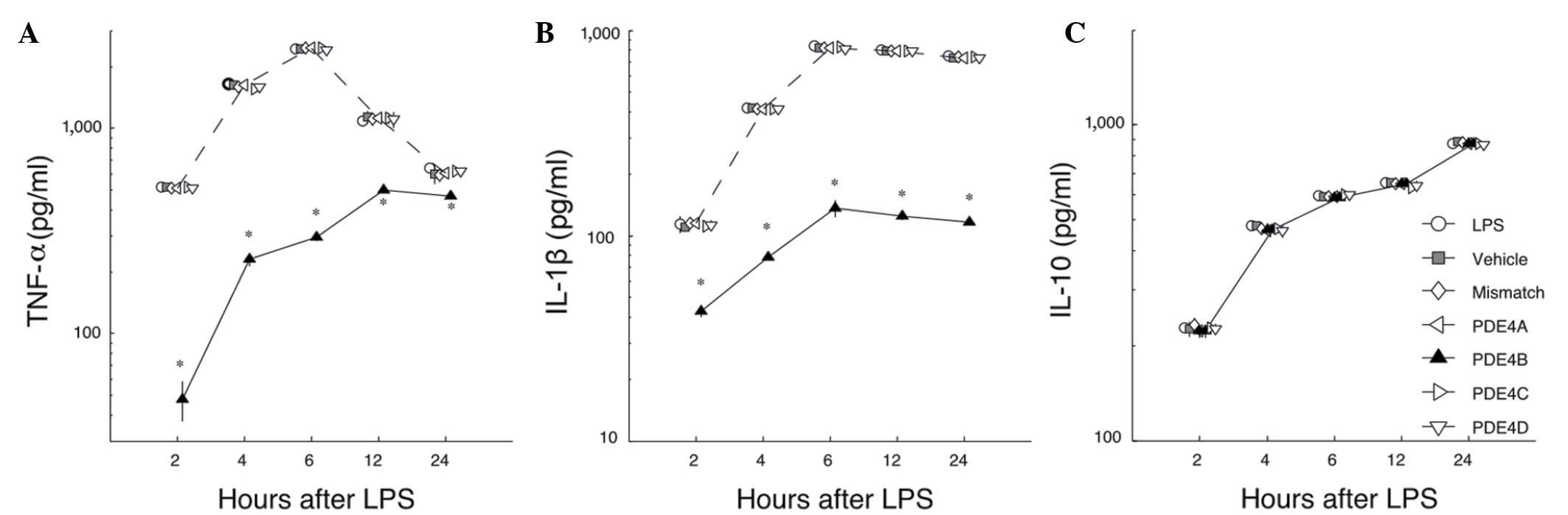
Effects of PDE4 subtype A-D siRNAs on
the LPS-induced release of cytokines
To study the effects of silencing PDE4 subtypes A-D
with siRNA on the LPS-induced release of cytokines, the levels of
TNF-α, IL-1β and IL-10 were measured by ELISA. First, in the LPS
group, microglia showed a strong increase in TNF-α production at 2
h after stimulation with LPS, then the amount increased markedly
and the maximum was observed at 6 h. After that, the amount of
TNF-α in the medium decreased gradually. Among the groups treated
with siRNAs, only PDE4B siRNA significantly inhibited the
production of TNF-α compared with the level in the LPS group
(P<0.05; Fig. 3A). Second, in the
LPS group a considerable amount of IL-1β was detected at 4 h. The
maximum level was reached at 6 h, and this level was maintained for
24 h. Among the groups treated with siRNAs, only the PDE4B group
showed a significantly reduced level of IL-1β (P<0.05; Fig. 3B). Third, in contrast to TNF-α and
IL-1β, the levels of IL-10 were not affected by any of the siRNAs
when compared with the LPS group (P>0.05; Fig. 3C). These data demonstrate that the
action of PDE4B-siRNA in primary microglia is highly selective,
inhibiting the production of TNF-α and IL-1β but not enhancing the
production of IL-10.
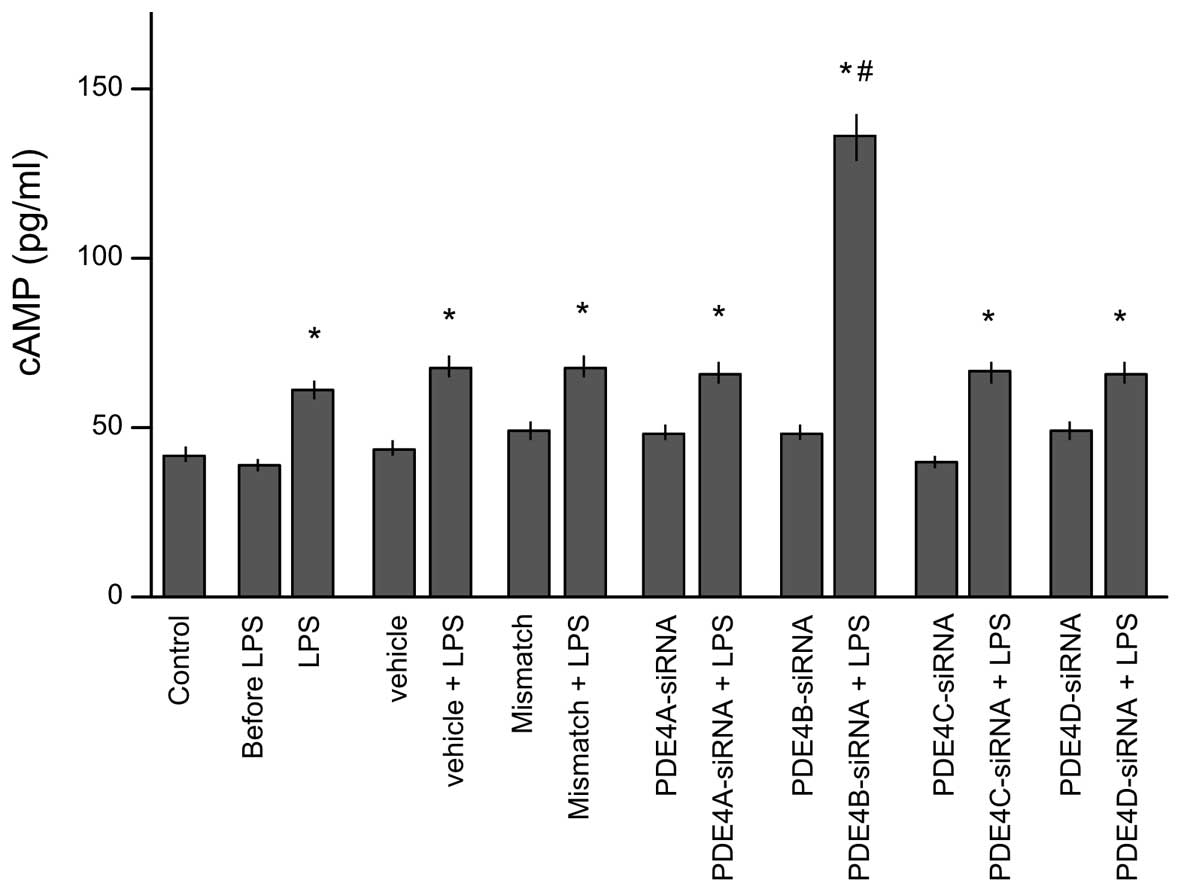
Effects of siRNA of PDE4 subtypes A-D
on the intracellular cAMP level
To test which subtype of PDE4 is potentially
responsible for the regulation of the intracellular cAMP level,
lysates were collected to determination of cAMP level after 30 min
of stimulation by LPS (Fig. 4).
Prior to stimulation with LPS, no clear differences were observed
in cAMP levels among the eight groups. LPS stimulation
significantly increased the cAMP levels in all groups compared with
those in the control group (P<0.05). However, only the
PDE4B-siRNA group showed a significantly higher intracellular cAMP
level than that in the LPS group (P<0.05). This indicates that
PDE4B is pivotal in the regulation of the cAMP level in microglial
cells.
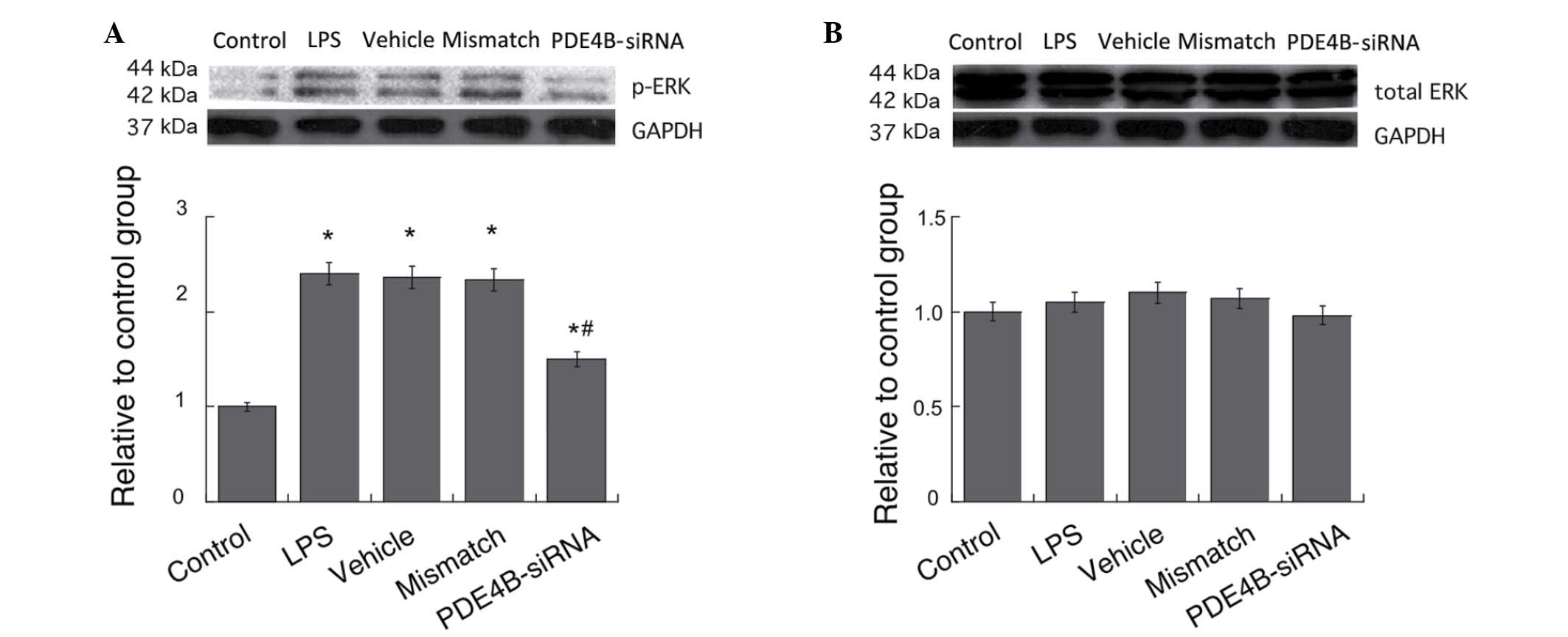
Effect of PDE4B-siRNA on the
LPS-induced activation of ERK in microglia
ERK is known to be involved in the regulation of
cytokine expression by influencing the activities of several
transcriptional factors (22,23). The
effects of PDE4B on ERK activities in microglial cells were
examined by western blotting. Treatment with LPS caused significant
increases in the p-ERK levels in the microglial cells compared with
those in the control group, and PDE4B-siRNA significantly
suppressed the elevation of the p-ERK level induced by LPS
treatment (P<0.05; Fig. 5A). No
significant differences in the total ERK levels were observed among
all groups (P>0.05; Fig. 5B).
Discussion
In this study, the expression of PDE4 subtypes A-D
was detected in primary microglia at the protein level.
Transfection of siRNA silenced the expression of all four subtypes
of PDE4 in microglial cells. However, the significantly elevated
levels of proinflammatory cytokines TNF-α and IL-1β could only be
suppressed by silencing PDE4B, and not by silencing PDE4A, PDE4C or
PDE4D. The significantly decreased level of cAMP induced by LPS
stimulation could only be elevated by silencing PDE4B, and not by
silencing PDE4A, PDE4C or PDE4D. Furthermore, the shift from ERK to
p-ERK that was induced by LPS stimulation was inhibited by the
silencing of PDE4B.
These results suggest that, among the four subtypes
of PDE4 only PDE4B plays a pivotal role in regulating the
inflammatory response in microglia. This result was consistent with
previous studies. Jin et al suggested that ablation of
PDE4B, but not PDE4A or PDE4D, significantly suppressed LPS-induced
TNF-α production in circulating monocytes and peritoneal
macrophages (24,25). Robichaud et al advised that
inhibition of PDE4D but not PDE4B may be responsible for the emetic
effects of non-selective PDE4 inhibitors; the reason may be that in
the CNS, PDE4D is linked to cAMP signaling of the 2-adrenoceptor in
noradrenergic neurons (26).
In comparison with previous studies, however,
attention was paid to several important points. To explore the
detailed functions of the four subtypes, RNAi was selected to knock
down the expression of PDE4A, PDE4B, PDE4C and PDE4D, respectively.
RNAi is a straightforward method to eliminate the function of any
gene of interest.
Furthermore, in this study, the intracellular cAMP
level was measured by ELISA. The results demonstrated that compared
with the LPS group, PDE4B silencing markedly enhanced cAMP
accumulation, while silencing of the other PDE4 subtypes did not
significantly affect the cAMP level in microglial cells, suggesting
that PDE4B plays a pivotal role in the regulation of the cAMP level
in microglial cells.
cAMP is a key intracellular second messenger, and
PDE4B siRNA would prevent PDE4B from hydrolyzing cAMP, thus
maintaining intracellular cAMP levels and providing an increased
stabilizing effect since they are significantly reduced following
LPS stimulation. Activation of the membrane G-protein coupled
receptor subunit Gsa causes cAMP levels to increase, which inhibits
the inflammatory response (27). Gsa
activates adenylyl cyclase, an enzyme with numerous downstream
effector systems associated with cell signaling. Rac activity is
downregulated via the cAMP-protein kinase A pathway, which leads to
reduced neutrophil migration (28–30).
Increased cAMP levels reduce the expression of adhesion molecules
(30) and disrupt chemokine-induced
chemotaxis (31). High cAMP levels
also inhibit leukocyte adhesion and migration (32), and downregulate phagocytosis and
nitric oxide production in macrophages (33).
It has been suggested that cAMP signaling is
regulated by an ERK-dependent pathway. ERK can influence the
activities of numerous transcriptional factors including cAMP
response element-binding protein and nuclear factor-κB (34,35).
Zhuang et al suggested that there is robust activation of
ERK in microglia (36). The
intensity of ERK activation is closely correlated with activation
of glial cells. It phosphorylates many downstream targets that lead
to inhibition of cell activation (36). In the present study, p-ERK intensity
was greatly increased by LPS stimulation compared with the control
group. The p-ERK protein expression in the PDE4B-silenced group was
decreased significantly compared with that in the LPS group. The
total ERK level did not differ significantly among the groups.
The present data about TNF-α, IL-1β and IL-10 are
consistent with previous findings and support the increased cAMP
concentration. Considering these findings together, the activation
of the ERK/MARK pathway may lead to the release of large quantities
of pro-inflammatory cytokines from the microglial cells.
The present study provides solid evidence that PDE4B
in microglial cells plays an important role in neuropathic pain
associated with inflammatory responses. Clinically, a selective PDE
inhibitor targeting PDE4B could be a promising candidate in a new
therapeutic strategy for neuropathic pain.
References
|
1
|
Gremo F, Sogos V, Ennas MG, Meloni A,
Persichini T, Colasanti M and Lauro GM: Features and functions of
human microglia cells. Adv Exp Med Biol. 429:79–97. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
WyssCoray T and Rogers J: Inflammation in
Alzheimer disease - a brief review of the basic science and
clinical literature. Cold Spring Harb Perspect Med.
2:a0063462012.PubMed/NCBI
|
|
3
|
Karagkouni A, Alevizos M and Theoharides
TC: Effect of stress on brain inflammation and multiple sclerosis.
Autoimmun Rev. 12:947–953. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Campbell JN and Meyer RA: Mechanisms of
neuropathic pain. Neuron. 52:77–92. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aloisi F: Immune function of microglia.
Glia. 36:165–179. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garden GA and Möller T: Microglia biology
in health and disease. J Neuroimmune Pharmacol. 1:127–137. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buttini M, Mir A, Appel K, Wiederhold KH,
Limonta S, Gebicke Haerter PJ and Boddeke HW: Lipopolysaccharide
induces expression of tumour necrosis factor alpha in rat brain:
Inhibition by methylprednisolone and by rolipram. Br J Pharmacol.
122:1483–1489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka J, Toku K, Matsuda S, Sudo S,
Fujita H, Sakanaka M and Maeda N: Induction of resting microglia in
culture medium devoid of glycine and serine. Glia. 24:198–215.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang B, Yang L, Konishi Y, Maeda N,
Sakanaka M and Tanaka J: Suppressive effects of phosphodiesterase
type IV inhibitors on rat cultured microglial cells: comparison
with other types of cAMP-elevating agents. Neuropharmacology.
42:262–269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Conti M and Jin SL: The molecular biology
of cyclic nucleotide phosphodiesterases. Prog Nucleic Acid Res Mol
Biol. 63:1–38. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Houslay MD: Underpinning compartmentalised
cAMP signalling through targeted cAMP breakdown. Trends Biochem
Sci. 35:91–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jarnagin K, Chanda S, Coronado D,
Ciaravino V, Zane LT, GuttmanYassky E and Lebwohl MG: Crisaborole
topical ointment, 2%: A nonsteroidal, topical, anti-inflammatory
phosphodiesterase 4 inhibitor in clinical development for the
treatment of atopic dermatitis. J Drugs Dermatol. 15:390–396.
2016.PubMed/NCBI
|
|
13
|
Pearse DD and Hughes ZA: PDE4B as a
microglia target to reduce neuroinflammation. Glia. Apr 1–2016.doi:
10.1002/glia.22986 (Epub ahead of print). View Article : Google Scholar
|
|
14
|
Flemming S, Schlegel N, Wunder C, Meir M,
Baar W, Wollborn J, Roewer N, Germer CT and Schick MA:
Phosphodiesterase 4 inhibition dose dependently stabilizes
microvascular barrier functions and microcirculation in a rodent
model of polymicrobial sepsis. Shock. 41:537–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin SL, Ding SL and Lin SC:
Phosphodiesterase 4 and its inhibitors in inflammatory diseases.
Chang Gung Med J. 35:197–210. 2012.PubMed/NCBI
|
|
16
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doré-Savard L, Roussy G, Dansereau MA,
Collingwood MA, Lennox KA, Rose SD, Beaudet N, Behlke MA and Sarret
P: Central delivery of Dicer-substrate siRNA: A direct application
for pain research. Mol Ther. 16:1331–1339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Röhl T and Kurreck J: RNA interference in
pain research. J Neurochem. 99:371–380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang H, Bielekova B, Okazaki H,
ClarenceSmith K, Johnson KP, Bergey G, Martin R and Dhib-Jalbut S:
The effect of vesnarinone on TNF alpha production in human
peripheral blood mononuclear cells and microglia: A preclinical
study for the treatment of multiple sclerosis. J Neuroimmunol.
97:134–145. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xue Y, Wang Y, Feng DC, Xiao BG and Xu LY:
Tetrandrine suppresses lipopolysaccharide-induced microglial
activation by inhibiting NF-kappaB pathway. Acta Pharmacol Sin.
29:245–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Zhao X, Cao J, Xue Q, Feng X, Liu
X, Zhang F and Yu B: Differential roles of PKA and Epac on the
production of cytokines in the endotoxin-stimulated primary
cultured microglia. J Mol Neurosci. 45:186–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi DC, Lee JY, Lim EJ, Baik HH, Oh TH
and Yune TY: Inhibition of ROS-induced p38MAPK and ERK activation
in microglia by acupuncture relieves neuropathic pain after spinal
cord injury in rats. Exp Neurol. 236:268–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li W, Li Y, Zhu S, Ji Q, Shu Y, Zhang L
and Liu J: Rosuvastatin attenuated the existing morphine tolerance
in rats with L5 spinal nerve transection through inhibiting
activation of astrocytes and phosphorylation of ERK42/44. Neurosci
Lett. 584:314–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin SL and Conti M: Induction of the
cyclic nucleotide phosphodiesterase PDE4B is essential for
LPS-activated TNF-alpha responses. Proc Natl Acad Sci USA.
99:7628–7633. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin SL, Lan L, Zoudilova M and Conti M:
Specific role of phosphodiesterase 4B in lipopolysaccharide-induced
signaling in mouse macrophages. J Immunol. 175:1523–1531. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robichaud A, Stamatiou PB, Jin SC,
Lachance N, MacDonald D, Laliberté F, Liu S, Huang Z, Conti M and
Chan CC: Deletion of phosphodiesterase 4D in mice shortens
alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate
of emesis. J Clin Invest. 110:1045–1052. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao F, Fleming JC, Golshani R, Pearse DD,
Kasabov L, Brown A and Weaver LC: A selective phosphodiesterase-4
inhibitor reduces leukocyte infiltration, oxidative processes and
tissue damage after spinal cord injury. J Neurotrauma.
28:1035–1049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Erdogan S, Aslantas O, Celik S and Atik E:
The effects of increased cAMP content on inflammation, oxidative
stress and PDE4 transcripts during Brucella melitensis infection.
Res Vet Sci. 84:18–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pearse DD, Pereira FC, Marcillo AE, Bates
ML, Berrocal YA, Filbin MT and Bunge MB: cAMP and Schwann cells
promote axonal growth and functional recovery after spinal cord
injury. Nat Med. 10:610–616. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagasawa SY, Takuwa N, Sugimoto N, Mabuchi
H and Takuwa Y: Inhibition of Rac activation as a mechanism for
negative regulation of actin cytoskeletal reorganization and cell
motility by cAMP. Biochem J. 385:737–744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sanz MJ, Cortijo J and Morcillo EJ: PDE4
inhibitors as new anti-inflammatory drugs: Effects on cell
trafficking and cell adhesion molecules expression. Pharmacol Ther.
106:269–297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lorenowicz MJ, FernandezBorja M and
Hordijk PL: cAMP signaling in leukocyte transendothelial migration.
Arterioscler Thromb Vasc Biol. 27:1014–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ninković J and Roy S: Morphine decreases
bacterial phagocytosis by inhibiting actin polymerization through
cAMP-, Rac-1- and p38 MAPK-dependent mechanisms. Am J Pathol.
180:1068–1079. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Emery AC and Eiden LE: Signaling through
the neuropeptide GPCR PAC1 induces neuritogenesis via a
single linear cAMP- and ERK-dependent pathway using a novel cAMP
sensor. FASEB J. 26:3199–3211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Enserink JM, Christensen AE, de Rooij J,
van Triest M, Schwede F, Genieser HG, Døskeland SO, Blank JL and
Bos JL: A novel Epac-specific cAMP analogue demonstrates
independent regulation of Rap1 and ERK. Nat Cell Biol. 4:901–906.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhuang ZY, Gerner P, Woolf CJ and Ji RR:
ERK is sequentially activated in neurons, microglia and astrocytes
by spinal nerve ligation and contributes to mechanical allodynia in
this neuropathic pain model. Pain. 114:149–159. 2005. View Article : Google Scholar : PubMed/NCBI
|