Introduction
Brucellae are facultative intracellular bacterial
pathogens (1–3). Although Brucella species lack
most of the classical virulence factors such as invasive proteases,
exotoxins, capsules fimbriae, virulence plasmids and lysogenic
phages (4), they are able to grow in
phagocytes. Similar to other intracellular bacterial pathogens,
including Shigella, Salmonella, Yersinia and
Mycobacterium, Brucella species are able to modulate
the apoptosis of host cells to benefit intracellular survival and
replication (5). To replicate in
macrophage cells, Brucella have evolved mechanisms such as
inhibition of apoptosis to modulate cell death machinery (6,7), which
in turn favors pathogen survival and replication (7,8); cell
death by apoptosis is a common response of mammalian cells to a
wide variety of bacterial infections (9).
Tumor necrosis factor (TNF)-α is important for the
autocrine stimulation of macrophage functions, which is important
for triggering specific immunity against a number of intracellular
pathogens. TNF-α is one of many physiological factors that are able
to trigger apoptosis (10).
Furthermore, TNF-α-induced apoptosis has been indicated to play a
role in a variety of pathologies linked to chronic inflammation and
auto-immune diseases (11,12). Analysis of Brucella infection
in mice indicates a crucial and central role for TNF-α in the
control of Brucella invasion and mouse recovery (13). Furthermore, TNF-α contributes to
resistance to intracellular Brucella at different stages of
infection, at the levels of innate and specific immunity (13).
Outer membrane protein (OMP)25 from Brucella
suis has been reported to inhibit the production of TNF-α by
human macrophages (14).
Furthermore, B. suis-infected macrophages secreted
significantly lower amounts of TNF-α than did macrophages infected
with a ΔOMP25 B. suis mutant (14). OMP31 has a significant homology (34%
identity) with Brucella OMP25 (15). In a previous study, OMP31 was
reported to be an important immunogenic major outer membrane
protein and antigen (16), which is
present in all Brucella species, with the exception of B.
abortus (17).
However, whether the inhibition of cellular
apoptosis is associated with OMP31 from Brucella melitensis
is unknown. It may be hypothesized that OMP31 from B.
melitensis benefits survival and replication in macrophages by
inhibiting apoptosis through TNF-α signaling following
Brucella infection. In the present study, to test this
hypothesis, an OMP31 gene deletion mutant based on B.
melitensis 16M was constructed and the apoptosis of RAW264.7
macrophages induced by the mutant or parent strain was assessed
using flow cytometry. To determine the mechanism of apoptosis
associated with OMP31 of B. melitensis, enzyme-linked
immunosorbent assay (ELISA) and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assays for the detection of the
levels of B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein
(Bax), caspase-2, −3, −6 and −9, TNF-α and cytochrome c (Cyt
c) were carried out.
Materials and methods
Bacterial strains and macrophage cell
line
The strains used in these experiments were B.
melitensis strain 16M (Library for Brucellosis of Chinese
Centre for Disease Control and Prevention) and Bacillus
subtilis strain BAA12545 (gifted by Professor Wenge Hu; College
of Life Sciences, Shihezi University, Shihezi, Xinjiang, China),
which was engineered for its sacB gene and used for the
screening of mutant strains by counter-selection. B.
melitensis was grown on Brucella agar (BD Biosciences,
Franklin Lakes, NJ, USA). Sucrose medium was utilized for
sacB counter-selection as previously described (18).
The RAW264.7 macrophage cell line was purchased from
the China Academy Typical Culture Preservation Committee cell
library (Shanghai, China). The cells were cultured at 37°C with 5%
CO2 in complete tissue culture medium (c-DMEM)
consisting of Dulbecco's modified Eagle's medium (American Type
Culture Collection, Manassas, VA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Construction of OMP31 gene deletion
mutant
Genomic DNA of B. melitensis strain 16M was
used as the template for amplification of OMP31 upstream and
downstream segments by PCR. The primer sequences used are shown in
Table I. The flanking sequences were
separately amplified, and overlap extension PCR (19) was then employed to combine the
flanking sequences to generate contiguous DNA sequences of the
OMP31 fragment. The fragment was cloned into a pMD18-T vector
(D101A; Takara Biotechnology Co., Ltd., Dalian, China). Following
SphI/XhoI digestion of the recombinants, the target
fragments were introduced into a pGEM-7zf (+) suicide vector
(P2251; Promega Corp., Beijing, China). The sacB gene of
Bacillus subtilis was amplified by PCR and inserted into the
recombinant pGEM-7zf (+) vector. Thus, the suicide plasmid
pGB-OMP31 was generated. The pGB-OMP31 plasmid was introduced into
B. melitensis strain 16M by electroporation as described
previously (20).
Ampicillin-resistant and sucrose-sensitive integrants were selected
according to the study of Campos et al (20). The mutant, designated 16MΔOMP31, was
identified by western blot assay and PCR tests (21) following subculture for 15
generations. Conventional microbiological tests, including assays
of lipopolysaccharide antigens, phage typing, dye sensitivities,
CO2 requirements, H2S production, and metabolic properties
(21), were carried out to identify
its phenotype.
 | Table I.Primer sequences for the construction
of the mutant Brucella melitensis strain 16MΔomp31. |
Table I.
Primer sequences for the construction
of the mutant Brucella melitensis strain 16MΔomp31.
| Target | Primer | Primer sequences
(5′→3′) | Restriction
site |
|---|
| Upstream | OMP31-N | F:
GCATGCCCCATCCTGTCGCTTTGTGT | SphI |
|
|
| R:
CTCGAGAATCACATTCGGCGAAAAAG | XhoI |
| Downstream | OMP31-C | F:
CTCGAGTTATCAGGCGGCGGGAATTG | XhoI |
|
|
| R:
GGATCCAGGCTTCGCTCGGTCACAGG | BamHI |
| SacB
gene | sacB | F:
GGATCCGGGCTGGAAGAAGCAGACCGCTA | BamHI |
|
|
| R:
GAGCTCGCTTATTGTTAACTGTTAATTGTCC | SacI |
| Detection for
Brucella | L7/L12 | F:
ATGGCTGATCTCGCAAAGA | – |
|
|
| R:
TTACTTGAGTTCAACCTTG | – |
| Detection for
mutant | DC | F:
CGTACATATTGGCGAGGGTG | – |
|
|
| R:
CCGTCAGGAAGGGTTCAGTG | – |
Macrophage infection and survival
assay
RAW264.7 macrophages were plated in 12-well plates
in DMEM without antibiotics at a concentration of 5×105
cells per well and incubated overnight at 37°C with 5% (vol/vol)
CO2. The RAW264.7 cells were then infected with B.
melitensis 16M or the mutant strain 16MΔOMP31 in triplicate
wells of a 24-well plate at a multiplicity of infection (MOI) of
100:1. Following 4 h of incubation at 37°C in an atmosphere
containing 5% CO2, the cells were washed three times
with phosphate-buffered saline (PBS, pH 7.0) and treated with 50
µg/ml gentamicin to kill extracellular Brucella. The
infected cells were then lysed with 0.1% Triton X-100 in PBS at 2,
4, 8, 12 and 24 h post infection. The number of viable colony
forming units (CFUs) was determined by plating a series of 1:10
dilutions on tryptic soy agar plates.
Determination of cell apoptosis
following infection by B. melitensis 16M or mutant
RAW264.7 cells cultured in 12-well plates were
infected with B. melitensis 16M or mutant strain at a MOI of 100:1
in triplicate wells as described above. At 2, 4, 8, 12 and 24 h
post infection, the RAW264.7 cells were washed with PBS and
harvested. Apoptosis was then investigated. Briefly, B.
melitensis 16M or mutant strain-infected RAW264.7 cells were
incubated with Annexin V and propidium iodide (PI) using an Annexin
V-FLUOS staining kit (Roche Diagnostics Corporation, Indianapolis,
IN, USA) at room temperature for 20 min. Immediately afterwards,
apoptotic RAW264.7 cells were detected using flow cytometry.
Detection of TNF-α by ELISA
Cells cultured in 12-well plates were infected with
B. melitensis 16M or mutant strain in triplicate wells as
described above. At 2, 4, 8, 12 and 24 h post infection, TNF-α
production in the culture supernatants was tested using an ELISA
kit (BD Biosciences).
Total RNA isolation and RT-qPCR
analysis of TNF-α, Bax and Bcl-2
Cells cultured in 12-well plates were infected with
B. melitensis 16M or mutant strain in triplicate wells as
described above. Total RNA of the RAW264.7 cells was isolated using
TRIzol reagent (CW0580Sl; CWBio, Beijing, China) and was further
purified using a Qiagen RNeasy mini kit (Qiagen, Inc., Valencia,
CA, USA), used according to the manufacturers' protocols. A
Nanodrop 2100 instrument (NanoDrop Technologies; Thermo Fisher
Scientific, Inc.) was used to assess the concentrations and quality
of the RNA samples. Reverse transcription, cDNA quantification and
cDNA amplification were performed exactly as described in the
instructions of the Super RT One Step RT-PCR kit (CW0742S; CWBio).
Specific primers used are listed in Table II. Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was used as a control. Relative mRNA levels
of a particular gene in mutant strains were normalized using the
2−ΔΔCq method with GAPDH as a reference gene (22).
 | Table II.Primer sequences for detection of
Bax, Bcl-2 and TNF-α by reverse transcription-quantitative
polymerase chain reaction. |
Table II.
Primer sequences for detection of
Bax, Bcl-2 and TNF-α by reverse transcription-quantitative
polymerase chain reaction.
| Primers | Primer sequences
(5′→3′) |
|---|
| GAPDH | F:
GCAGTGGCAAAGTGGAGATT |
|
| R:
CCTTGACTGTGCCGTTGAAT |
| TNF-α | F:
GCCTCCCTCTCATCAGTTCTAT |
|
| R:
CACTTGGTGGTTTGCTACGAC |
| Bax | F:
GCCTTTTTGCTACAGGGTTT |
|
| R:
TGCTGTCCAGTTCATCTCCA |
| Bcl-2 | F:
GACTTCTCTCGTCGCTACCG |
|
| R:
ACAATCCTCCCCCAGTTCAC |
Detection of Cyt c by ELISA
RAW264.7 cells cultured in 12-well plates were
infected with B. melitensis 16M or mutant strain at a MOI of
100:1 in triplicate wells as described above. At 12 h post
infection, Cyt c from the cells was detected using a Cyt
c ELISA kit (Elabscience, Wuhan, China) according to the
manufacturer's protocol.
Detection of caspase-3, −8 and −9 by
ELISA
RAW264.7 cells cultured in 12-well plates were
infected with B. melitensis 16M or mutant strain at a MOI of
100:1 in triplicate wells as described above. At 4 h post
infection, the concentrations of caspase-3, −8 and −9 were measured
using caspase-3, −8 and −9 ELISA kits (BioVision, Inc., Milpitas,
CA, USA) according to the manufacturer's instructions.
Statistical analysis
To determine the statistical significance of the
immune response to the parent and mutant, differences were analyzed
using analysis of variance tests and least significant difference
post-hoc tests, with SPSS statistical analysis software (version
13.0; SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Mutant strain 16MΔOMP31 has the same
phenotype as the parent strain
The OMP31 gene deletion mutant based on B.
melitensis 16M was successfully constructed. To assess the
mutant strain, conventional microbiology tests were carried out to
identify its phenotype. These results showed that the
lipopolysaccharide of the mutant strain remained smooth and the
mutant exhibited the same traits as Brucella melitensis 16M
(data not shown), including lipopolysaccharide antigens, phage
typing, dye sensitivities, CO2 requirements,
H2S production, and metabolic properties.
Deletion of OMP31 impairs the
intracellular survival ability of Brucella following infection of
RAW264.7 cells
Brucella has developed the ability to inhibit
apoptosis to benefit intracellular replication. To assess the
intracellular replication of the bacteria in RAW264.7 macrophages,
the number of CFUs was counted following infection with Brucella
melitensis 16M or the mutant strain. The results demonstrated
that at 2–10 h post-infection, particularly at 4 h post-infection,
parent stain impaired the ability of the bacteria to replicate in
RAW264.7 cells. However, at 10–24 h post infection, the OMP31
mutant strain impaired the ability of the bacteria to replicate in
RAW264.7 cells.
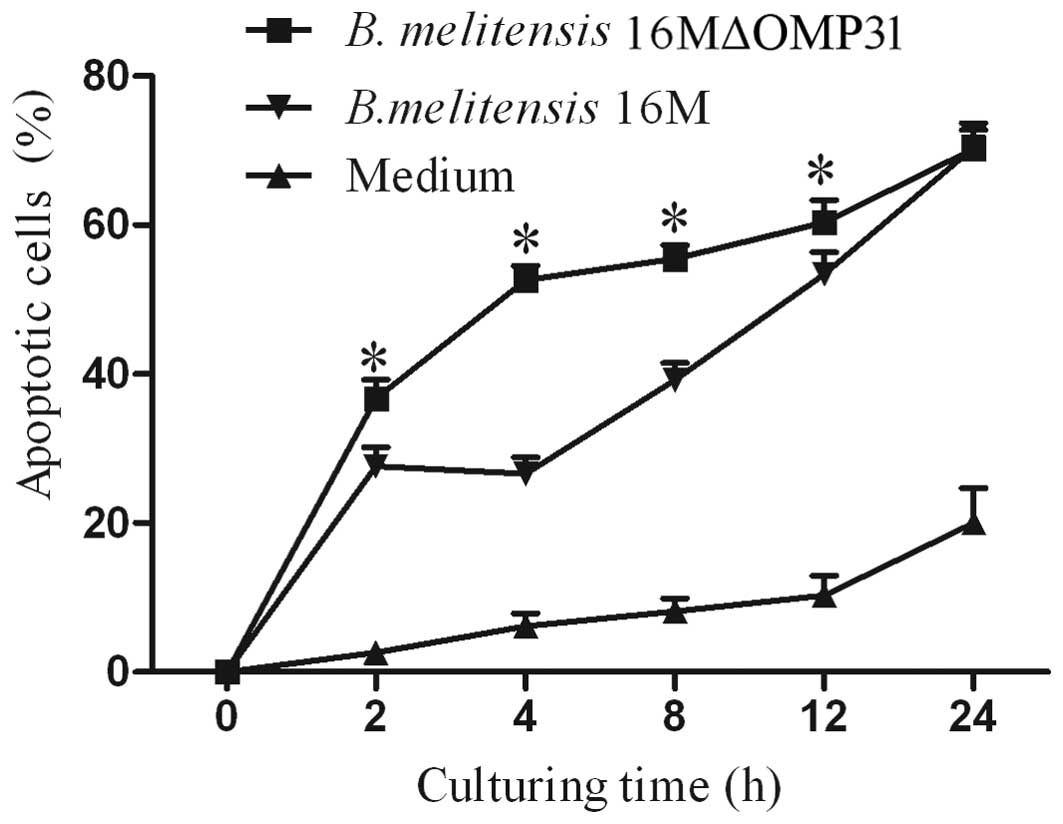
Mutant strain 16MΔOMP31 increases the
apoptosis of RAW264.7 cells compared with the parent strain
Whether the deletion of OMP31, in addition to
impairing the intracellular colony formation of Brucella in
macrophages, also affected the apoptosis level was investigated. To
evaluate the apoptosis of RAW264.7 cells induced by B.
melitensis 16M or the mutant, flow cytometric analysis was
conducted. The results demonstrated that the deletion of
OMP31 increased the proportion of apoptotic RAW264.7 cells
from 2 h post infection (Fig. 1). At
2–24 h post-infection, the mutant strain triggered more apoptosis
cells than the parent strain. However, at 2–4 h post-infection, the
mutant strain promoted RAW264.7 apoptosis and the parent strain
inhibited apoptosis slightly. At 4–24 h post-infection, both the
parent and mutant strains promoted RAW264.7 apoptosis.
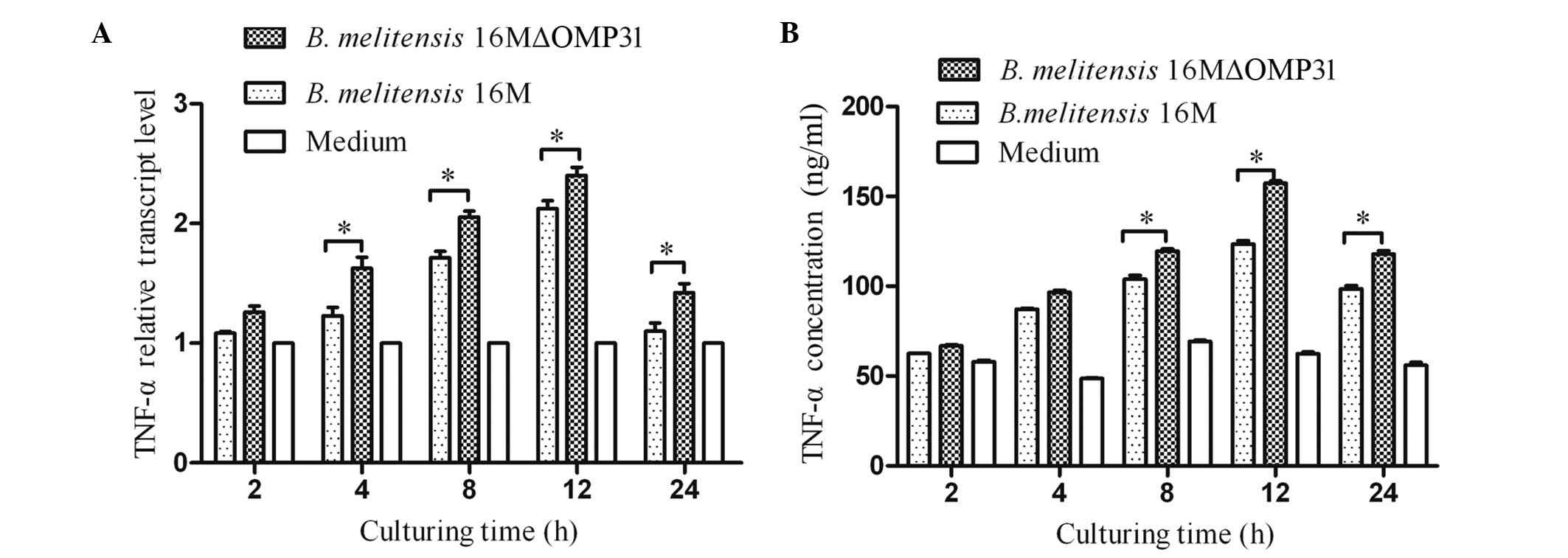
Mutant strain 16MΔOMP31 increases the
TNF-α levels of RAW264.7 cells compared with the parent strain
The cytokine TNF-α plays a key role in immunity and
inflammation by inducing cellular responses such as apoptosis
(23). Therefore, following the
detection of apoptosis, the TNF-α levels (RNA and secretion)
induced by B. melitensis 16M or the mutant strain were
detected. The results showed that the deletion of OMP31
increased the levels of TNF-α for the mutant strain in comparison
with those for B. melitensis 16M from 4 h after infection
for RNA (Fig. 2A), and 8 h post
infection for protein (Fig. 2B). At
2–12 h post-infection, the concentration and transcriptional level
of TNF-α increased rapidly, triggered by both mutant stain and
parent strain, although the mutant stain induced greater TNF-α
expression.
Mutant strain 16MΔOMP31 increases
caspase-3 and −8 levels in RAW264.7 cells compared with the parent
strain
Triggering of the TNF receptor-1 (TNFR-1) by TNF-α
may induce classical apoptosis by activating the initiator protease
caspase-8 in the death receptor pathway (24). TNF-α induced apoptosis is mediated
via the cell surface receptor TNFR-1 and involves the formation of
two signaling complexes that sequentially activate NF-κB and
caspases (25). Other studies have
suggested that TNF-α may trigger apoptosis via an additional
mechanism, involving constituents of acidic vesicles that can
generate ceramides as second messengers such as Bcl-2 and
activation of caspase-3 (26,27).
Therefore, caspase-3 and 8 were detected by ELISA. The levels of
caspase-3 and −8 were increased after 4 h post infection with the
mutant strain 16MΔOMP31 (Fig. 3), to
a greater extent than with the parent strain.
Mutant strain 16MΔOMP31 increases Bax
expression and reduces Bcl-2 levels of RAW264.7 cells compared with
the parent strain
Bax, a member of the Bcl-2 protein family, is
involved in the control of apoptotic events, and may accelerate
apoptosis (28). Bcl-2 prolongs cell
survival following the Bax-induced release of Cyt c
(29). High levels of Bcl-2 inhibit
apoptosis by attenuating the release of Cyt c while high
levels of Bax induce apoptosis by binding to mitochondrial
membranes and increasing the permeability of the membranes,
facilitating the release of Cyt c (30). Cyt c activates caspase
cascades in the cytosol, ultimately leading to cell death (31). To identify the possible pathway
underlying the effects of OMP31, following the increase of TNF-α
expression and apoptosis percentage, the transcriptional levels of
Bcl-2 and Bax were detected. The present results for Bcl-2
(Fig. 4A) showed that the deletion
of OMP31 reduced the bcl-2 level from 8 to 24 h post
infection, although not at 4 h post infection. The results for Bax
(Fig. 4B) revealed that the deletion
of OMP31 raised the Bax level from 2 to 24 h post infection,
but not at 4 h post infection. At 4–24 h post-infection, the mutant
strain and parent strain both impaired Bcl-2 transcription and
induced Bax transcription. Similarly, the mutant strain induced a
greater change than the parent strain.
Mutant strain 16MΔOMP31 increases the
Cyt c level of RAW264.7 cells compared with the parent strain
Cyt c is an essential component of the
mitochondrial respiratory chain. It is a soluble protein, localized
in the intermembrane space, and is loosely attached to the surface
of the inner mitochondrial membrane. Cyt c release from
mitochondria is a key event that has an important role in
initiating the process of apoptosis in mammalian cells (32). To further investigate the pathway
involved in the apoptosis induced by the deletion of OMP31,
Cyt c release from infected cells was detected in the
present study. The results showed that the deletion of OMP31
raised the concentration of Cyt c released after infection
for 12 h (Fig. 4C). At 2–12 h
post-infection, the concentration of Cyt c rapidly
increased, triggered by both the mutant and parent strains.
Mutant strain 16MΔOMP31 increases
caspase-9 levels in RAW264.7 cells compared with the parent
strain
Caspases are a family of proteases with major roles
in apoptosis. They are considered as downstream effectors of
apoptosis, with caspase activation being an irreversible step in
apoptotic signaling. Caspase-3, −6 and −7, known as executioner
caspases, are downstream of the caspase-8 or −9 activation cascade
(33,34). In a previous experiment of the
present study, caspase-3 and −8 levels were found to be raised by
the mutant strain. To further define the pathway induced by the
deletion of OMP31, following the observation of a high level
of Cyt c release, the levels of caspase-9 were detected
(Fig. 4D). The results showed that
caspase-9 levels were increased more than caspase-8 levels at 4 h
post infection with the mutant strain. The mutant strain triggered
more caspase-3 and −9 than the parent strain, and the concentration
of caspase-9 was greater than that of caspase-3.
Discussion
In 1999, Caron et al reported that
Brucella species released a specific, protease-sensitive
inhibitor of TNF-α expression (35).
It has since been reported that OMP25 from B. suis inhibits
the production of TNF-α by human macrophages (14). Furthermore, B. suis-infected
macrophages were shown to secrete significantly less TNF-α than did
macrophages infected with a ΔOMP25 B. suis mutant (14). TNF-α is important for the autocrine
stimulation of macrophage functions, which is important in the
triggering of specific immunity against intracellular pathogens
such as Brucella. TNF-α contributes to resistance to
intracellular Brucella infection, via both innate and
specific immunity (13). In the
present study, the number of CFUs and TNF-α levels in macrophages
infected with mutant and parent strains of B. melitensis
were detected and the results showed that the deletion of
OMP31 increased the secretion of TNF-α and impaired
intracellular colony formation.
TNF-α is able to trigger apoptosis (10). Triggering of TNF receptor-1 (TNFR-1)
by TNF-α induces classical apoptosis by activating the initiator
protease caspase-8 in the death receptor pathway (24). TNF-α-induced apoptosis is mediated
via the cell surface receptor TNFR-1, and involves the formation of
two signaling complexes that sequentially activate NF-κB and
caspases (25). Other studies have
suggested that TNF-α may trigger apoptosis via an additional
mechanism, involving constituents of acidic vesicles that generate
ceramides as second messengers for Bcl-2 and activation of
caspase-3 (26,27). In the present study, the flow
cytometry results indicated that the mutant strain induced higher
levels of apoptosis than did the parent strain. The results of the
colony formation, TNF-α and flow cytometry experiments indicate
that OMP31 contributes to the inhibition of apoptosis triggered by
TNF-α and benefits the survival and replication of B.
melitensis in RAW264.7 cells.
Caspase are cysteine proteases that cleave protein
substrates at the sites of aspartic acid residues, and play a
central role in the regulation and execution of apoptosis (36). To investigate the classical apoptosis
route that is triggered by TNF-α (24), caspase-3 and −8 were detected and the
results indicated that the classic pathway was triggered by TNF-α
and involved OMP31 from Brucella.
Cyt c release from mitochondria has been
observed in cells undergoing apoptosis following induction by
signals including Fas and TNF activation, growth factors and
exposure to chemotherapeutic drugs (37). The overexpression of Bcl-2 or Bcl-xL
blocks the release of Cyt c and inhibits the apoptotic
response (38,39). Bax is a member of the Bcl-2 protein
family, which can accelerate apoptosis (28). Bax mostly exists in the cytosol and
translocates to mitochondria following an apoptotic stimulus
(29). The mitochondrial apoptosis
pathway is regulated by members of the Bcl-2 protein family. Bcl-2
prolongs the survival of cells following the Bax-induced release of
Cyt c (29). Notably, the
present study also detected another pathway, the mitochondrial
pathway (31,40), that is induced by TNF-α and is
influenced by OMP31 from Brucella. All the results obtained
for Cyt c, Bcl-2, Bax and caspase-9 demonstrated that
apoptosis was triggered by TNF-α, and was more dependent upon the
mitochondrial death pathway than the classical apoptosis
pathway.
Notably, the results of the present study suggest
that TNF-α induces caspases directly and also indirectly via
mitochondria, with both pathways contributing to the apoptosis of
RAW264.7 cells following infection by B. melitensis. In all,
the present study indicated that OMP31 promoted apoptosis mediated
by TNF-α-mitochondria-apoptosis pathway, not only
TNF-α-caspase8-caspase3-pathway.
In summary, the cellular death response triggered by
cytotoxic agents depends on the type and dose of chemotherapeutic
stress within the cellular context and may involve classic
apoptosis. In common with most intracellular bacteria,
Brucella uses apoptosis inhibition as a strategy to
replicate and survive in host cells. By constructing an
OMP31 deletion mutant based on B. melitensis and
analyzing the apoptosis induced by mutant and parent, the present
study indicated that OMP31 from B. melitensis contributes to
the inhibition of apoptosis to benefit survival and replication in
RAW264.7 cells. Significantly, the results suggest that the pathway
inhibited by OMP31 was triggered by TNF-α, and TNF-α may trigger
downstream pathways, specifically the classical or mitochondrial
death pathways. Further studies are required to investigate how and
when the downstream pathway is triggered.
Acknowledgements
This study was supported by grants from the National
Science & Technology Pillar Program (grant no. 2013BAI05B05),
the National Nature Science Foundation of China (grant nos.
31060334 and 31260596) and the Key Discipline of Pathogenic Biology
and Scientific Research Start-up Funding (grant no.
PXY-BSQD-2015006), both from Pingdingshan University.
References
|
1
|
Schurig GG, Sriranganathan N and Corbel
MJ: Brucellosis vaccines: Past, present and future. Vet Microbiol.
90:479–496. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boschiroli ML, Foulongne V and O'Callaghan
D: Brucellosis: A worldwide zoonosis. Curr Opin Microbiol. 4:58–64.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Franco MP, Mulder M, Gilman RH and Smits
HL: Human brucellosis. Lancet Infect Dis. 7:775–786. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roop RM II, Gee JM, Robertson GT,
Richardson JM, Ng WL and Winkler ME: Brucella stationary-phase gene
expression and virulence. Annu Rev Microbiol. 57:57–76. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao LY and Kwaik YA: The modulation of
host cell apoptosis by intracellular bacterial pathogens. Trends In
Microbiol. 8:306–313. 2000. View Article : Google Scholar
|
|
6
|
Häcker G and Fischer SF: Bacterial
anti-apoptotic activities. FEMS Microbiol Lett. 21:1–6. 2002.
View Article : Google Scholar
|
|
7
|
Fernandez-Prada CM, Zelazowska EB,
Nikolich M, Hadfield TL, Roop RM II, Robertson GL and Hoover DL:
Interactions between Brucella melitensis and human phagocytes:
Bacterial surface O-polysaccharide inhibits phagocytosis, bacterial
killing and subsequent host cell apoptosis. Infect Immun.
71:2110–2119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gross A, Terraza A, Ouahrani-Bettache S,
Liautard JP and Dornand J: In vitro Brucella suis infection
prevents the programmed cell death of human monocytic cells. Infect
Immun. 68:342–351. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zychlinsky A: Apoptosis in bacterial
pathogenesis. Biochem Soc Trans. 24(Suppl): 591S1996.
|
|
10
|
Aggarwal BB: Signalling pathways of the
TNF superfamily: A double-edged sword. Nat Rev Immunol. 3:745–756.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin M, Wheeler MD, Kono H, Bradford BU,
Gallucci RM, Luster MI and Thurman RG: Essential role of tumor
necrosis factor alpha in alcohol-induced liver injury in mice.
Gastroenterology. 117:942–952. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maeda S, Chang L, Li ZW, Luo JL, Leffert H
and Karin M: IKKbeta is required for prevention of apoptosis
mediated by cell-bound but not by circulating TNFalpha. Immunity.
19:725–737. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dornand J, Gross A, Lafont V, Liautard J,
Oliaro J and Liautard JP: The innate immune response against
Brucella in humans. Vet Microbiol. 90:383–394. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jubier-Maurin V, Boigegrain RA, Cloeckaert
A, Gross A, Alvarez-Martinez MT, Terraza A, Liautard J, Köhler S,
Rouot B, Dornand J and Liautard JP: Major outer membrane protein
Omp25 of Brucella suis is involved in inhibition of tumor necrosis
factor alpha production during infection of human macrophages.
Infect Immun. 69:4823–4830. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vaíno N, Cloeckaert A, Zygmunt MS and
Dubray G: Cloning, nucleotide sequence, and expression of the
Brucella melitensis omp31 gene coding for an immunogenic major
outer membrane protein. Infec Immun. 64:3744–3751. 1996.
|
|
16
|
Ghasemi A, Salari MH, Zarnani AH, Pourmand
MR, Ahmadi H, Mirshafiey A and Jeddi-Tehrani M: Immune reactivity
of Brucella melitensis-vaccinated rabbit serum with recombinant
Omp31 and DnaK proteins. Iranian J Microbiol. 5:19–23. 2013.
|
|
17
|
Cloeckaert A, Debbarh HS, Vizcaíno N,
Saman E, Dubray G and Zygmunt MS: Cloning, nucleotide sequence, and
expression of the Brucella melitensis bp26 gene coding for a
protein immunogenic in infected sheep. FEMS Microbiol Lett.
140:139–144. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pelicic V, Reyrat JM and Gicquel B:
Generation of unmarked directed mutations in mycobacteria, using
sucrose counter-selectable suicide vectors. Mol Microbiol.
20:919–925. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pelicic V, Reyrat JM and Gicquel B: A
general method of in vitro preparation and specific mutagenesis of
DNA fragments: Study of protein and DNA interactions. Nucleic Acids
Res. 16:7351–7367. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Campos E, Cravero SL, Delgui L, Mora I,
Kahn N, Arese AI and Rossetti OL: Brucella abortus INTA2, a novel
strain 19 (Delta)bp26: luc (Delta)bmp18 double mutant lacking drug
resistance markers. Vet Microbiol. 87:1–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alton GG, Jones LM and Pietz DE:
Laboratory techniques in brucellosis. Monogr Ser World Health
Organ. 55:1–163. 1975.PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang P, Qiu W, Dudgeon C, Zambetti GP, Yu
J and Zhang L: PUMA is directly activated by NF-kappaB and
contributes to TNF-alpha-induced apoptosis. Cell Death differ.
16:1192–1202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ashkenazi A: Targeting death and decoy
receptors of the tumour-necrosis factor superfamily. Nat Rev
Cancer. 2:420–430. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Micheau O and Tschopp J: Induction of TNF
receptor I-mediated apoptosis via two sequential signaling
complexes. Cell. 114:181–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dbaibo GS, Perry DK, Gamard CJ, Platt R,
Poirier GG, Obeid LM and Hannun YA: Cytokine response modifier A
(CrmA) inhibits ceramide formation in response to tumor necrosis
factor (TNF)-alpha: CrmA and Bcl-2 target distinct components in
the apoptotic pathway. J Exp Med. 185:481–490. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Monney L, Olivier R, Otter I, Jansen B,
Poirier GG and Borner C: Role of an acidic compartment in
tumor-necrosis-factor-alpha-induced production of ceramide,
activation of caspase-3 and apoptosis. Eur J Biochem. 251:295–303.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wolter KG, Hsu YT, Smith CL, Nechushtan A,
Xi XG and Youle RJ: Movement of Bax from the cytosol to
mitochondria during apoptosis. J Cell Biol. 139:1281–1292. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rossé T, Olivier R, Monney L, Rager M,
Conus S, Fellay I, Jansen B and Borner C: Bcl-2 prolongs cell
survival after Bax-induced release of cytochrome c. Nature.
391:496–499. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parone PA, James D and Martinou JC:
Mitochondria: Regulating the inevitable. Biochimie. 84:105–111.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Antonsson B: Bax and other pro-apoptotic
Bcl-2 family ‘killer-proteins’ and their victim the mitochondrion.
Cell Tissue Research. 306:347–361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu K, Shu D, Song N, Gai Z, Yuan Y, Li J,
Li M, Guo S, Peng J and Hong H: The role of cytochrome c on
apoptosis induced by Anagrapha falcifera multiple nuclear
polyhedrosis virus in insect Spodoptera litura cells. PloS One.
7:e408772012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sadowski-Debbing K, Coy JF, Mier W, Hug H
and Los MJ: Caspases - their role in apoptosis and other
physiological processes as revealed by knock-out studies. Archivum
Immunologiae Et Therapiae Experimentalis. 50:19–34. 2002.PubMed/NCBI
|
|
34
|
Slee EA, Harte MT, Kluck RM, Wolf BB,
Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES
and Green DR: Ordering the cytochrome c-initiated caspase cascade:
hierarchical activation of caspases−2, −3, −6, −7, −8, and −10 in a
caspase−9−dependent manner. J Cell Biol. 144:281–292. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Caron E, Gross A, Liautard JP and Dornand
J: Brucella species release a specific, protease-sensitive,
inhibitor of TNF-alpha expression, active on human macrophage-like
cells. J Immunol. 156:2885–2893. 1996.PubMed/NCBI
|
|
36
|
Cryns V and Yuan J: Proteases to die for.
Genes Dev. 12:1551–1570. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reed JC: Cytochrome c: Can't live with it
- can't live without it. Cell. 91:559–562. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: A
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|