Introduction
Diabetes is a group of metabolic diseases, which is
characterized by high levels of blood glucose and blood fat over a
long period of time (1). Previous
studies have indicated that the glycolipids joint toxicity results
in increased oxidative stress and endoplasmic reticulum stress, as
well as in protein misfolding, thus leading to the activation of
pancreatic islet β cell apoptosis (2,3).
Insulin, which is only secreted by pancreatic islet β cells, is an
essential hormone for metabolic processes, including the metabolism
of glucose, amino acids and fatty acids in the liver, muscles,
adipose tissues and the brain (4,5).
Increased blood sugar levels induce the transcription, synthesis
and secretion of insulin, while genetic and environmental factors
result in impaired insulin function and secretion (6,7).
Therefore, a defects in insulin secretion is the major
pathophysiological mechanism of diabetes. However, the mechanisms
through which high blood sugar leads to pancreatic islet β cell
damage and further promotes the progression of diabetes remain
unclear.
Nucleolar protein NOM1, which contains an MIF4G
domain and an MA3 domain, was first isolated from the bone marrow
of children with acute myeloid leukemia (8,9). It has
been previously demonstrated that proteins containing the MIF4G or
MA3 domains are associated with the cell proliferation and growth,
and serve pivotal roles in cell apoptosis and protein translation
(10). NOM1 is highly conserved in a
variety of species, including in yeast and in humans (11). Recently, the role of NOM1 has been
reported to be associated with pancreatic development by regulating
ribosome biogenesis in zebrafish (12), indicating that NOM1 may be involved
in diabetes progression. However, the correlation between the NOM1
gene and diabetes has yet to be fully described.
In the present study, we analyzed the effect of NOM1
expression on pancreatic islet β cell apoptosis using a gene
knockdown method under the condition of high glucose concentration.
This study aimed to investigate the effect of NOM1 on pancreatic
islet β cell proliferation and insulin expression during diabetes
progression. Thus, the current study may provide a basis for
understanding the pathogenesis of diabetes and developing
therapeutic targets for diabetes in clinical practice.
Materials and methods
Cell culture
The mouse derived pancreatic β cells, MIN6, were
purchased from the American Type Culture Collection (Masassas, VA,
USA) and cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS), 6 mg/ml penicillin
G, 5 mg/ml streptomycin sulfate, 2 mM L-glutamine and 50 µM
β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA) at 37°C and
5% CO2.
MIN6 cell response to high glucose
concentration
MIN6 cells were cultured in DMEM supplemented with 5
mM glucose (Sigma-Aldrich) and seeded in 6-well plates at a density
of 1×106 cells/well. Subsequent to culturing for 3 days,
the cells were washed with serum-free and glucose-free medium, and
then were incubated in glucose-free RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with glucose concentrations
between 5 and 25 mM glucose. The medium was also supplemented with
0.2% bovine serum albumin, 10 mM HEPES, 2 mM L-glutamine, 1 mM
sodium pyruvate, 50 µM β-mercaptoethanol, 100 U/ml penicillin, and
100 µg/ml streptomycin (all from Sigma-Aldrich). Subsequently, the
expression levels of NOM1 and a cell apoptosis-associated protein
caspase-3 in MIN6 cells was measured by reverse
transcription-quantitative polymerase chair reaction (RT-qPCR) and
western blot analysis after 72 h.
Cell proliferation assay
The effects of NOM1 and glucose on MIN6 cell
proliferation ability were assessed using MTT assay, according to a
previously described method (13).
Briefly, MIN6 cells transfected with small interfering RNA
(siRNA)-NOM1 or its negative control (GenePharma, Shanghai, China)
at the logarithmic stage were cultured in DMEM mixed with 10% FBS.
Transfection was performed using Lipofectamine 2000 regent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. Cells were planted into 96-well plates
at a density of 5×103 cells per well. After culturing
for 24 h, the cells were centrifuged at 225 × g for 10 min at 4°C,
and then the supernatant was removed. Next, 20 µl MTT was added to
each well and then cultured for 4 h, followed by addition of 150 µl
dimethyl sulfoxide to the cells for 10 min. The absorbance of cells
in each well was observed at 570 nm under an absorption
spectrophotometer (Hitachi U-3110; Hitachi, Tokyo, Japan). All
experiments were conducted in triplicate.
Glucose-stimulated insulin
secretion
MIN6 cells were transfected with siRNA-NOM1, and
then incubated for 72 h at 37°C. The MIN6 cells were then washed
three times with phosphate-buffered saline (PBS) buffer (pH 7.4)
and incubated for 2 h in Krebs-Ringer bicarbonate HEPES buffer
(KRBH-BSA; including 120 mM NaCl, 4 mM
KH2PO4, 20 mM HEPES, 1 mM MgCl2, 1
mM CaCl2, 5 mM NaHCO3 and 0.5% BSA, pH 7.4)
containing 5 mM glucose. Subsequently, the medium was replaced with
fresh KRBH-BSA supplemented with 5 and 25 mM glucose, followed by
incubation for 1 h. Finally, the secreted insulin was detected
using a radioimmunoassay kit (EMD Millipore, Billerica, MA, USA)
according to the manufacturer's instructions (14).
RT-qPCR
The mRNA expression levels of insulin in MIN6 cells
treated with different concentration of glucose and siRNA-NOM1 were
detected as previously described (15). Briefly, MIN6 cells from different
groups were collected at 48 h, ground in liquid nitrogen and then
washed three times with PBS buffer (pH 7.4). Total RNA from MIN6
cells was extracted using TRIzol extraction reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) as previously described (16), and then RNase-free DNase I (Promega
Corp., Madison, WI, USA) was added to remove DNA from the sample.
The concentration and purity of extracted RNA were detected using a
SMA4000 UV-Vis spectrophotometer (Merinton, Shanghai, China) at 260
nm. The purified RNA of 0.5 µg/µl was reverse transcribed into cDNA
synthesis with the PrimeScript 1st Strand cDNA Synthesis kit
(Takara, Dalian, China). Primers used for target amplification are
shown in Table I, and all primers
were synthesized by GenePharma. The mRNA expression levels were
then detected using SYBR Green Real-Time PCR Master Mix
(Invitrogen; Thermo Fisher Scientific, Inc.) in a total volume of
20 µl containing 1 µl cDNA from the PCR product, 10 µl SYBR Premix
EX Taq, 1 µl of each primer (10 µM) and 7 µl double-distilled
H2O. The PCR reaction was performed at 50°C for 2 min,
95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C
for 1 min. Melting curve analysis of the amplification products was
subsequently performed at the end of each PCR analysis in order to
confirm that only one product was amplified and detected. Data were
analyzed according to the 2−ΔΔCq method (17). β-actin was selected as the internal
control.
 | Table I.Primers used for targets quantitative
polymerase chair reaction amplification in this study. |
Table I.
Primers used for targets quantitative
polymerase chair reaction amplification in this study.
| Gene | Primer | Sequence (5′-3′) |
|---|
| Insulin 1 | Sense |
GAAGTGGAGGACCCACAAGTG |
|
| Antisense |
CTGAAGGTCCCCGGGGCT |
| Insulin 2 | Sense |
AGCCCTAAGTGATCCGCTACAA |
|
| Antisense |
AGTTGCAGTAGTTCTCCAGCTG |
| β-actin | Sense |
GACGTTGACATCCGTAAAGA |
|
| Antisense |
GCCAGAGCAGTAATCTCCTT |
Western blot analysis
Cell apoptotic proteins from MIN6 cells were
investigated with western blotting analysis as previously described
(18). Briefly, MIN6 cells
transfected with siRNA-NOM1 and treated with glucose (5 or 25 mM)
were cultured for 48 h and then lysed with radioimmunoprecipitation
assay buffer (Sangon Biotech Co. Ltd., Shanghai, China) containing
phenylmethylsulfonyl fluoride. Next, the samples were centrifuged
at 225 × g for 5 min at 4°C. The supernatant was collected to
measure the concentration of proteins using a bicinchoninic acid
protein assay kit (Pierce; Thermo Fisher Scientific, Inc.,
Rockford, IL, USA). Subsequently, a total of 20 µg protein per cell
lysate was subjected to 12% sodium dodecylsulfate-polyacrylamide
gel electrophoresis and transferred onto a polyvinylidene fluoride
membrane (EMD Millipore). The membrane was then blocked in
Tris-buffered saline-Tween 20 (TBST) mixed with 5% non-fat milk for
1 h. Subsequently, the sample was incubated overnight at 4°C with
rabbit anti-human antibodies against NOM1 (cat. no. HPA019866;
dilution 1:100; Sigma-Aldrich), B-cell lymphoma 2 (Bcl-2; cat. no.
PRS3335; dilution 1:100; Sigma-Aldrich), Bcl-2-associated X protein
(Bax2; cat. no. B3428; dilution 1:100; Sigma-Aldrich) and cleaved
caspase-3 (cat. no. ab2302; dilution 1:1,000; Abcam, Cambridge,
UK), followed by incubation with horseradish peroxidase-labeled
goat anti-rabbit secondary antibody (cat. no. ab6721; dilution,
1:1,000; Abcam) at room temperature for 1 h. The membrane was then
washed three times with 1X TBST buffer for 10 min. Finally,
detection was performed on X-ray films following addition of a
chromogenic substrate BeyoECL Plus (Beyotime Institute of
Biotechnology, Haimen, China) using an enhanced chemiluminescence
method. GAPDH (cat. no. ab181602; 1:1,000 dilution; Abcam) was
selected as the internal control.
Statistical analysis
All data in the present study are presented as the
mean ± standard error of the mean. Statistically significant
differences between two groups were analyzed using Student's
t-test. P<0.05 was considered to demonstrate a statistically
significant difference.
Results
Effects of NOM1 and glucose on MIN6
cells
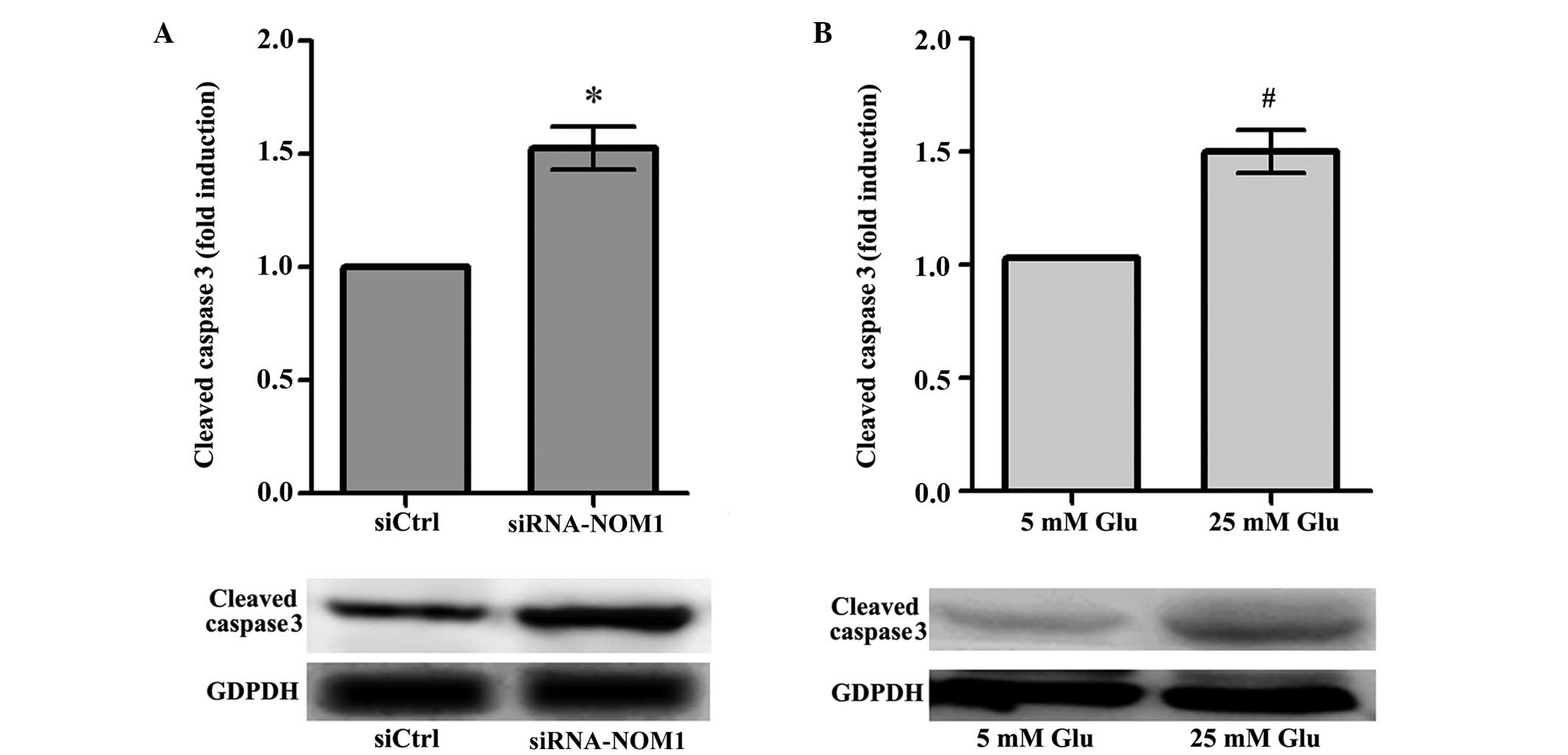
The effect of NOM1 in MIN6 cells was analyzed using
transfection of cells with a siRNA-NOM1 vector, and measured by
RT-qPCR and western blot. Compared with the control group (siCtrl),
downregulation of NOM1 expression resulted in a significantly
increase in the expression of cleaved caspase-3 in MIN6 cells
(P<0.05; Fig. 1A), suggesting
that NOM1 may inhibit the expression of cell apoptosis-associated
factors in MIN6 cells. In addition, the effect of different
concentrations of glucose on MIN6 cells was analyzed (Fig. 1B). The results showed that, following
treatment with 25 mM glucose, the cleaved caspase-3 expression in
MIN6 cells significantly increased compared with that in the 5 mM
glucose treatment group (P<0.05). Thus, a high glucose
concentration may lead to increased expression of MIN6 cell
apoptosis-associated factors.
Cell proliferation assay
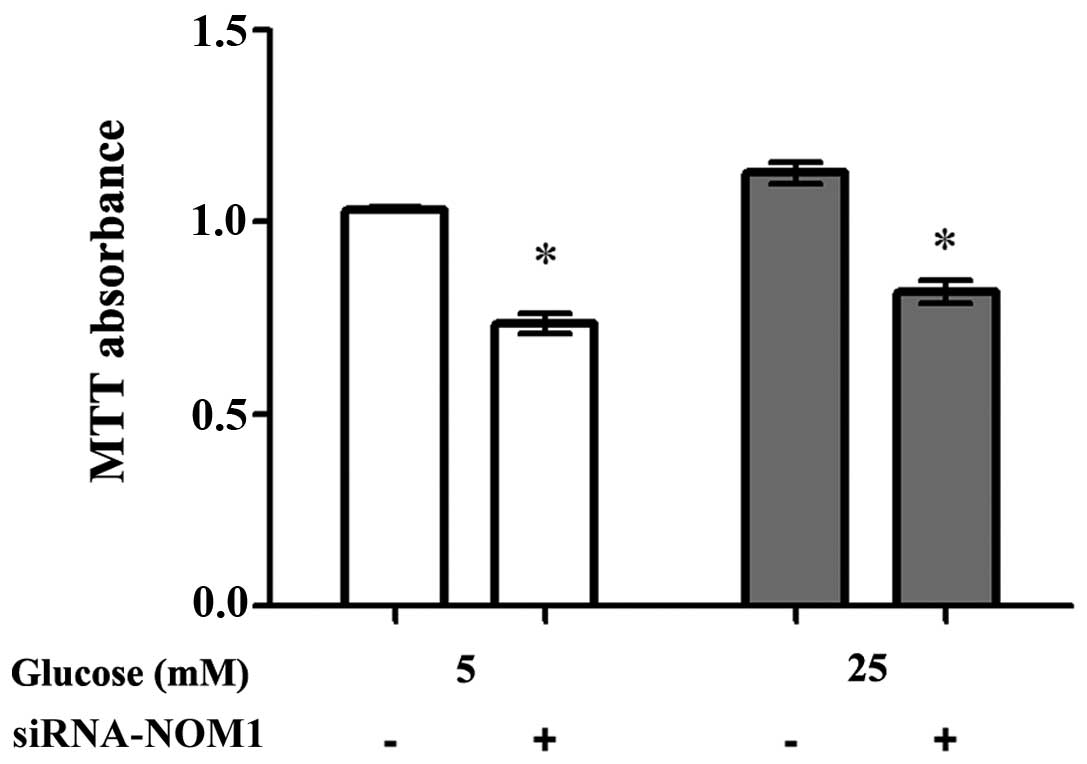
siRNA-NOM1 was transfected into MIN6 cells treated
with different concentrations of glucose (5 and 25 mM), and the
cell proliferation ability of MIN6 cells in each group was assessed
using an MTT assay (Fig. 2). When
treated with 5 mM glucose, downregulation of NOM1 expression
significantly decreased the cell proliferation ability of MIN6
cells compared with the negative control (P<0.05). A similar
tendency was observed when MIN6 cells were treated with 25 mM
glucose. These results indicated that NOM1 may serve a crucial role
in the regulation of MIN6 cell proliferation ability.
Glucose-stimulated insulin
secretion
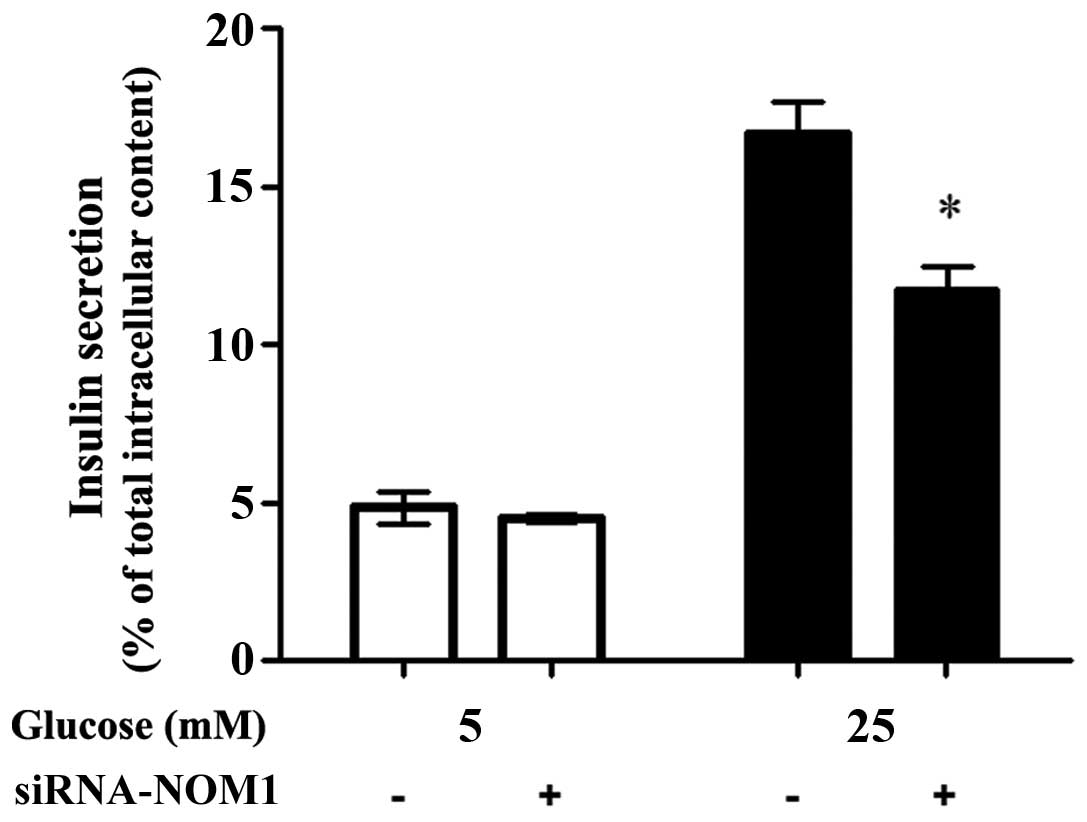
As shown in Fig. 3,
when MIN6 cells were treated with 5 mM glucose, no significant
difference was observed in insulin secretion between the siCtrl and
siRNA-NOM1 groups. When MIN6 cells were treated with 25 mM glucose,
insulin secretion in MIN6 cells transfected with siRNA-NOM1 was
significantly decreased when compared with that in the cells
transfected with siCtrl (P<0.05; Fig.
3). However, insulin secretion in MIN6 cells transfected with
siRNA-NOM1 was significantly higher in cells treated with 25 Mm
glucose compared with those treated with 5 mM glucose.
Insulin 1 and insulin 2 mRNA
expression levels in MIN6 cells
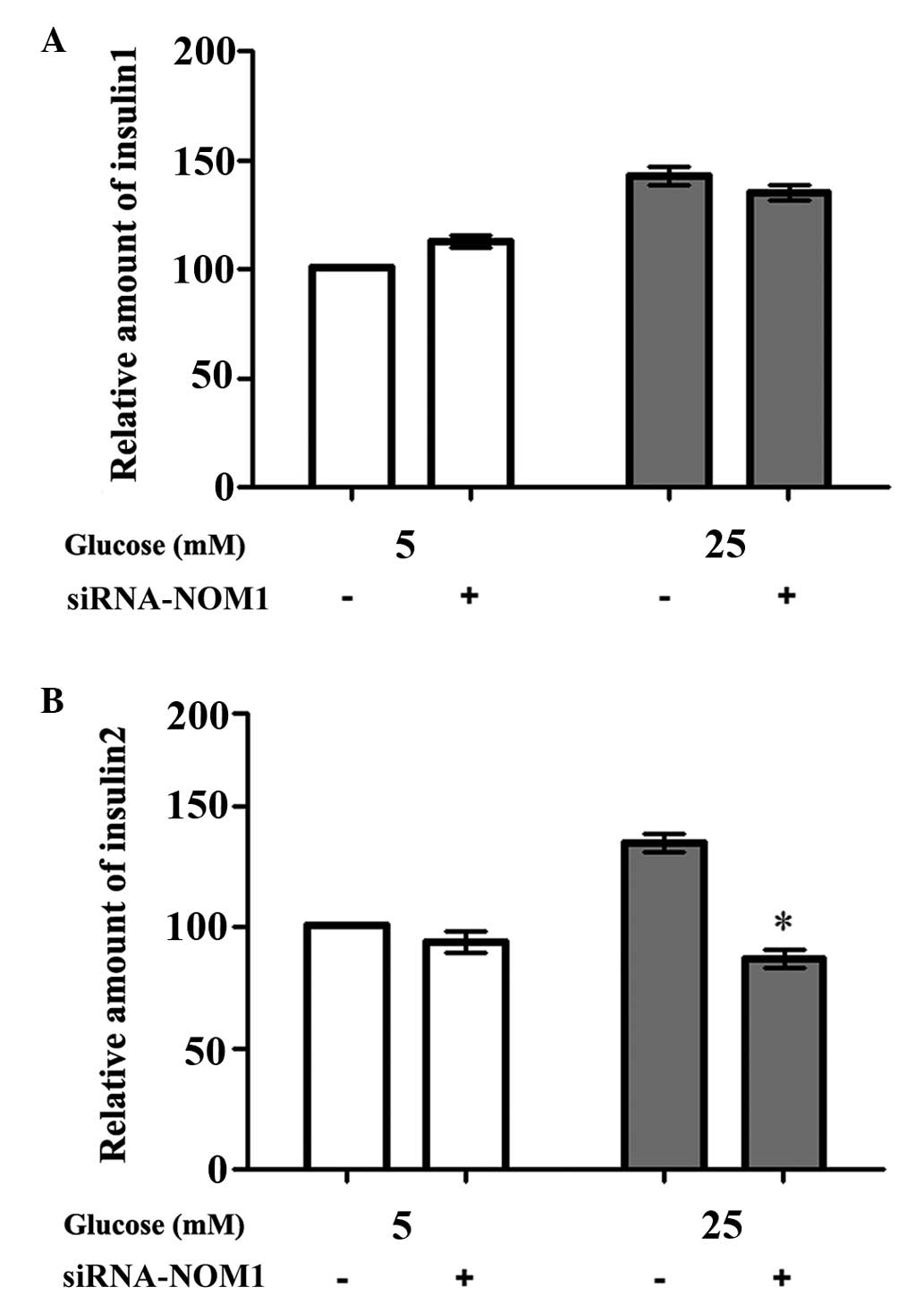
RT-qPCR analysis was used to detect the mRNA
expression levels of insulin 1 and insulin 2 in the different
transfection and glucose treatment groups (Fig. 4). The results showed that there was
no significant difference in insulin 1 mRNA expression among the
control and siRNA-NOM1-transfected groups, upon treatment of MIN6
cells with 5 or 25 mM glucose (P>0.05; Fig. 4A). However, when MIN6 cells were
treated with 25 mM glucose after transfection for 48 h, the insulin
2 mRNA expression in the siRNA-NOM1-transfected group was
significantly reduced compared with the control group (P<0.05;
Fig. 4B). However, there was no
significant difference in insulin 2 mRNA expression between the two
groups when MIN6 cells were treated with 5 mM glucose.
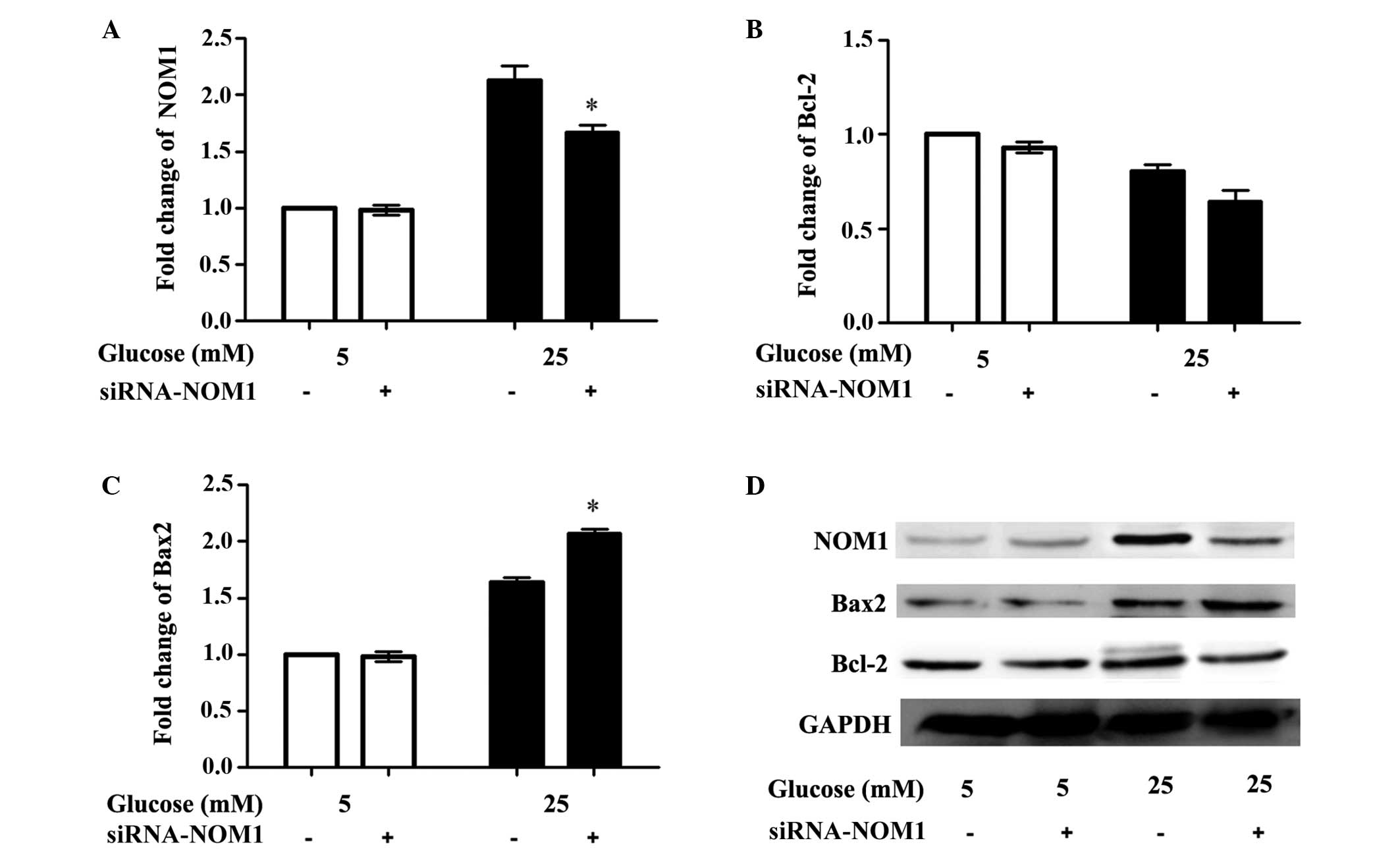
Western blot analysis
The expression levels of NOM1 and the cell
apoptosis-associated proteins caspase-3, Bcl-2 and Bax in MIN6
cells from each experimental group were detected using western
blotting analysis (Fig. 5). The
results demonstrated that, when MIN6 cells were treated with 5 mM
glucose, there were no significant differences in the levels of
cell apoptotic proteins, including Bcl-2, Bax2, and NOM1, between
the control and transfection groups (P>0.05). By contrast, when
cells were treated with 25 mM glucose, NOM1 expression in the
siRNA-NOM1 group was significantly reduced compared with the
control group (P<0.05; Fig. 5A),
whilst there was no significant difference in Bcl-2 expression
among the different transfection groups (P>0.05; Fig. 5B). In addition, Bax2 expression in
the siRNA-NOM1 group was significantly increased compared with the
control group (P<0.05; Fig.
5C).
Discussion
Diabetes, characterized by increased levels of
glucose in the blood and urine, is a metabolic disorder that
involves the disturbance of sugar, fat and protein metabolisms
resulting from impairment in the secretion and function of insulin
(19,20). The present study analyzed the role of
NOM1 expression in the apoptosis and insulin secretion of
pancreatic islet MIN6 β cells. The results showed that knockdown of
NOM1 was able to significantly inhibit MIN6 cell proliferation and
significantly contribute to MIN6 cell apoptosis through increasing
cleaved caspase-3 and Bax2 expression levels, but decreasing Bcl-2
expression (P<0.05). Furthermore, NOM1 knockdown was found to
significantly decrease insulin secretion and the expression of
insulin 2 mRNA in MIN6 cells cultured with a high glucose
concentration (25 mM; P<0.05).
Since diabetes indicates a sensitivity to high sugar
levels, the current study investigated the effect of glucose in
NOM1-expressing cells by indirectly assessing the cleaved caspase-3
level in MIN6 cells (21). The
results demonstrated that a high glucose concentration of 25 mM led
to increased caspase-3 expression in MIN6 cells; since NOM1 may be
negatively correlated to the cleaved caspase-3 level, the NOM1
expression was affected by 25 mM glucose in MIN6 cells. Insulin is
a secreted peptide that regulates homeostasis in mammals, while
insulin biosynthesis is controlled by glucose at many levels
(22). Panda et al observed
that the mRNA level of mouse insulin 2 was correlated with insulin
expression (23). In the present
study, NOM1 knockdown significantly decreased the insulin 2 mRNA
expression in MIN6 cells, implying that downregulation of NOM1 may
suppress insulin expression in MIN6 cells.
Pancreatic islet β cell proliferation is positively
correlated to insulin secretion during diabetes progression
(24). In the present study,
knockdown of NOM1 significantly inhibited MIN6 cell proliferation,
indicating that upregulation of NOM1 may promote MIN6 β cell
proliferation. In addition, it has been previously demonstrated
that caspase family proteins are cysteine proteases and they are
widely involved in cell apoptosis (25). Caspase-3 is the apoptotic factor that
can be activated by upstream key cellular proteins, such as
cytochrome c and other signal proteins (26). Furthermore, Bcl-2 and Bax2 are two
cell apoptosis-associated proteins that belong to the Bcl-2 family
protein (27). Overexpression of
anti-apoptotic Bcl-2 or downregulation of pro-apoptotic Bax2 has
been shown to inhibit apoptosis in various cells (28). Therefore, the Bcl-2/Bax ratio has
become an index for cell apoptosis in a number of diseases. For
instance, Federici et al have proved that caspase-3
expression and Bcl-2/Bax ratio were altered during human pancreatic
islets cell apoptosis induced by Arg972 polymorphism
(29). The present study revealed
that NOM1 knockdown was able to significantly increase cleaved
caspase-3 expression and decrease Bcl-2/Bax2 ratio in MIN6 cells,
suggesting that upregulation of NOM1 may inhibit MIN6 β cell
apoptosis.
In conclusion, the results presented in the current
study suggested that NOM1 may serve pivotal roles in pancreatic
islet β cell proliferation and insulin secretion by affecting cell
apoptosis. The present study may provide a basis for the
understanding of pathogenesis and development of diabetes therapies
in clinical practice. However, further experimental studies are
required for in-depth investigation of the underlying
mechanism.
References
|
1
|
Henderson DC, Cagliero E, Gray C,
Nasrallah RA, Hayden DL, Schoenfeld DA and Goff DC: Clozapine,
diabetes mellitus, weight gain and lipid abnormalities: A five-year
naturalistic study. Am J Psychiatry. 157:975–981. 2014. View Article : Google Scholar
|
|
2
|
Wajchenberg BL: Beta-cell failure in
diabetes and preservation by clinical treatment. Endocr Rev.
28:187–218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang S and Kaufman RJ: The impact of the
unfolded protein response on human disease. J Cell Biol.
197:857–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kondo T, Sasaki K, Matsuyama R, MorinoKoga
S, Adachi H, Suico MA, Kawashima J, Motoshima H, Furukawa N, Kai H
and Araki E: Hyperthermia with mild electrical stimulation protects
pancreatic β-cells from cell stresses and apoptosis. Diabetes.
61:838–847. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boroughs LK and DeBerardinis RJ: Metabolic
pathways promoting cancer cell survival and growth. Nat Cell Biol.
17:351–359. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu Z, Gilbert ER and Liu D: Regulation of
insulin synthesis and secretion and pancreatic Beta-cell
dysfunction in diabetes. Curr Diabetes Rev. 9:25–53. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saltiel AR and Kahn CR: Insulin signalling
and the regulation of glucose and lipid metabolism. Nature.
414:799–806. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woods WG, Neudorf S, Gold S, Sanders J,
Buckley JD, Barnard DR, Dusenbery K, DeSwarte J, Arthur DC, Lange
BJ, et al: A comparison of allogeneic bone marrow transplantation,
autologous bone marrow transplantation and aggressive chemotherapy
in children with acute myeloid leukemia in remission. Blood.
97:56–62. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buchwald G, Schüssler S, Basquin C, Le Hir
H and Conti E: Crystal structure of the human eIF4AIII-CWC22
complex shows how a DEAD-box protein is inhibited by a MIF4G
domain. Proc Natl Acad Sci USA. 110:E4611–E4618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simmons HM, Ruis BL, Kapoor M, Hudacek AW
and Conklin KF: Identification of NOM1, a nucleolar, eIF4A binding
protein encoded within the chromosome 7q36 breakpoint region
targeted in cases of pediatric acute myeloid leukemia. Gene.
347:137–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alexandrov A, Colognori D and Steitz JA:
Human eIF4AIII interacts with an eIF4G-like partner, NOM1,
revealing an evolutionarily conserved function outside the exon
junction complex. Genes Dev. 25:1078–1090. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin W, Chen Z, Zhang Y, Yan R, Yan G, Li
S, Zhong H and Lin S: Nom1 mediates pancreas development by
regulating ribosome biogenesis in zebrafish. PloS One.
9:e1007962014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Huang W, Huang S, Du J and Huang C:
Screening of anti-cancer agent using zebrafish: Comparison with the
MTT assay. Biochem Biophys Res Commun. 422:85–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haddow JE, Palomaki GE, Knight GJ,
Cunningham GC, Lustig LS and Boyd PA: Reducing the need for
amniocentesis in women 35 years of age or older with serum markers
for screening. N Engl J Med. 330:1114–1118. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vandesompele J, De Preter K, Pattyn F,
Poppe B, Van Roy N, De Paepe A and Speleman F: Accurate
normalization of real-time quantitative RT-PCR data by geometric
averaging of multiple internal control genes. Genome Biol.
3:Research0034. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rio DC, Ares M, Hannon GJ and Nilsen TW:
Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb
Protoc: pdb. prot5439. 2010. View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Withers DJ, Gutierrez JS, Towery H, Burks
DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, et al:
Disruption of IRS-2 causes type 2 diabetes in mice. Nature.
391:900–904. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Virdi N, Daskiran M, Nigam S, Kozma C and
Raja P: The association of self-monitoring of blood glucose use
with medication adherence and glycemic control in patients with
type 2 diabetes initiating non-insulin treatment. Diabetes Technol
Ther. 14:790–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beals JM, DeFelippis MR, Kovach PM and
Jackson JA: Insulin. In: Pharmaceutical Biotechnology: Fundamentals
and ApplicationsCrommelin DJA, Sindelar RD and Meibohm B: 4th
edition. Springer; New York, NY: pp. 255–275. 2013
|
|
21
|
Malik VS, Popkin BM, Bray GA, Després JP,
Willett WC and Hu FB: Sugar-sweetened beverages and risk of
metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes
Care. 33:2477–2483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bai H, Kang P and Tatar M: Drosophila
insulin-like peptide-6 (dilp6) expression from fat body extends
lifespan and represses secretion of drosophila insulin-like
peptide-2 from the brain. Aging Cell. 11:978–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Panda AC, Kulkarni SD, Muralidharan B,
Bakthavachalu B and Seshadri V: Novel splice variant of mouse
insulin2 mRNA: Implications for insulin expression. FEBS Lett.
584:1169–1173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lumelsky N, Blondel O, Laeng P, Velasco I,
Ravin R and McKay R: Differentiation of embryonic stem cells to
insulin-secreting structures similar to pancreatic islets. Science.
292:1389–1394. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Q, Li F, Liu X, Shi W, Liu FF,
O'Sullivan B, He Z, Peng Y, Tan AC, Zhou L, et al: Caspase
3-mediated stimulation of tumor cell repopulation during cancer
radiotherapy. Nat Med. 17:860–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bagci E, Vodovotz Y, Billiar TR,
Ermentrout GB and Bahar I: Bistability in apoptosis: Roles of bax,
bcl-2 and mitochondrial permeability transition pores. Biophys J.
90:1546–1559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vaux DL, Cory S and Adams JM: Bcl-2 gene
promotes haemopoietic cell survival and cooperates with c-myc to
immortalize pre-B cells. Nature. 335:440–442. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Federici M, Hribal ML, Ranalli M, Marselli
L, Porzio O, Lauro D, Borboni P, Lauro R, Marchetti P, Melino G and
Sesti G: The common Arg972 polymorphism in insulin
receptor substrate-1 causes apoptosis of human pancreatic islets.
FASEB J. 15:22–24. 2001.PubMed/NCBI
|