Introduction
It has been well-documented that opioid-induced
hyperalgesia (OIH) is a potential risk factor for the development
of chronic pain following surgery based on the results from basic
and clinical studies (1–4). Many patients undergoing surgery
experience moderate to severe acute postoperative pain possibly
caused by OIH in addition to inflammatory and neuropathic pain
(1). OIH is defined as a state of
nociceptive sensitization characterized by a paradoxical response,
in which a patient receiving opioids to treat pain might have an
increased sensitivity to painful stimuli (2). In a systematic review and
meta-analysis, Fletcher and Martinez concluded that exposure to a
high dose of short-acting remifentanil was associated with the
development of hyperalgesia and led to significantly increased
acute pain after surgery (3).
Although OIH is generally caused by neuroplastic
changes in the peripheral and central nervous system, the precise
molecular mechanism of OIH is not well understood (4). A cellular mechanism involving the rapid
and prolonged upregulation of N-methyl-D-aspartate (NMDA) receptor
function by remifentanil has been reported to contribute to the
clinical development of remifentanil-induced hyperalgesia (5). Modulation of acute OIH has been
reported with NMDA receptor antagonists, α2 agonists and
cyclooxygenase (COX) inhibitors in clinical studies, as previously
reviewed (6). Data from basic
research have shown that dexmedetomidine produces antihyperalgesic
effects by inhibiting the phosphorylation of NMDA receptor subunit
2B (NR2B), and that increased tyrosine phosphorylation of NR2B in
the spinal cord is associated with remifentanil-induced
postoperative hyperalgesia (7).
Another proposed mechanism for the regulation of
NMDA receptor function involves COX inhibitors. COX inhibitors have
been found to antagonize the NMDA receptor, inhibit the synthesis
of prostaglandin (PGs), and thereby reduce the production of
inflammatory mediators and decrease peripheral sensitization
(8). PGs have been shown to
stimulate the release of the excitatory amino acid glutamate in
spinal cord dorsal horns (9) and
promote NMDA receptor activation (10), which is one of the main mechanisms of
remifentanil-induced hyperalgesia (11). In summary, the mechanisms of both
α2 adrenergic receptor agonists and COX inhibitors in
preventing OIH probably involve the regulation of NMDA
receptors.
Thus, the present study examined the hypothesis that
α2 adrenergic receptor agonists and COX inhibitors would
have synergetic effects on preventing the hyperalgesia induced by
high-dose remifentanil in patients. This study aimed to compare the
effect of using dexmedetomidine alone or combined with flurbiprofen
axetil in reducing the hyperalgesic response after high-dose
infusion of remifentanil in patients undergoing
laparoscopic-assisted vaginal hysterectomy (LAVH).
Materials and methods
Study subjects
This study was approved by the Ethics Committee of
Fujian Provincial Hospital (Fujian, China) and registered
(ChiCTR-TRC-14004837) at Chictr.org. After
written informed consent was obtained, 95 adult women aged from 18
to 60 years, with American Society of Anesthesiologists physical
status I or II, who were diagnosed with hysteromyoma and underwent
elective LAVH were enrolled in this study. Patients who met the
inclusion criteria (n=90) were randomly divided into three groups
(each n=30) using a computer-generated random number table. The
exclusion criteria included narcotic analgesic abuse,
opioid-related medication, morbid obesity, contradictory to
patient-controlled intravenous analgesia (PCIA), allergy to
dexmedetomidine or flurbiprofen axetil and significant psychiatric
conditions. A day before surgery, all patients were instructed
about the use of a 100-mm linear visual analog scale (VAS) and
patient-controlled analgesia (PCA) device (Apollo Science
Instrument Co., Ltd., Jiangsu, China). They were instructed to
perform a self-delivered analgesia procedure once they felt pain.
The quantitative sensory testing (QST) procedure using an
Electronic von Frey (EVF) device (IITC Life Science Inc., Woodland
Hills, CA, USA) was carefully explained in detail by an
anesthesiologist who was not aware of the grouping.
Intervention protocols
Patients were sedated with midazolam (2–3 mg) upon
arrival at the operating room and standard monitoring and
bispectral index (BIS) monitoring (BIS Vista; Medtronic,
Minneapolis, MN, USA) were performed. At 15 min prior to anesthesia
induction, the hyperalgesia and dexmedetomidine group (Group HD)
were given a continuous infusion of dexmedetomidine (Hengrui
Pharmaceutical Co., Ltd., Lianyungang, China) as an initial dose of
0.5 µg/kg within 10 min, followed by a continuous infusion of 0.6
µg/kg/h, and the hyperalgesia, dexmedetomidine and flurbiprofen
axetil group (Group HDF) received flurbiprofen axetil (1.5 mg/kg;
Tide Pharmaceutical Co., Ltd., Beijing, China) 10 min before
anesthesia induction in combination with dexmedetomidine infusion.
The remaining patients constituted the hyperalgesia group (Group
H). Anesthesia was induced with an inhalation agent. The anesthetic
circuit of the ventilator was prefilled with 7% sevoflurane
(Maruishi Pharmaceutical Co., Ltd., Chuoku, Japan) in 7 l/min
oxygen for 3 min. Induction was then performed with the vital
capacity rapid inhalation induction technique. Following a deep
exhalation to the residual volume, patients were asked to take a
forced inspiration, to hold it as long as possible and then to
breathe spontaneously or with assisted ventilation. At 2 min after
the beginning of induction, fresh gas flow was reduced to 4 l/min
and sevoflurane was set to a rate of 3%. Remifentanil (1 µg/kg;
Humanwell Pharmaceutical Co., Ltd., Yichang, China) was then
administered over 1 min followed by an infusion of 0.5 µg/kg/min.
When the BIS value was <50, rocuronium (0.9 mg/kg; NV Organon,
Oss, The Netherlands) was administered intravenously to facilitate
intubation. After tracheal intubation, anesthesia was maintained
with intravenous remifentanil infusion at 0.3 µg/kg/min and
sevoflurane inhalation. The concentration of sevoflurane was
adjusted (0.5% stepwise titration) according to the target BIS
between 40 and 60. The continuous infusion of dexmedetomidine in
Groups HD and HDF was terminated at 30 min prior to the end of
surgery, while the remifentanil infusion in all groups was
continued until the final stitch. If hypotension (mean arterial
pressure, <60 mm Hg) or bradycardia (heart rate, <45
beats/min) occurred more than 5 min after fluid resuscitation, the
patient was treated with 10 mg ephedrine or 0.5 mg atropine. For
prophylaxis of postoperative nausea and vomiting (PONV), 4 mg
ondansetron (Tianheng Pharmaceutical Co., Ltd., Ningbo, China) was
given intravenously 15 min before the end of surgery. Upon
completion of surgery, neuromuscular blockade was reversed with 1
mg neostigmine and 0.5 mg atropine intravenously. After the
recovery of adequate spontaneous ventilation and a response to
verbal commands such as eye opening, extubation was performed. A
PCIA pump was applied to all patients when the surgery was
completed. The PCIA pump contained sufentanil (150 µg; Humanwell
Pharmaceutical Co., Ltd.), ondansetron (8 mg) and normal saline in
a total volume of 100 ml, and was set to deliver a 2-ml bolus dose
with a 10-min lockout interval with a background infusion of 1
ml/h. The patients were transferred to the post-anesthesia care
unit, where the VAS pain scores at 30 min and 1 h after surgery
were evaluated and the mechanical pain threshold was recorded at 1
h after surgery. If the patient sought more extensive pain control
or if the VAS score was >6, sufentanil 5 µg was administered as
a rescue dose.
Outcome measurements
Primary outcomes were the mechanical pain threshold
and the area of secondary hyperalgesia at 24 h after surgery. The
mechanical pain threshold was determined using an EVF device (rigid
tips) on the midpoint of the inner forearm, 2 cm above the level of
the umbilicus (preoperative) and 2 cm from the incision site
(around the umbilicus, postoperative) at four points (horizontally
and perpendicularly). The rigid tip of the probe was pressed at a
right angle and force exerted against the testing area within the
patient's tolerance. The probe was removed when the patient
perceived pain, and the compact system automatically stored and
displayed the testing results. The area of secondary hyperalgesia
was tested with the rigid tip around the surgical incision. It was
determined by testing along linear paths, quadrilaterally at a
distance of 5 cm around the incision at 24 h after surgery. Along
linear paths, stimulation attempts started at 5 cm from the
incision site and gradually moved toward the site, moving 0.5 cm
closer each time. Each attempt was applied with increasing force
until a readout value of ~30 g was reached (30 g was defined as the
hyperalgesic pain threshold) and stopped when the patient perceived
pain. The stimulation was stopped 1 cm from the incision if no
change in sensation occurred. The distance from the incision to
where sensations changed was measured, and an average of four
assessments was calculated and used for statistical comparisons.
The hyperalgesic area (A) was calculated using the formula:
A=πr2, where r was the average distance to the site. The
secondary outcomes included time to first analgesic requirement,
postoperative analgesic consumption, VAS pain score, and incidence
of associated side effects including PONV, hypotension, bradycardia
and post-anesthetic shivering. General assessments include
demographic characteristics (age, weight and height), awakening and
extubation time (defined as time from remifentanil discontinuation
to patients' responses to a verbal command and extubation,
respectively), dose of remifentanil, volume of sevoflurane and
duration of anesthesia and surgical procedure.
Statistical analysis
All data were processed using Statistical Package
for Social Sciences (SPSS) software, version 20.0 (IBM SPSS,
Armonk, NY, USA). The normality of distribution for each variable
was verified using the Shapiro-Wilk test. Numeric variables were
described as the mean ± standard deviation or median (interquartile
range, IQR) while categorical variables were expressed as number
(percentage, %). The demographic characteristics, surgery-related
information and time to first analgesic requirement were analyzed
using one-way analysis of variance, with post hoc Bonferroni
corrections. Two-way repeated measures analysis of variance
followed by post hoc Bonferroni corrections was used to
analyze the difference between the treatments at different time
points. For analgesic-related adverse effects, differences between
groups were compared with Chi-square or Fisher's exact test, and
pairwise Kruskal-Wallis H test was used to further analyze the
deviation between groups. P<0.05 was considered to indicate a
statistically significant difference. Power calculation indicated
that to yield a power of 80% with a significance level of 5% based
on the 20% reduction in hyperalgesic area from a preliminary
experiment, there should be ≥26 subjects in each group.
Results
Recruitment and clinical
characteristics of patients in the three groups
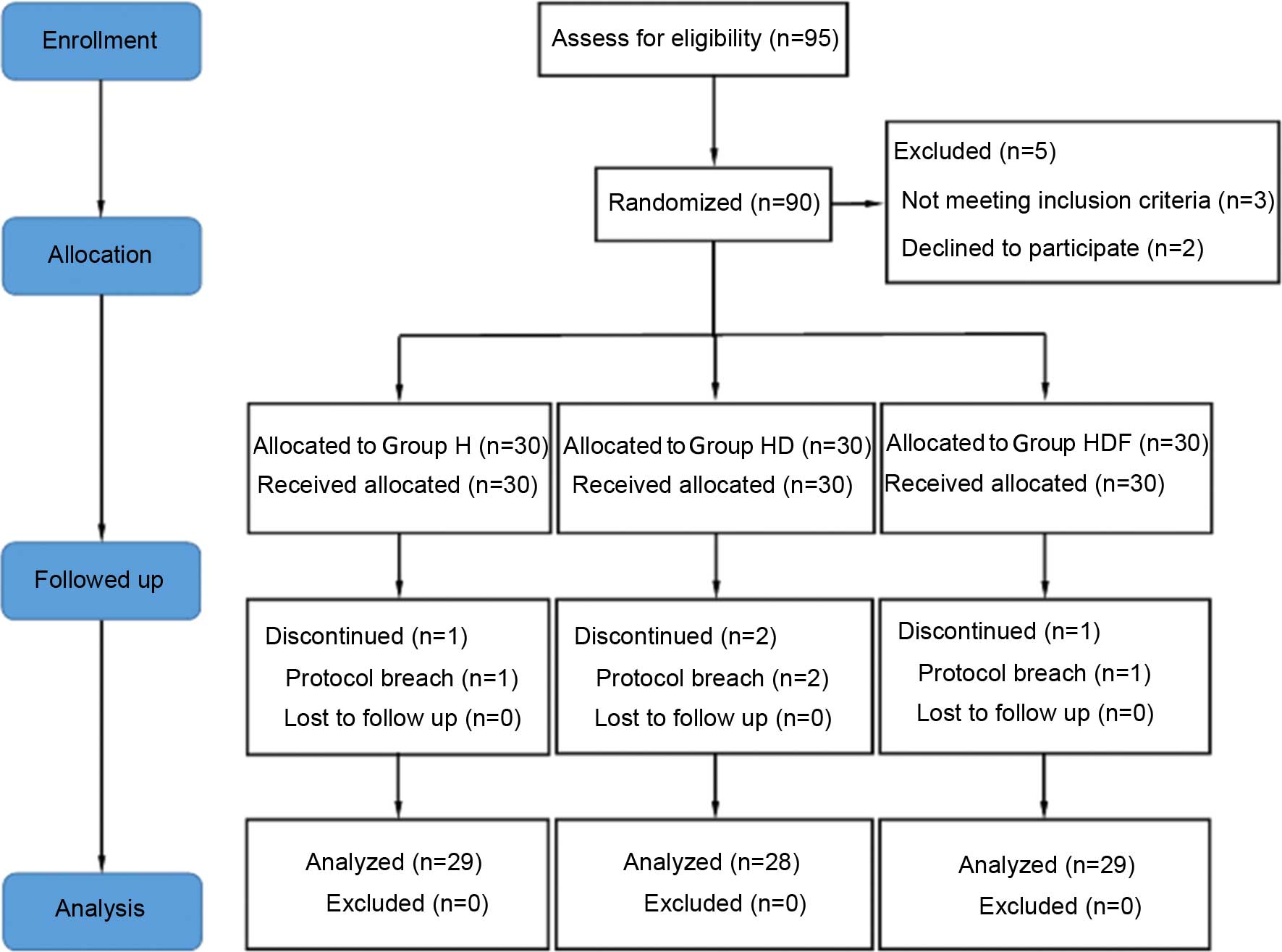
Of the 95 recruited participants, 5 patients were
declined during eligibility assessments for not meeting the
inclusion criteria. A total of 90 patients were considered eligible
and received study medication following randomization. Four of the
90 patients initially enrolled were withdrawn because of conversion
to open surgery. The remaining 86 patients completed the study and
were included in the statistical analysis (Fig. 1). There were no significant
differences in age, weight, height, duration of anesthesia and
surgery, awakening time, extubation time and remifentanil
consumption among the three groups (Table I). The mean volume (%) of sevoflurane
was significantly lower in Group HD and Group HDF compared with
Group H (both P<0.05 vs. Group H; Table I).
 | Table I.Patient demographic characteristics
and surgery-related parameters. |
Table I.
Patient demographic characteristics
and surgery-related parameters.
| Variables | Group H (n=29) | Group HD (n=28) | Group HDF (n=29) |
|---|
| Age (year) |
45.8±3.6 |
45.9±2.9 |
46.7±4.9 |
| Weight (kg) |
58.4±8.7 |
59.8±7.8 |
60.0±6.2 |
| Height (cm) |
159.3±5.0 |
158.4±4.6 |
160.4±3.9 |
| Duration of
anesthesia (min) |
135.6±18.3 |
138.3±20.6 |
129.6±18.9 |
| Duration of surgery
(min) |
117.1±17.0 |
121.1±20.6 |
113.2±16.2 |
| Awakening time
(min) |
13.0±3.6 |
14.6±3.7 |
14.2±3.5 |
| Extubation time
(min) |
13.7±3.5 |
15.1±3.4 |
14.7±3.5 |
| Remifentanil
consumption (µg) |
2,442.1±541.5 |
2,524.9±476.9 |
2,388.2±381.1 |
| Mean volume of
sevoflurane (%) |
1.59±0.17 |
1.20±0.16a |
1.13±0.19a |
Per-incisional mechanical pain
threshold around the umbilicus and postoperative pain score
measured using a VAS
The mechanical pain threshold at the midpoint of the
inner forearm was not significantly different among the three
groups at the preoperative and 1, 6 and 24 h postoperative time
points, respectively (data not shown). Mechanical pain threshold at
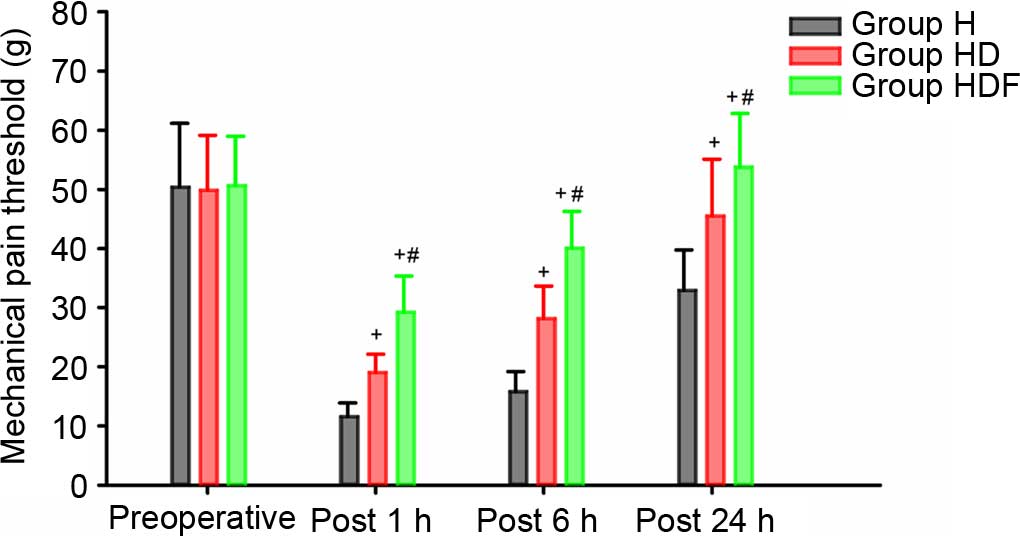
the incision site was not significantly different among the three
groups preoperatively, but was significantly lower at 1, 6 and 24 h
postoperative time points in Group H compared with those in the
other two groups (all P<0.05; Fig.
2). Furthermore, the mechanical pain threshold in Group HDF at
the three post-operative time points were all significantly higher
compared with those of Group HD (all P<0.05; Fig. 2).
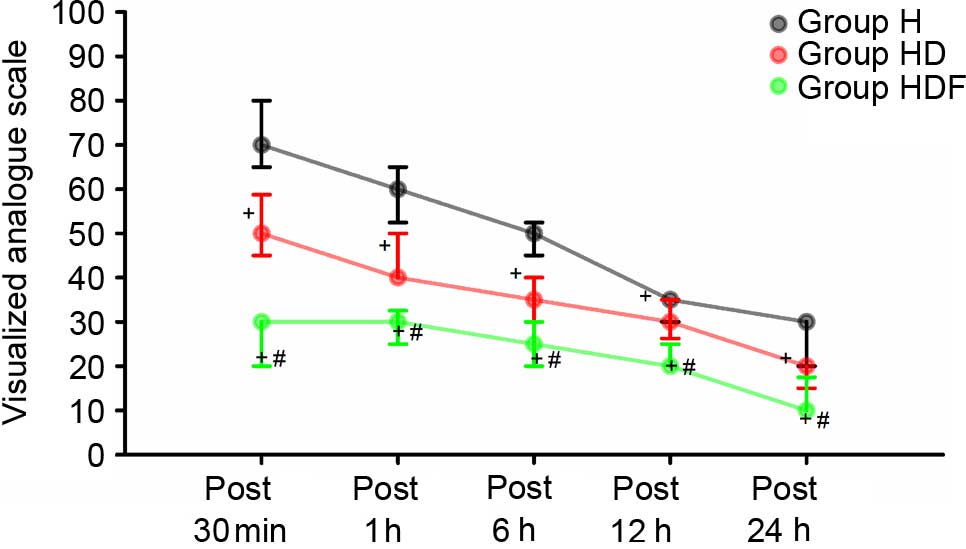
VAS scores at 30 min, and 1, 6, 12 and 24 h were
higher in Group H than in the other two groups (P<0.05; Fig. 3). In addition, VAS scores at above
time points were significantly lower in Group HDF than in the other
two groups (all P<0.05; Fig.
3).
Perioperative anesthetic parameters
and perioperative anesthetic-related adverse events within 24 h
after surgery
The time to first analgesic requirement was
significantly shorter in Group H compared with the other two groups
(both P<0.05), and it was significantly longer in Group HDF
compared with Group HD (P<0.05; Table II). The area of secondary
hyperalgesia around the incision site at 24 h after surgery was
greater in Group H than in the other two groups (both P<0.05),
and it was significantly smaller at 24 h after the surgery in Group
HDF than in Group HD (P<0.05; Table
II). The total sufentanil consumption was significantly higher
in Group H at all time points than those in other two groups (all
P<0.05) and significantly lower in Group HDF at these time
points compared with those of Group HD (all P<0.05; Table II).
 | Table II.First analgesic requirement time,
hyperalgesic area and sufentanil consumption during the
postoperative 24 h. |
Table II.
First analgesic requirement time,
hyperalgesic area and sufentanil consumption during the
postoperative 24 h.
| Variables | Group H (n=29) | Group HD (n=28) | Group HDF (n=29) |
|---|
| First analgesic
requirement (min) | 11.38±3.85 |
22.18±6.46a |
54.14±14.83a,b |
| Hyperalgesic area
(cm2) postoperative 24 h | 12.56
(10.17–19.63) | 9.08
(8.04–11.5)a | 3.14
(3.14–4.52)a,b |
| Sufentanil
consumption (µg) |
|
|
|
| Postoperative 30
min | 11.0
(8.0–11.0) | 8.0
(5.0–8.0)a | 0.0
(0.0–0.0)a,b |
|
Postoperative 1 h | 18.5
(15.5–18.5) | 12.5
(9.5–12.5)a | 1.5
(1.5–4.5)a,b |
|
Postoperative 6 h | 44.0
(41.0–44.0) | 26.0
(26.0–29.0)a | 15.0
(11.3–15.0)a,b |
|
Postoperative 12 h | 65.0
(62.0–68.0) | 38.0
(35.0–38.0)a | 27.0
(24.0–27.0)a,b |
|
Postoperative 24 h | 86.0
(83.0–86.0) | 55.0
(53.0–56.0)a | 45.0
(42.0–45.0)a,b |
The incidence of hypotension and bradycardia was
significantly higher in Group HD when compared with Group H (both
P<0.05). Post-anesthetic shivering was significantly higher in
Group H than in Group HD and Group HDF (both P<0.05). The
incidence of PONV was significantly lower in Group HD than in Group
H (P<0.05; Table III).
 | Table III.Perioperative anesthetic-related
adverse events. |
Table III.
Perioperative anesthetic-related
adverse events.
| Variables | Group H (n=29) | Group HD
(n=28) | Group HDF
(n=29) |
|---|
| Hypotension
perioperatively | 6
(20.7) | 15
(53.6)a | 14 (48.3) |
| Bradycardia
perioperatively | 7
(24.1) | 16
(57.1)a | 15 (51.7) |
| Shivering within 1
h after surgery | 15 (51.7) | 5
(17.9)a | 4
(13.8)a |
| PONV within 24 h
postoperatively | 16 (55.2) | 6
(21.4)a | 7
(24.1) |
Discussion
In the present study, the effects of combined
dexmedetomidine and flurbiprofen axetil treatment on
remifentanil-induced hyperalgesia in patients undergoing LAVH were
investigated. It was found that dexmedetomidine had protective
effects against remifentanil-induced hyperalgesia, and the
combination of dexmedetomidine and flurbiprofen axetil had a
synergetic effect in preventing the hyperalgesia induced by a high
dose of remifentanil in patients undergoing LAVH.
This conclusion was reached on the basis of the
results obtained from the present study that a relatively large
dose of intra-operative remifentanil induced postoperative
hyperalgesia, which is revealed as a reduction of the postoperative
peri-incisional mechanical pain threshold, an extension of
secondary hyperalgesia, enhanced pain intensity, a shorter time to
first postoperative analgesic requirement and high sufentanil
consumption. Compared with patients in group H, the patients in
Group HD had a greater peri-incisional mechanical pain threshold,
reduced area of secondary hyperalgesia, and lower VAS scores and
sufentanil consumption. Furthermore, the mechanical pain threshold
was much higher and the area of secondary hyperalgesia was much
smaller in Group HDF compared with Group HD. In addition, VAS
scores were significantly lower and sufentanil consumption was
significantly less in Group HDF compared with Group HD.
Dexmedetomidine is a highly selective α2
adrenergic receptor agonist, acting on the central and peripheral
nervous system, which possesses hypnotic, sedative, anxiolytic,
sympatholytic, analgesic and antihyperalgesic characteristics
(12). Studies have shown that
dexmedetomidine produces an antihyperalgesic effect by suppressing
the phosphorylation of the NR2B subunit of the NMDA receptor
(7) and increased tyrosine
phosphorylation of NR2B in the spinal cord is associated with
remifentanil-induced postoperative hyperalgesia (13). Dexmedetomidine also selectively
inhibits excitatory postsynaptic potential via NMDA receptors, and
inhibits synaptic transmission mediated by primary afferent A and C
fibers to produce an antinociceptive effect (14). Moreover, dexmedetomidine has been
shown to decrease hyperalgesia in neuropathic pain by increasing
acetylcholine levels in the spinal cord (15) and suppressing glial cell
proliferation of the spinal cord (16), which has been considered to amplify
pain signals and activate the adjacent nerve cells involved in
peripheral and central sensitization (17). A clinical study conducted by Lee
et al (18) showed that
dexmedetomidine administration reduced the hyperalgesia induced by
high doses of remifentanil, and the findings of the present study
are consistent with this. Dexmedetomidine was given at an initial
dose of 0.5 µg/kg within 10 min prior to the induction of
anesthesia, followed by a continuous infusion of 0.6 µg/kg/h in the
current study, instead of an initial dose of 1 µg/kg within 10 min,
followed by a continuous infusion of 0.7 µg/kg/h, which was a much
higher dose. Furthermore, in the present study, sevoflurane was
used as an induction agent instead of propofol, which has been
reported to reduce OIH (19). Using
the aforementioned optimized protocol, it was found that the
relatively low dose of dexmedetomidine used in the present study
was effective in reducing remifentanil-induced hyperalgesia, while
a lower dose may reduce the incidence of the side effects such as
bradycardia and hypotension. Therefore, a low dosage of
dexmedetomidine could be a potential choice for physicians to use
in their clinical practice to reduce the side effects and increase
the tolerance of patients.
There have been many proposed mechanisms for OIH.
Each type of inhibitor blocks only one of the several mechanisms
involved in OIH and single drug administration may not be
sufficient to effectively prevent hyperalgesia development. Based
on this rational, a combination of dexmedetomidine and flurbiprofen
axetil treatment was used in patients undergoing surgery in the
present study, and it was found that the combination treatment had
a synergetic effect in the prevention of remifentanil-induced
hyperalgesia, supporting this hypothesis. Flurbiprofen axetil is a
non-selective COX inhibitor carried in lipid microspheres, which
has analgesic and anti-hyperalgesic effects through blocking spinal
PG synthesis. Flurbiprofen axetil formulated in emulsified lipid
microspheres has demonstrated a high affinity for inflammatory
tissues to achieve targeted drug therapy and prolonged duration of
action (20). PGs directly sensitize
the spinal nociceptive system by depolarizing deep spinal cord
dorsal horn neurons (21). Excessive
release of excitatory amino acids has been reported to be
associated with opioid-induced hyperalgesia (22) and PGs are able to stimulate the
release of excitatory amino acid glutamate in spinal cord dorsal
horns. Moreover, activation of NMDA receptors has found to be
associated with the elevated COX-2 expression in the spinal dorsal
horn upon the initiation of nociceptive stimulation (23). Therefore, inhibition of PG production
would be expected to block opioid-induced hyperalgesia. Based on
these previous findings, it may be proposed that flurbiprofen
axetil exerted its relieving effects on remifentanil-induced
postoperative hyperalgesia by reducing the synthesis and release of
PG and inhibiting the activation of NMDA receptors by excitatory
amino acids. A crossover study of healthy subjects suggested that
both COX-1 and COX-2 inhibitors, but particularly COX-2 inhibitor,
can relieve pinprick hyperalgesia (24). COX inhibitors are able to attenuate
opioid-induced tolerance, hyperalgesia and dependence (25–27). To
the best of our knowledge, the present study is the first to report
that there was a notably decreased hyperalgesic area, a predicting
factor for the development of chronic postsurgical pain (28), in hyperalgesic patients treated with
dexmedetomidine and flurbiprofen axetil. Therefore, this study has
not only demonstrated for the first time the effect of flurbiprofen
axetil in decreasing the hyperalgesic area, but has also suggested
a possible pathway through which hyperalgesia could be
prevented.
The QST protocol was established by the German
Network on Neuropathic Pain to investigate the somatosensory
thresholds in healthy subjects and in patients with neuropathic
pain (29). However, most studies
have used indirect evidence, such as greater postoperative pain and
analgesic consumption for evaluating OIH, which might be less
sensitive and objective than QST. Previous studies of hyperalgesia
have used QST performed with traditional von Frey Monofilaments
(VFMs) (11,18,30). In
the present study, an EVF device was used for quantitative sensory
testing to explore mechanical hyperalgesia, which is more reliable
and rapid than traditional VFM in exploring mechanical pain
thresholds (31). Using this
methodology, it was found that the incidence of hypotension and
bradycardia in Group HD and Group HDF was higher than that in Group
H. Therefore, dexmedetomidine combined with a high dose of
remifentanil should be used with caution. Furthermore, it is
important to determine an optimized infusion rate of
dexmedetomidine that could maximize the anesthetic and
analgesic-sparing effect, while minimizing the incidence of adverse
cardiovascular side effects.
Dexmedetomidine also showed a significant antiemetic
effect, which could be explained by the direct antiemetic effect of
α2 adrenergic receptor agonists due to a decreased level
of catecholamine and/or its opioid-sparing effect. In this study,
it was found that post-anesthetic shivering was associated with
high-dose remifentanil and could be prevented by dexmedetomidine.
The anti-shivering action of dexmedetomidine results from reduced
vasoconstriction and an increase in the shivering threshold
(32). A previous study has
demonstrated that parecoxib administered 30 min prior to the
induction of anesthesia effectively reduced the incidence and
severity of remifentanil-induced shivering (33). However, whether flurbiprofen axetil
has a protective effect against post-anesthetic shivering requires
further investigation.
There were several limitations in this study. First,
the effect of flurbiprofen axetil alone on the prevention of OIH
requires further investigation. Secondly, the usage of a background
infusion might decrease the sensitivity to sufentanil consumed
during the observation period. However, since the dosage of
sufentanil was only 1.5 µg/h, the impact should be minimal.
Finally, it would also be interesting to study the synergetic
effect of combined dexmedetomidine and flurbiprofen axetil
treatment in patients receiving major open surgery.
In summary, high doses of intra-operative
remifentanil are associated with postoperative hyperalgesia, which
is efficiently alleviated or even prevented by dexmedetomidine or
co-administration of flurbiprofen axetil. This synergetic effect
could also help to reduce the usage of remifentanil during surgery
to further prevent its excessive use resulting in OIH. Flurbiprofen
axetil (COX inhibitor) decreased the secondary hyperalgesia that
may contribute to the development of chronic pain. Therefore, the
use of a combination of dexmedetomidine and COX inhibitor as an
adjunctive agent to propofol-remifentanil-based general anesthesia
might be a promising method for preventing or attenuating OIH.
Acknowledgements
The authors would like to thank the staff from the
department of Gynecology in Fujian Provincial Hospital for their
support and cooperation.
References
|
1
|
Wu CL and Raja SN: Treatment of acute
postoperative pain. Lancet. 377:2215–2225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Angst MS and Clark JD: Opioid-induced
hyperalgesia: A qualitative systematic review. Anesthesiology.
104:570–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fletcher D and Martinez V: Opioid-induced
hyperalgesia in patients after surgery: A systematic review and a
meta-analysis. Br J Anaesth. 112:991–1004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee M, Silverman SM, Hansen H, Patel VB
and Manchikanti L: A comprehensive review of opioid-induced
hyperalgesia. Pain Physician. 14:145–161. 2011.PubMed/NCBI
|
|
5
|
Zhao M and Joo DT: Enhancement of spinal
N-methyl-D-aspartate receptor function by remifentanil action at
delta-opioid receptors as a mechanism for acute opioid-induced
hyperalgesia or tolerance. Anesthesiology. 109:308–317. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chu LF, Angst MS and Clark D:
Opioid-induced hyperalgesia in humans: Molecular mechanisms and
clinical considerations. Clin J Pain. 24:479–496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng Y, Cui S, Liu Y, Zhang J, Zhang W,
Zhang J, Gu X and Ma Z: Dexmedetomidine prevents
remifentanil-induced postoperative hyperalgesia and decreases
spinal tyrosine phosphorylation of N-methyl-d-aspartate receptor 2B
subunit. Brain Res Bull. 87:427–431. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tröster A, Sittl R, Singler B, Schmelz M,
Schüttler J and Koppert W: Modulation of remifentanil-induced
analgesia and postinfusion hyperalgesia by parecoxib in humans.
Anesthesiology. 105:1016–1023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Rielly DD and Loomis CW: Increased
expression of cyclooxygenase and nitric oxide isoforms, and
exaggerated sensitivity to prostaglandin E2, in the rat lumbar
spinal cord 3 days after L5-L6 spinal nerve ligation.
Anesthesiology. 104:328–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmadi S, Muth-Selbach U, Lauterbach A,
Lipfert P, Neuhuber WL and Zeilhofer HU: Facilitation of spinal
NMDA receptor currents by spillover of synaptically released
glycine. Science. 300:2094–2097. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joly V, Richebe P, Guignard B, Fletcher D,
Maurette P, Sessler DI and Chauvin M: Remifentanil-induced
postoperative hyperalgesia and its prevention with small-dose
ketamine. Anesthesiology. 103:147–155. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blaudszun G, Lysakowski C, Elia N and
Tramèr MR: Effect of perioperative systemic α2 agonists on
postoperative morphine consumption and pain intensity: Systematic
review and meta-analysis of randomized controlled trials.
Anesthesiology. 116:1312–1322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu X, Wu X, Liu Y, Cui S and Ma Z:
Tyrosine phosphorylation of the N-Methyl-D-Aspartate receptor 2B
subunit in spinal cord contributes to remifentanil-induced
postoperative hyperalgesia: The preventive effect of ketamine. Mol
Pain. 5:762009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Faber ES, Chambers JP and Evans RH:
Depression of NMDA receptor-mediated synaptic transmission by four
alpha2 adrenoceptor agonists on the in vitro rat spinal cord
preparation. Br J Pharmacol. 124:507–512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kimura M, Saito S and Obata H:
Dexmedetomidine decreases hyperalgesia in neuropathic pain by
increasing acetylcholine in the spinal cord. Neurosci Lett.
529:70–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu B, Zhang WS, Yang JL, Lû N, Deng XM, Xu
H and Zhang YQ: Evidence for suppression of spinal glial activation
by dexmedetomidine in a rat model of monoarthritis. Clin Exp
Pharmacol Physiol. 37:e158–e166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu L, Ji F, Liang J, He H, Fu Y and Cao
M: Inhibition by dexmedetomidine of the activation of spinal dorsal
horn glias and the intracellular ERK signaling pathway induced by
nerve injury. Brain Res. 1427:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee C, Kim YD and Kim JN: Antihyperalgesic
effects of dexmedetomidine on high-dose remifentanil-induced
hyperalgesia. Korean J Anesthesiol. 64:301–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singler B, Tröster A, Manering N,
Schüttler J and Koppert W: Modulation of remifentanil induced
postinfusion hyperalgesia by propofol. Anesth Analg. 104:1397–1403.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohmukai O: Lipo-NSAID preparation. Adv
Drug Deliv Rev. 20:203–207. 1996. View Article : Google Scholar
|
|
21
|
Baba H, Kohno T, Moore KA and Woolf CJ:
Direct activation of rat spinal dorsal horn neurons by
prostaglandin E2. J Neurosci. 21:1750–1756. 2001.PubMed/NCBI
|
|
22
|
Wen ZH, Chang YC, Cherng CH, Wang JJ, Tao
PL and Wong CS: Increasing of intrathecal CST excitatory amino
acids concentration following morphine challenge in
morphine-tolerant rats. Brain Res. 995:253–259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li SQ, Xing YL, Chen WN, Yue SL, Li L and
Li WB: Activation of NMDA receptor is associated with up-regulation
of COX-2 expression in the spinal dorsal horn during nociceptive
inputs in rats. Neurochem Res. 34:1451–1463. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lenz H, Raeder J, Draegni T, Heyerdahl F,
Schmelz M and Stubhaug A: Effects of COX inhibition on experimental
pain and hyperalgesia during and after remifentanil infusion in
humans. Pain. 152:1289–1297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akbari E: The role of cyclo-oxygenase
inhibitors in attenuating opioid-induced tolerance, hyperalgesia,
and dependence. Med Hypotheses. 78:102–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koppert W, Wehrfritz A, Körber N, Sittl R,
Albrecht S, Schüttler J and Schmelz M: The cyclooxygenase isozyme
inhibitors parecoxib and paracetamol reduce central hyperalgesia in
humans. Pain. 108:148–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tröster A, Sittl R, Singler B, Schmelz M,
Schüttler J and Koppert W: Modulation of remifentanil-induced
analgesia and postinfusion hyperalgesia by parecoxib in humans.
Anesthesiology. 105:1016–1023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hinrichs-Rocker A, Schulz K, Järvinen I,
Lefering R, Simanski C and Neugebauer EA: Psychosocial predictors
and correlates for chronic post-surgical pain (CPSP)-a systematic
review. Eur J Pain. 13:719–730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rolke R, Baron R, Maier C, Tölle TR,
Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür IC,
et al: Quantitative sensory testing in the German Research Network
on Neuropathic Pain (DFNS): Standardized protocol and reference
values. Pain. 123:231–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee C, Lee HW and Kim JN: Effect of oral
pregabalin on opioid-induced hyperalgesia in patients undergoing
laparo-endoscopic single-site urologic surgery. Korean J
Anesthesiol. 64:19–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tena B, Escobar B, Arguis MJ, Cantero C,
Rios J and Gomar C: Reproducibility of Electronic Von Frey and Von
Frey monofilaments testing. Clin J Pain. 28:318–323. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bajwa SJ, Gupta S, Kaur J, Singh A and
Parmar S: Reduction in the incidence of shivering with
perioperative dexmedetomidine: A randomized prospective study. J
Anaesthesiol Clin Pharmacol. 28:86–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elmawgood AA, Rashwan S and Rashwan D:
Effect of parecoxib on remifentanil induced postoperative
shivering. Egypt J Anaesthesiol. 30:399–403. 2014. View Article : Google Scholar
|