Introduction
Despite years of intensive clinical and experimental
investigation, subarachnoid hemorrhage (SAH) remains a lethal
complication of a ruptured intracranial aneurysm. For survivors of
the initial bleeding, delayed cerebral ischemia frequently occurs
and is the primary cause of subsequent morbidity and mortality
(1). However, the mechanism of
delayed cerebral ischemia following SAH is poorly understood
(2). Previous studies have typically
focused on vasospasm of the large cerebral arteries; however, a
double-blind, randomized clinical trial of the endothelin receptor
antagonist clazosentan showed no effect on clinical outcome,
despite inhibiting angiographic vasospasm (3).
An explanation for these results could be that
cerebral microcirculation and its regulatory mechanisms are
directly affected by SAH (4),
particularly pericytes, the primary cell type controlling
microcirculation in brain parenchyma (5). A number of studies have addressed the
role of microcirculatory dysfunction during SAH, where arteriolar
constriction is typically observed (6,7).
However, these results and insights have not been confirmed in
vitro. Until recently, in vitro observation and
quantitative functional assessment of cerebral microcirculation
following SAH were limited by the absence of suitable models.
The present study introduces whole-mount retinal
microvasculature to study brain microcirculation following
experimental SAH in vitro. Artificial blood-filled
cerebrospinal fluid (BSCF) was applied to the mount to test the
hypothesis that the presence of subarachnoid blood affects the
contractile properties of pericytes containing cerebral
microcirculation during the early phase of SAH.
Materials and methods
Experimental animals
All protocols used were approved by the Ethics
Committee of the Southwest Hospital (Chongqing, China) and
performed in accordance with the guidelines of the eighth edition
of the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (8). Sixty-five
six-week old male Sprague-Dawley rats (Experimental Animal Center,
Third Military Medical University, Chongqing, China), weighing
between 200 and 250 g, were used in the present study. All rats
were kept in a quiet room at 23–25°C, 70% humidity and a 12 h
light/dark cycle and were allowed ad libitum access to
normal rat chow and filtered water.
BCSF preparation
BCSF was prepared as previously described (9). Briefly, equal volumes of arterial blood
and normal cerebrospinal fluid from donor rats was mixed and
incubated in a 37°C water bath for 24 h. Then, the mixed samples
were centrifuged at 10,000 × g for 20 min at room temperature.
Finally, the supernatant was collected and stored at 4°C until use.
BCSF was freshly prepared under aseptic conditions prior to the
experiments.
Whole-mount retina preparation
Whole-mount retina was prepared as previously
described (10). Briefly, rats were
anaesthetized through intraperitoneal injection of ketamine (50
mg/kg, Gutian Pharmaceutical Co., Ltd., Fujian, China) and xylazine
(10 mg/kg, Sigma-Aldrich, Shanghai, China) prior to sacrifice by
decapitation. Following sacrifice, the eyes were immediately
enucleated. A small cut was then made in the sclera, close to the
cornea, and the eyeball was submerged in Ames' medium
(Sigma-Aldrich) equilibrated with 95% O2 and 5%
CO2. Then, the retina were carefully dissected from the
pigment epithelium and attached, ganglion cell side-up, to a
MF-Millipore Membrane Filter (EMD Millipore, Billerica, MA, USA;
cat. no. AABP02500) with a 2 mm diameter hole in the center for
microvascular observation during imaging.
Time-lapse photography
The whole-mount retina preparation was transferred
into a 0.5 ml imaging chamber on the fixed stage of an upright
microscope (Leica DM LFSA; Leica Microsystems GmbH, Wetzlar,
Germany). The preparation was continuously superfused with
oxygenated bicarbonate-buffered Ames' medium at 35°C. Microvessels
were viewed at ×400 magnification with the aid of a ×40
water-immersion objective. Following a 2.67 min control period,
microvessels were exposed to the experimental perfusate for 5.33
min, then re-exposed to the control perfusate. To facilitate the
detection of pericyte contractions, time-lapse images were captured
at 8 sec intervals using a digital camera running Image-Pro Plus
software version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
The small number of pericytes (<5%) that spontaneously
contracted and relaxed were excluded from analysis. Based on the
knowledge that 20% of the length of microvessels of the rat retina
are within 30 µm of a bifurcation (11), the probability of responding
pericytes being located near microvessel branch points (≤30 µm) was
calculated as previously described (12). Lumen diameters at sites adjacent to
contracting pericytes were measured using Image-Pro Plus software.
During exposure to experimental perfusates, lumen diameters were
measured at the time of maximum change in responsive vessels. As
contracting pericytes can cause microvascular lumens to move out of
the narrow depth of focus, only lumens that remained in focus
throughout the experiment were included in the analysis.
In vitro culture of retinal
pericytes
Microvessel pericytes were obtained from rat retina
as previously described (13). Rat
retinas were dissected as described above. The tissue was then
washed in a phosphate-buffered saline (PBS) solution supplemented
with antibiotics (100 U/ml penicillin and 100 U/ml streptomycin),
then minced and incubated in PBS solution with 0.1% collagenase,
0.2% trypsin and 0.02% glucose (all purchased from Sigma-Aldrich).
Next, the solution was homogenized for ~60 min at 37°C on a shaking
platform. The suspension was then filtered through 100 µm nylon
mesh to remove large tissue fragments and washed in Dulbecco's
modified Eagle's medium (DMEM, Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) containing 10% fetal bovine serum
(Hyclone; GE Healthcare Life Sciences) and antibiotic solution (100
U/ml penicillin and 100 µg/ml streptomycin). Each culture cell dish
was seeded from 4 retinas. Pure cultures of pericytes were
incubated at 37°C with 5% CO2 atmosphere, DMEM with 20%
fetal bovine serum was changed daily (days 3, 4, and 5) until day
5, after which the medium was changed every 3 days. When primary
pericyte cultures were near confluence (>80%), they were further
propagated by treatment with 1 ml of 0.25% (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and 0.02% EDTA for 3 min
and then were split into a 1:3 ratios to passage to 6-well plates.
In the present study, cultured pericytes were identified using
α-smooth muscle actin (α-SMA) as a marker and morphological images
were identified after the 3rd passage, and used between the 5th and
9th passages.
Collagen gel contraction assay
The collagen gel contraction assay was performed as
described previously (14). Rat-tail
tendon type I collagen (Shengyou Biotechnology Co., Ltd., Hangzhou,
China) was diluted with DMEM to 2 mg/ml and adjusted to pH 7.4. To
promote gel detachment, 12-well plates were precoated with 1%
agarose. Retinal pericyte cultures were trypsinized when 80%
confluent and liberated cells were counted using a hemocytometer.
The trypsinized solution was then diluted to 1×105
cells/ml with DMEM. Equal volumes of collagen and cell solution
were then combined, and 1 ml containing 1 mg collagen and
5×104 cells was pipetted into each well. Then, gels were
polymerized at 37°C and then released from the dish edges with a
fine needle. Diameters were recorded at three time points: Prior to
BCSF incubation, and BCSF incubation at 24 and 48 h, with
contraction measured relative to the initial gel diameter. All
assays were repeated 3x with triplicate wells for each experimental
condition.
Western blotting
Pericytes harvested from the collagen gel were
suspended in ice-cold PBS at 1.25×106 cells/ml. Proteins
were extracted by Total Protein Extraction Kit for Cultured Cells
(Boster Biological Technology, Wuhan, China), following the
instruction manual. Western blot analysis was then performed as
previously described (15).
Equivalent protein amounts (30 µg) were loaded in each lane of 10%
SDS-PAGE gels. After gel electrophoresis, protein was transferred
onto a nitrocellulose membrane, which was then blocked by 5%
non-fat milk blocking buffer for 2 h at room temperature. The
following primary antibodies were diluted to incubate with the
membrane under gentle agitation at 4°C overnight: Anti-α-SMA
(1:1,000; cat. no. ab32575; rabbit monoclonal; Abcam, Cambridge,
UK). Then, a secondary antibody (horseradish peroxidase-conjugated
goat anti-rabbit IgG; 1:1,000; cat. no. ab6721; Abcam) was
incubated with the nitrocellulose membrane for 2 h at room
temperature. Chemiluminescent detection was performed to identify
the immune bands with an ELC Plus kit (cat. no. PRN2232; GE
Healthcare Life Sciences). The resulting blots were scanned and
semi-quantitatively analyzed in a blind fashion using Image J
software version 1.48 (https://imagej.nih.gov/ij/). Anti-β-tubulin (1:4,000;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used as an
internal control for all the experiments and any changes observed
were expressed as a percentage of the readings on day 0.
Intracellular calcium
([Ca2+]i) microfluorimetry
[Ca2+]i imaging was performed
as previously described (16). The
buffer solution for [Ca2+]i measurement was
pH adjusted to 7.4 with NaOH and contained (in mmol/l): 145 NaCl, 1
CaCl2, 3 KCl, 1 MgCl2, 10 HEPES and 10
glucose. EGTA (0.1 mmol/l) was included in the Ca2+-free
extracellular buffer. Cells were loaded into the microfluorimeter
with the Ca2+-specific fluorescence indicator
fura-2-acetoxymethylester (3 mmol/l) and incubated for 30 min at
room temperature in the buffer solution. After loading, coverslips
were placed on the bottom of a 600 ml Plexiglas perfusion chamber
with openings at either end for perfusion and aspiration. The cells
were perfused with the buffer solution for 10 min prior to the
experiment to allow de-esterification of the dye. Digital
[Ca2+]i imaging was performed through video
microfluorimetry using a charge-coupled device (Princeton
Instruments, Inc., Trenton, NJ, USA) attached to a Nikon Eclipse
microscope with a Nikon CFI Super Fluor Objectives (Nikon
Corporation, Tokyo, Japan) and MetaMorph System version 5.0
(Universal Imaging Corp, Downingtown, PA, USA). Imaging was
performed with alternating excitation wavelengths of 340 and 380
nm. Background fluorescence obtained from a cell-free portion of
the same coverslip was subtracted from all recordings to normalize
the data prior to calculation of the ratio between the 340:380 nm
recordings. The ratio values were then converted into
[Ca2+]i as previously described (16).
Statistical analysis
The results are expressed as the mean ± standard
error of the mean. The statistical significance of the differences
between groups was calculated using one-way analysis of variance
followed by a Newman-Keuls test. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SPSS software (version 16.0; SPSS,
Inc., Chicago, IL, USA).
Results
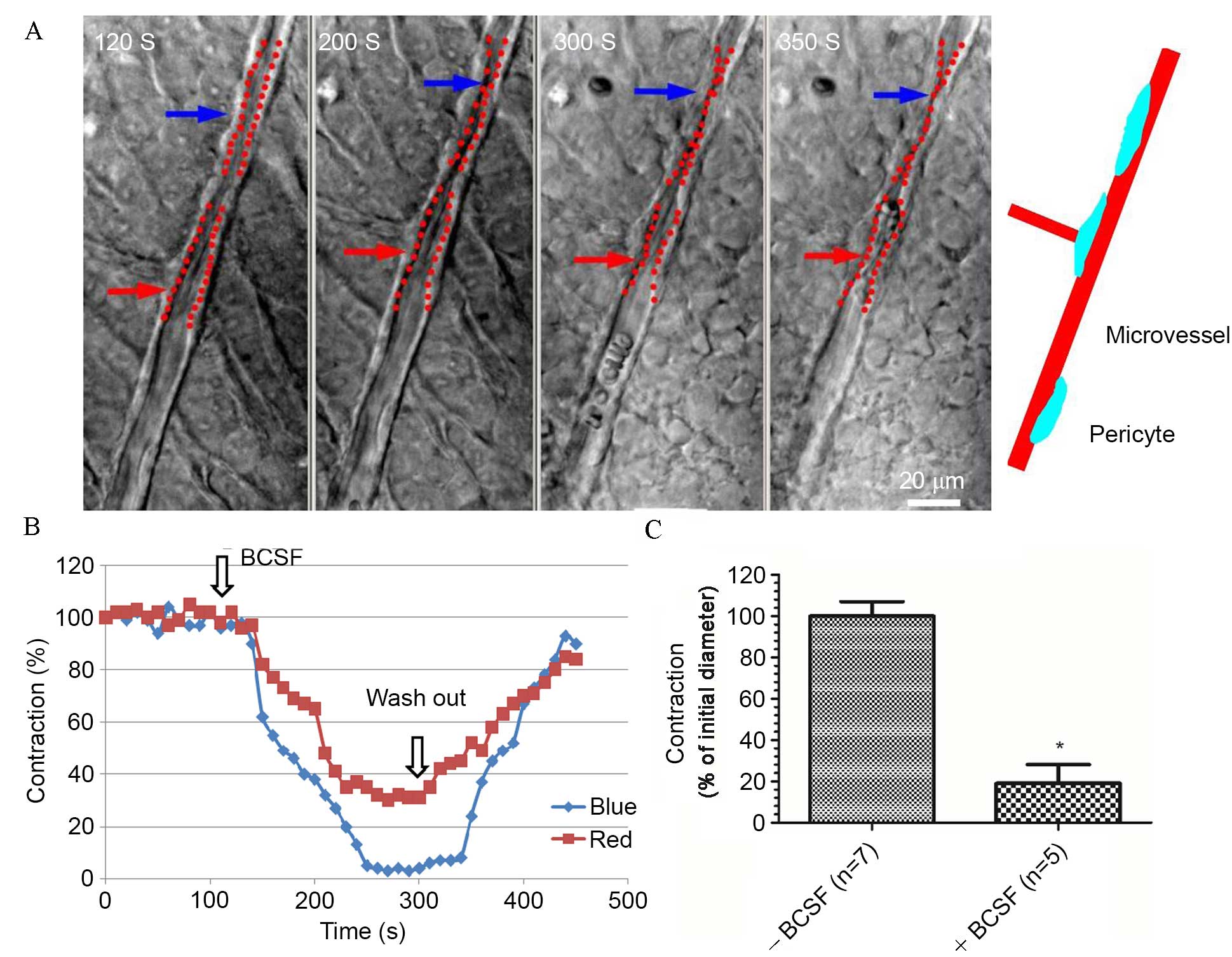
BCSF induced contraction of retinal
microvessels
To test the hypothesis that SAH would affect the
physiology of retinal microvessels, BSCF was used to mimic SAH
in vitro. It was observed that exposure of retinal
microvessels to BCSF caused increased contraction of the
microvessels (Fig. 1). However,
contraction diminished and the microvessels relaxed after the BSCF
was washed out, showing that the effects of BSCF were reversible
(Fig. 1A and B). The mean
contraction percentage of retinal microvessels following BCSF
exposure was significantly lower compared with prior to BCSF
exposure (P<0.01; Fig. 1C).
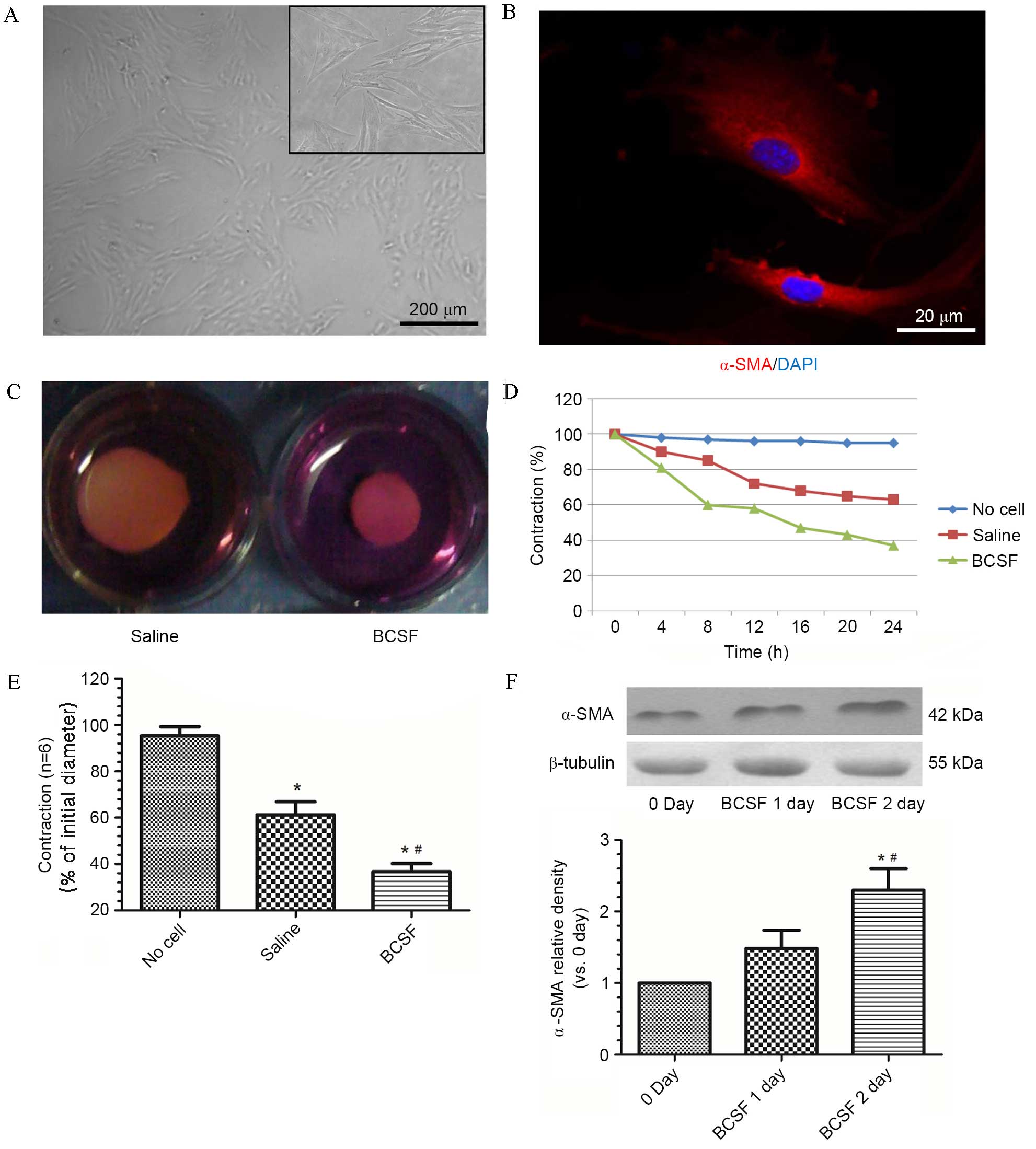
BCSF induced contraction of
pericyte-populated collagen gel and increased α-SMA expression
Cultured pericytes were identified using α-SMA as a
marker and morphological images after the 3rd passage (Fig. 2A and B). With the addition of
pericytes, collagen gels contracted to 63% of their initial
diameter at 24 h (P<0.05; Fig.
2C-E). The addition of BCSF significantly accelerated
pericyte-populated collagen gel contraction at 24 h to 36%
(P<0.05; Fig. 2C-E). The
increased contraction induced by BCSF exhibited a time-dependent
trend over 24 h in groups treated with saline and BCSF (Fig. 2D). The BCSF-treated pericytes group
and saline-treated control pericyte group from the collagen gel
expressed different levels of α-SMA. Western blots identified that
the expression of α-SMA was significantly upregulated over a 2-day
period of incubation with BCSF (P<0.05, Fig. 2F).
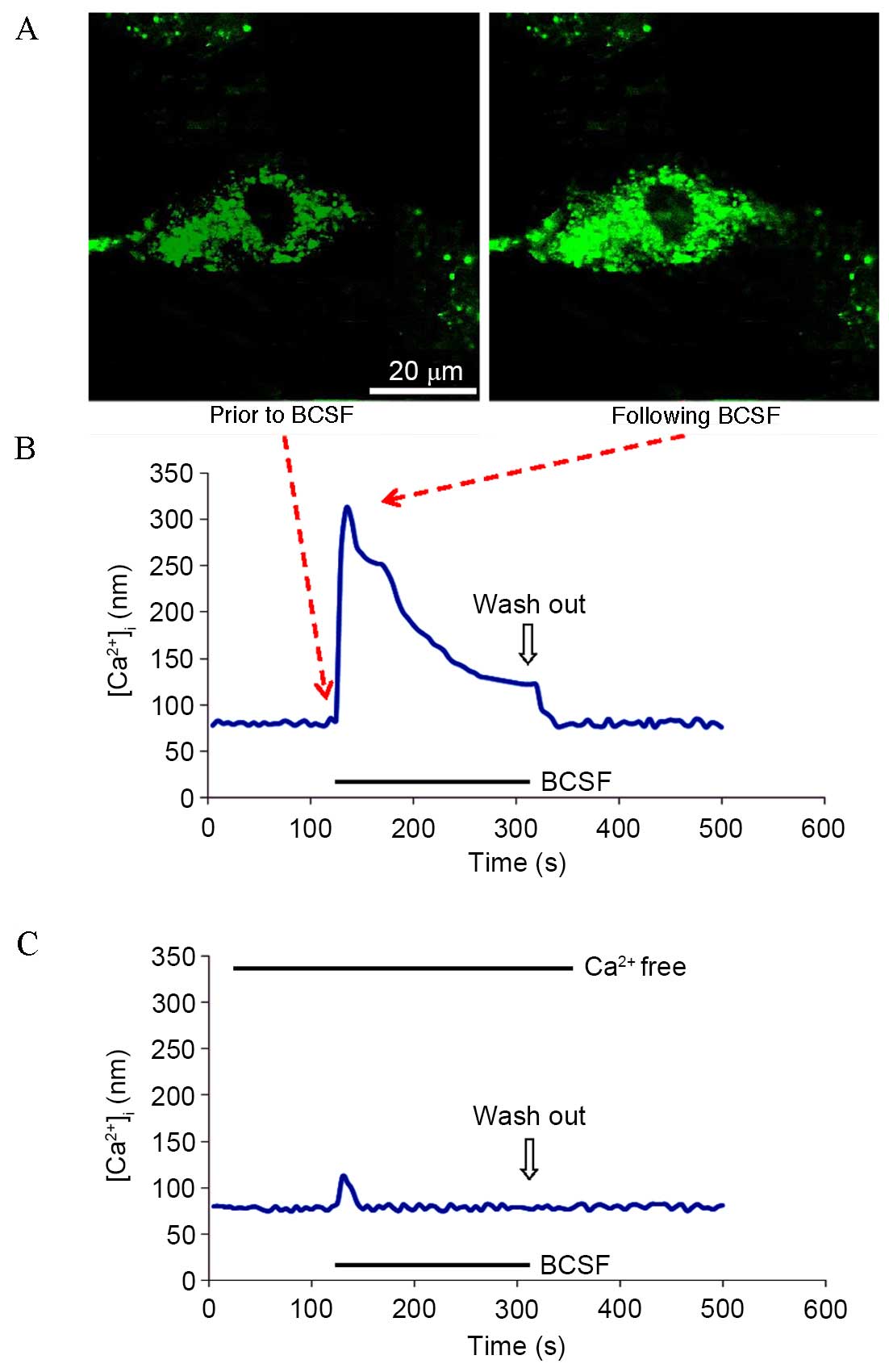
BCSF induced
[Ca2+]i elevation in cultured retinal
pericytes
Microfluorimetry analysis identified that pericyte
exposure to BCSF produced a peak [Ca2+]i
response, followed by a sustained plateau phase in the presence of
extracellular Ca2+ (Fig. 3A
and B). BCSF exposure in the absence of extracellular
Ca2+ dramatically decreased the peak
[Ca2+]i response, producing only a small
transient peak without a plateau phase (Fig. 3C).
Discussion
In the present study, it was identified that BCSF
exposure induced retinal microvessel contraction and that this
contraction was resolved by BCSF wash-out. In addition, BCSF
exposure accelerated retinal pericyte-populated collagen gel
contraction and increased the expression of α-SMA in a
time-dependent manner. Furthermore, BCSF induced a calcium influx
in cultured retinal pericytes.
Numerous studies of SAH in the past decades have
focused on vasospasm in the primary arteries (4,17,18). It
was widely accepted that the ischemia that prognosticates poor
outcome was caused by cerebral vasospasm (18). However, this classical theory of
SAH-induced vasospasm has been fiercely challenged by a clinical
trial of clazosentan, which, although reversing angiographic
vasospasm, failed to improve patient outcome (19). Previous clinical studies have showed
that numerous patients develop angiographic vasospasm following
aneurysmal SAH, but only a small number will go on to develop
cerebral ischemia and infarction (18,20–22).
Angiographic vasospasm does not always correlate with poor clinical
outcome (18,19), this may be attributable to the
changes in microcirculation following SAH (6,23,24).
Previous studies have demonstrated that the cerebral
microvasculature is significantly affected by SAH (6,7,25). Uhl et al (26) reported, for the first time, direct
visualization of cerebral microcirculatory changes in patients
following SAH with orthogonal polarization spectral imaging.
Similar observations were described by Friedrich et al
(27). These studies showed that SAH
is associated with a micro-vasospasm, primarily affecting
arterioles, with a reduction of diameter in pial vessels and an
overall decrease in microvessel density. It is postulated that the
increased intracranial pressure following SAH leads to compression
in cerebral microvessels, resulting in a reduction in microvessel
density (28). However, due to
technical limitations, these studies showed no direct evidence of
microvessel dysfunction following SAH.
The present study identified that SAH may be
associated with increased contractility of microvessels, as
indicated by enhanced vasoconstriction in response to BCSF. As
microvessels lack smooth muscle, blood flow is frequently assumed
to be regulated by precapillary arterioles. However, the majority
(65%) of adrenergic innervation of central nervous system (CNS)
blood vessels terminates near microvessels rather than arterioles,
and in the muscle and brain a dilatory signal propagates from
vessels near metabolically active cells to precapillary arterioles,
suggesting that blood flow control is initiated in microvessels
(4,5,7).
Pericytes in CNS microvessels contain contractile proteins and can
initiate such signaling (29,30). In
the present study it was observed that pericytes can control
microvessel diameter in whole-mount retinal microvasculature.
It has often been postulated that pericytes are
contractile cells and contribute to the regulation of blood flow at
the microvascular level (29,30). The
BCSF-induced microvessel observed in the present study may be
attributable to the release of a vasoactive agent from underlying
glia and neurons. In order to exclude this cause, the effect of
BCSF on cultured pericytes was tested with a gel contraction assay.
This identified that BCSF accelerated pericyte-populated collagen
gel contraction over 24 h. This confirms that BCSF affects
microvessel function through enhancing pericyte contraction. Future
studies into the underlying mechanisms and role of
pericyte-containing microvessel disturbances in the pathophysiology
of delayed cerebral ischemia should be explored.
In conclusion, the present study demonstrates an
increased contractility of the pericytes and reduced diameter of
microvessels in the presence of BCSF in vitro. These
findings indicate that pericyte contraction and microvascular
dysfunction is induced following SAH, which may lead to an
increased susceptibility to SAH-induced ischemia.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 30801186, 81501002
and 81220108009) and the National Basic Research Program of China
(program 973, grant no. 2014CB541600).
References
|
1
|
Woitzik J, Dreier JP, Hecht N, Fiss I,
Sandow N, Major S, Winkler M, Dahlem YA, Manville J, Diepers M, et
al: Delayed cerebral ischemia and spreading depolarization in
absence of angiographic vasospasm after subarachnoid hemorrhage. J
Cereb Blood Flow Metab. 32:203–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Macdonald RL: Delayed neurological
deterioration after subarachnoid haemorrhage. Nat Rev Neurol.
10:44–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyers PM and Connolly ES Jr: Stroke:
Disappointing results for clazosentan in CONSCIOUS-2. Nat Rev
Neurol. 7:660–661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen S, Feng H, Sherchan P, Klebe D, Zhao
G, Sun X, Zhang J, Tang J and Zhang JH: Controversies and evolving
new mechanisms in subarachnoid hemorrhage. Prog Neurobiol.
115:64–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen Y, Li Q, Tang J, Feng H and Zhang JH:
The evolving roles of pericyte in early brain injury after
subarachnoid hemorrhage. Brain Res. 1623:110–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tso MK and Macdonald RL: Subarachnoid
hemorrhage: A review of experimental studies on the
microcirculation and the neurovascular unit. Transl Stroke Res.
5:174–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ostergaard L, Aamand R, Karabegovic S,
Tietze A, Blicher JU, Mikkelsen IK, Iversen NK, Secher N, Engedal
TS, Anzabi M, et al: The role of the microcirculation in delayed
cerebral ischemia and chronic degenerative changes after
subarachnoid hemorrhage. J Cereb Blood Flow Metab. 33:1825–1837.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Institute of Laboratory Animal Resources
(US), . Committee on Care, Use of Laboratory Animals and National
Institutes of Health (US)Division of Research Resources: Guide for
the care and use of laboratory animals. 8th. National Academies
Press; Washington, DC: 2011
|
|
9
|
Foley PL, Takenaka K, Kassell NF and Lee
KS: Cytotoxic effects of bloody cerebrospinal fluid on cerebral
endothelial cells in culture. J Neurosurg. 81:87–92. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun W, Deng Q, Levick WR and He S: ON
direction-selective ganglion cells in the mouse retina. J Physiol.
576:197–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawamura H, Kobayashi M, Li Q, Yamanishi
S, Katsumura K, Minami M, Wu DM and Puro DG: Effects of angiotensin
II on the pericyte-containing microvasculature of the rat retina. J
Physiol. 561:671–683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu DM, Kawamura H, Sakagami K, Kobayashi M
and Puro DG: Cholinergic regulation of pericyte-containing retinal
microvessels. Am J Physiol Heart Circ Physiol. 284:H2083–H2090.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu G, Meng C, Pan M, Chen M, Deng R, Lin
L, Zhao L and Liu X: Isolation, purification, and cultivation of
primary retinal microvascular pericytes: A novel model using rats.
Microcirculation. 21:478–489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oishi K, Kamiyashiki T and Ito Y:
Isometric contraction of microvascular pericytes from mouse brain
parenchyma. Microvasc Res. 73:20–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Zhang Y, Tang J, Liu F, Hu Q, Luo
C, Tang J, Feng H and Zhang JH: Norrin protected blood-brain
barrier via frizzled-4/β-catenin pathway after subarachnoid
hemorrhage in rats. Stroke. 46:529–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wurm A, Pannicke T and Reichenbach A: Ca2+
microfluorimetry in retinal Müller glial cells. Methods Mol Biol.
935:257–270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caner B, Hou J, Altay O, Fujii M and Zhang
JH: Transition of research focus from vasospasm to early brain
injury after subarachnoid hemorrhage. J Neurochem. 123 Suppl
2:S12–S21. 2012. View Article : Google Scholar
|
|
18
|
Macdonald RL, Pluta RM and Zhang JH:
Cerebral vasospasm after subarachnoid hemorrhage: The emerging
revolution. Nat Clin Pract Neurol. 3:256–263. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wong GK and Poon WS: Clazosentan for
patients with subarachnoid haemorrhage: Lessons learned. Lancet
Neurol. 10:871–872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cossu G, Messerer M, Oddo M and Daniel RT:
To look beyond vasospasm in aneurysmal subarachnoid haemorrhage.
Biomed Res Int. 2014:6285972014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou J and Zhang JH: Does prevention of
vasospasm in subarachnoid hemorrhage improve clinical outcome? No.
Stroke. 44(6 Suppl 1): S34–S36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hansen-Schwartz J, Vajkoczy P, Macdonald
RL, Pluta RM and Zhang JH: Cerebral vasospasm: Looking beyond
vasoconstriction. Trends Pharmacol Sci. 28:252–256. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Terpolilli NA, Brem C, Bühler D and
Plesnila N: Are we barking up the wrong vessels? Cerebral
microcirculation after subarachnoid hemorrhage. Stroke.
46:3014–3019. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naraoka M, Matsuda N, Shimamura N, Asano K
and Ohkuma H: The role of arterioles and the microcirculation in
the development of vasospasm after aneurysmal SAH. Biomed Res Int.
2014:2537462014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chai WN, Sun XC, Lv FJ, Wan B and Jiang L:
Clinical study of changes of cerebral microcirculation in cerebral
vasospasm after SAH. Acta Neurochir Suppl. 110:225–228.
2011.PubMed/NCBI
|
|
26
|
Uhl E, Lehmberg J, Steiger HJ and Messmer
K: Intraoperative detection of early microvasospasm in patients
with subarachnoid hemorrhage by using orthogonal polarization
spectral imaging. Neurosurgery. 52:1307–1317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Friedrich B, Müller F, Feiler S, Schöller
K and Plesnila N: Experimental subarachnoid hemorrhage causes early
and long-lasting microarterial constriction and microthrombosis: An
in-vivo microscopy study. J Cereb Blood Flow Metab. 32:447–455.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen S, Chen Y, Xu L, Matei N, Tang J,
Feng H and Zhang JH: Venous system in acute brain injury:
Mechanisms of pathophysiological change and function. Exp Neurol.
272:4–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peppiatt CM, Howarth C, Mobbs P and
Attwell D: Bidirectional control of CNS capillary diameter by
pericytes. Nature. 443:700–704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hall CN, Reynell C, Gesslein B, Hamilton
NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM, Lauritzen M
and Attwell D: Capillary pericytes regulate cerebral blood flow in
health and disease. Nature. 508:55–60. 2014. View Article : Google Scholar : PubMed/NCBI
|