Introduction
Osteanagenesis, which is also known as bone tissue
engineering, is a novel and multidisciplinary field (1). By employing the fundamental principles
of biology, medical science and tissue engineering, it is possible
to remodel injured bone tissue or cure bone diseases (2). Resulting from trauma or physiological
and pathological bone resorption, bone defects are a global heath
issue, the treatment of which can be challenging. Among
craniofacial and plastic surgeries, bone defects caused by trauma
are a common clinical problem, and osteanagenesis may be beneficial
in repairing injuries and improving the quality of life of patients
(3). Osteanagenesis is also used to
treat and repair bone mass in various bone diseases caused by
gender, age and infection, including osteoporosis, osteopenia and
tooth loss as a result of periodontitis (4).
Bone formation is a lengthy and strictly regulated
process associated with embryonic development, reconstruction of
bone tissue and bone fracture repair (5). Classical bone biology theories claim
that mature osteoblasts are formed from BMSCs. During bone
formation, bone precursor cells are differentiated into mature
osteoblasts that can compound and secrete bone matrix (6), and are subsequently mineralized and
imbedded into bone matrix. During the embryonic development
process, BMSCs participate in bone formation through membranous
ossification and entochondrostosis (7). Membranous ossification predominantly
occurs in craniofacial bones, parts of the cartilage and mandibles.
During the developmental process of these tissues, BMSCs are able
to directly differentiate into osteoblasts, whereas
entochondrostosis is employed in the development process of torso
and limb bones (8). Initially,
through accumulation, proliferation and differentiation into
chondrocytes, BMSCs begin to form bones (9). Cartilage cells are then gradually
divided into hypertrophic chondrocytes. With the mineralization of
deep stromas and in-growth of blood vessels, chondrocytes die and
are replaced by osteoblasts, and mature bones are formed.
As a bioactivator, soy isoflavone is a type of
flavonoid compound and a secondary metabolite formed during the
growth of soybeans (10). Although
it is extracted from plants, it has the same structure as estrogen;
therefore, it is also known as phytoestrogen. Soy isoflavone can
occur in various types, including daidzin, daidzein, genistin,
genistein, glycitin and glycitein (11). Glycitin is antibacterial, antiviral
and estrogenic (10), and has been
demonstrated to have preventative effects on alcoholism,
cardiovascular and cerebrovascular diseases and some types of
cancer (12,13). It can also reduce the number of
tumors and alleviate or avoid macteric syndrome caused by a
decrease of estrogen (14), and has
demonstrated anti-aging effects (15). The aim of the present study was to
investigate whether glycitin regulates osteoblast formation from
BMSCs through TGF-β or AKT signaling pathways.
Materials and methods
Isolation and culture of primary
BMSCs
A total of 24 healthy male New Zealand white rabbits
(age, 6 months; weight, 1–1.5 kg) were purchased from the
Experimental Animal Center of Jilin City (Jilin, China). Rabbits
were housed at 22–24°C (relative humidity, 55–70%) with natural
light and air circulation, and were allowed free access to food and
water. All animal procedures were conducted in strict accordance
with the Animal Ethical Standard, and the present study was
approved by the Experimental Animal Center of Beihua University,
Jilin Province Ethics Committee (Jilin, China).
BMSCs were isolated from New Zealand white rabbits
using the method described previously by Li et al (14). Initially, the femurs and tibias were
removed, and flushed bone marrow cells were acquired via Percoll
density gradient centrifugation (1.073 g/ml). Flushed bone marrow
cells were washed with phosphate-buffered saline (PBS) and seeded
into 25-cm2 cell culture flasks. Flushed bone marrow
cells were incubated with L-Dulbecco's modified Eagle medium (DMEM)
supplemented with 10% fetal bovine serum and 1%
penicillin-streptomycin at 37°C in an atmosphere containing 5%
CO2 for 48 h, and subsequently incubated with DMEM for
48 h. Cells were detached using 0.25% trypsin and 0.02% EDTA (Merck
Millipore, Darmstadt, Germany) and centrifuged at 2,000 × g
for 5 min. Suspended cells were gathered, seeded on 6-well plates
at 1.5–2×106 cells/well and incubated for two days.
Authenticating BMSCs
BMSCs were fixed using 5% precooled paraformaldehyde
for 30 min at 4°C and incubated with hematoxylin (Merck Millipore)
for 10 min. BMSCs were washed with water for 10 min, and 95% ethyl
alcohol and xylene were used to dehydrate and clear BMSCs,
respectively. BMSCs were observed using light microscopy (D5300;
Nikon Corp., Tokyo, Japan).
Assessment of primary BMSCs
growth
BMSCs (1–2×106 cells or
1–2×104 per well) were cultured in 6- or 96-well culture
plates overnight at 37°C in an atmosphere containing 5%
CO2. Glycitin (Merck Millipore) was added to the wells
at final concentrations of 0.01, 0.5, 1, 5 and 10 µM and cultured
for 7 days.
In cells cultured in 6-well culture plates, BMSCs
were determined using Oil Red O staining and observed via light
microscopy at 510 nm. BMSCs were fixed using 5% precooled
paraformaldehyde for 30 min at 4°C and stained with 0.6% (w/v) Oil
Red O solution for 15 min at room temperature. Cells stained with
Oil Red O were washed with water (3×5 min) to remove unbound dye,
and culture dishes were stained with 1 ml isopropyl alcohol for 10
min.
In cells cultured in 96-well culture plates, BMSCs
were determined via MTT assay. A total of 20 µl MTT (5 g/l) were
added to each well and cultured for 4 h. The supernatant was
removed and 200 µl dimethylsulfoxide were added to each well for 15
min. Optical density (OD) was measured using a microplate
spectrophotometer (model 680; Bio-Rad Laboratories, Inc., Hercules,
CA, USA) at 570 nm. Proliferation rate was calculated using: OD
treated / OD control × 100%.
Measurement of collagen type 1 (Col I)
and alkaline phosphatase (ALP) using reverse-transcription
polymerase chain reaction (RT-PCR)
Total RNA was extracted from BMSCs treated with
glycitin (0, 0.5, 1 and 5 µM) using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Total RNA (1–2
µg) was used to transcribe cDNA using a SYBR PrimeScript RT-PCR kit
(Takara Bio, Inc., Otsu, Japan), according to the manufacturer's
protocol PCR was performed on an ABI 7500 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). PCR thermal
cycling was performed as follows: Amplification at 94°C for 1 min,
followed by 40 cycles of amplification at 94°C for 30 sec,
annealing at 58°C for 45 sec, and extension at 72°C for 30 sec.
Primers were designed as follows: Col I, forward
5′-TGACCTCAAGATGTGCCACT-3′ and reverse 5′-GGGAGTTTCCATGAAGCCAC-3′;
and β-actin forward 5′-CGTGCGGGACATCAAGGA-3′ and reverse
5′-AGGAAGGAGGGCTGGAACA-3′. Subsequently, 7500 Fast Real-Time PCR
system software was used to analyze crossing threshold (Cq) values
using the second derivative maximum method (16).
Measurement of ALP activity
BMSCs (1–2×106 cells) were cultured in
6-well plates overnight at 37°C in an atmosphere containing 5%
CO2. Glycitin was added to the wells at final
concentrations of 0, 0.5, 1 and 5 µM and cultured for 7 days. Cells
were washed with ice-cold PBS and lysed via the repeated
freeze-thaw method. Supernatant was analyzed using an ALP kit
(Sangon Biotech Co., Ltd., Shanghai, China). ALP activity was
calculated according to the formula: Treated group / control ×
100%.
Western blotting for TGF-β and
phosphorylated AKT (p-AKT)
Proteins were extracted from BMSCs treated with
glycitin (0, 0.5, 1 and 5 µM) by grinding with protease inhibitors.
BMSCs were lysed using RIPA lysis buffer and protein content was
measured using a micro-Bradford assay kit (Sangon Biotech Co.,
Ltd.). Protein samples (50–100 µg) were separated using 10–12%
SDS-PAGE and transferred onto a polyvinylidene fluoride membrane.
Membranes were incubated with 5% non-fat milk for 2 h at room
temperature, and were subsequently incubated with antibodies
against TGF-β (1:500; sc-146), p-AKT (1:500; sc-135,650) and
β-actin (1:1,000; sc-130,656; all Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) at 4°C for 24 h. Membranes were washed three times
with Tris-buffered saline with Tween-20 and subsequently incubated
with an anti-rabbit secondary antibody (1:5,000; C2247; Applygen
Technologies Inc., Beijing, China) at 37°C for 30 min at room
temperature and were visualized with enhanced chemiluminescent
reagent (ECL Plus; P0018A; Beyotime Institute of Biotechnology,
Haimen, China). Proteins were quantified using Image Lab 4.1
(Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation
using SPSS software (version 18.0; SPSS, Inc., Chicago, IL, USA).
Statistical differences were analyzed using Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Authentication of BMSCs
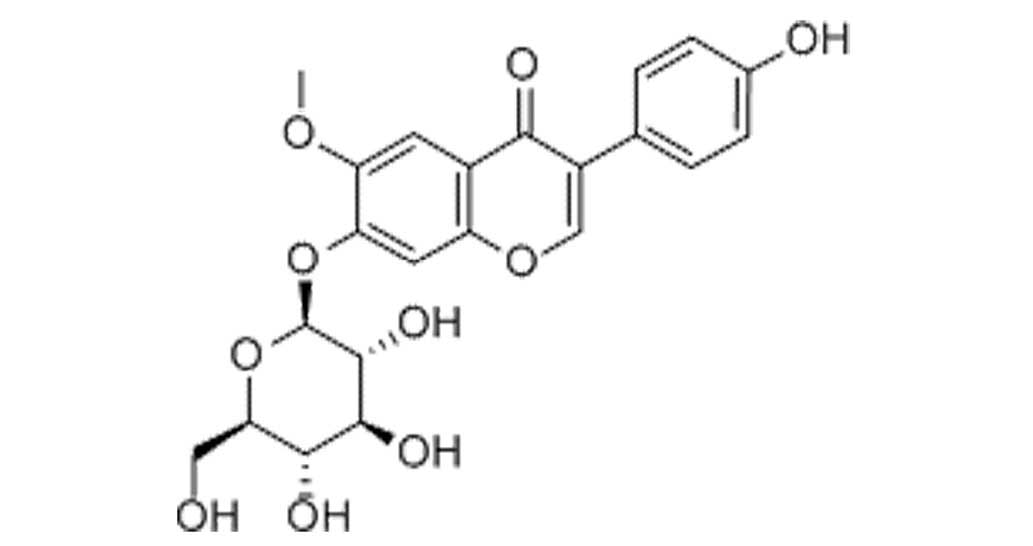
The constitutional formula of glycitin is displayed
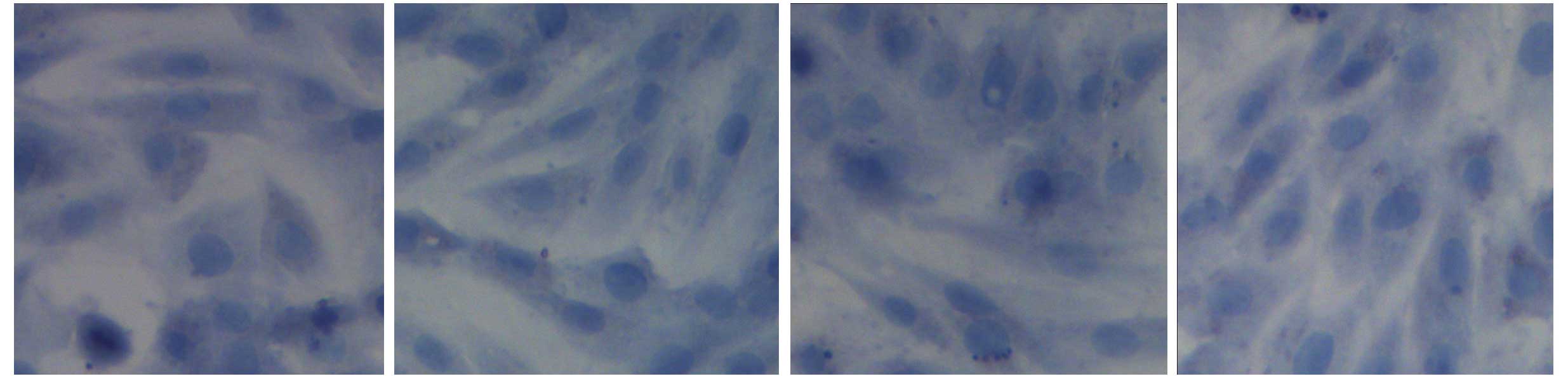
in Fig. 1. BMSCs appeared
homogeneously mazarine blue, which demonstrated that BMSCs were
successful extracted, indicating that BMSCs were successfully
separated and cultivated (Fig.
2).
Glycitin promotes BMSC
proliferation
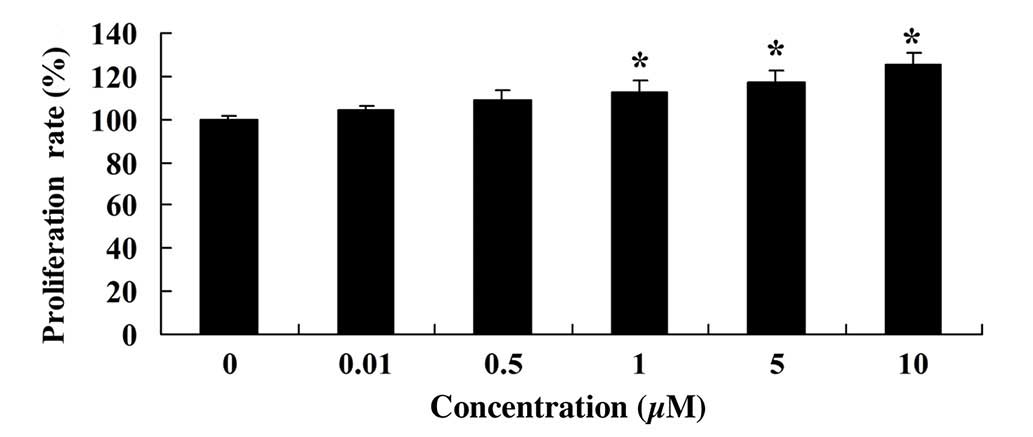
MTT assay was used to measure the effect of glycitin
on the proliferation of BMSCs. As shown in Fig. 3, glycitin increased the proliferation
of BMSCs, with statistical significance detected after treatment
with 1 and 5 µM glycitin (P=0.0023 and P=0.0004, respectively).
Glycitin increases adipogenic
differentiation of BMSCs
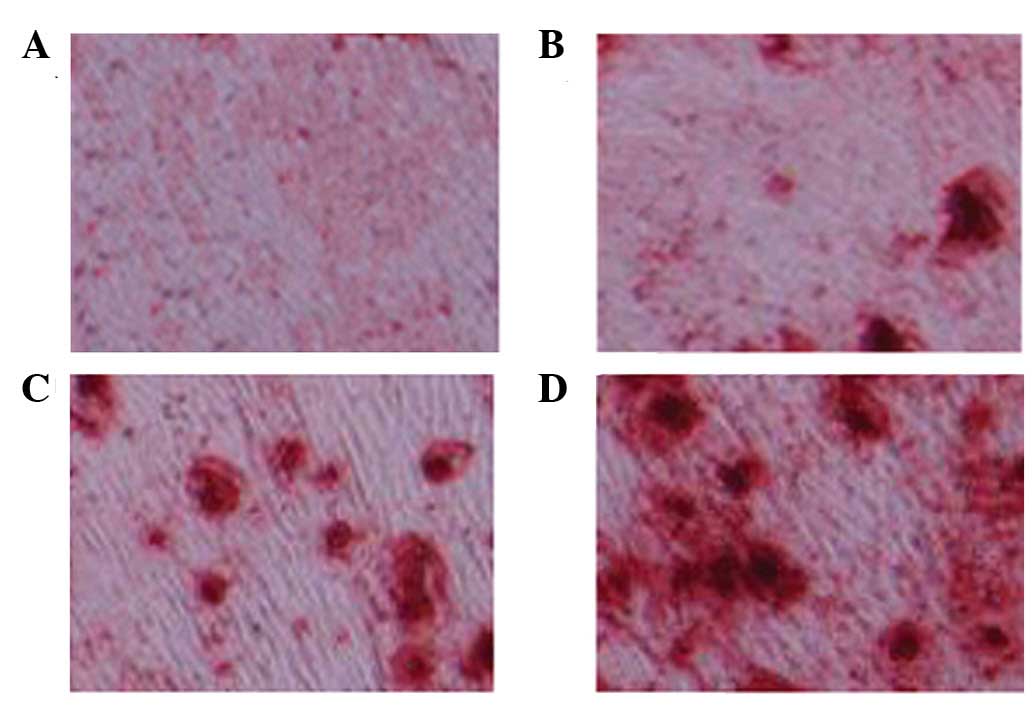
As shown in Fig. 4,
Oil Red O staining was observed in every group. A marked increase
in red staining, and therefore osteoblasts, was detected following
treatment with 1 and 5 µM glycitin.
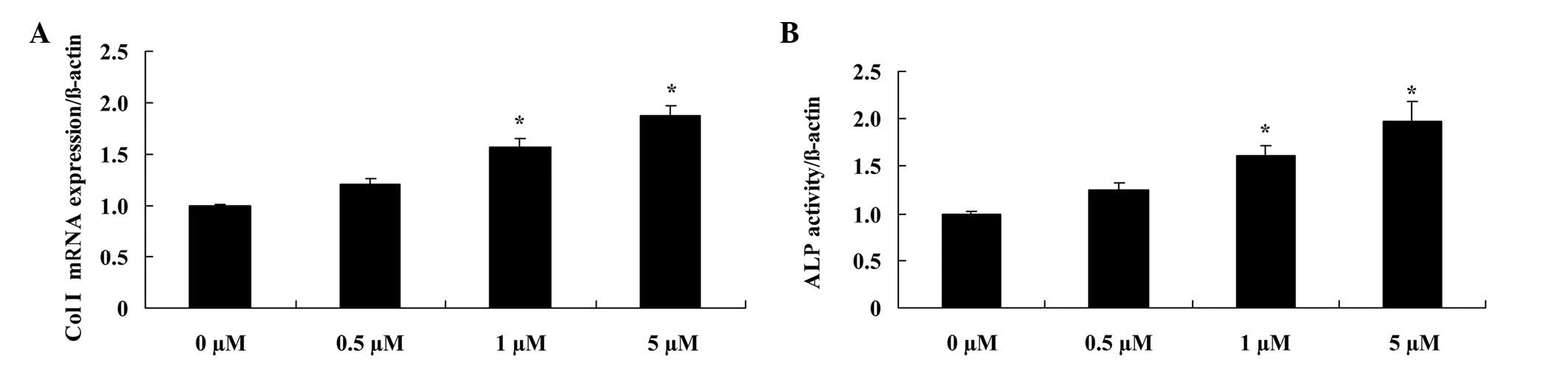
Glycitin increases Col I mRNA
expression and ALP activity in BMSCs
In order to elucidate the effect of glycitin on the
mRNA expression levels and activity of Col I and ALP, respectively,
RT-PCR and ALP kits were used. The results demonstrated that
administration of 1 and 5 µM glycitin significantly promoted Col I
mRNA expression (P=0.0079 and P=0.0031, respectively) and ALP
activity in BMSCs (P=0.0049 and P=0.0023, respectively; Fig. 5).
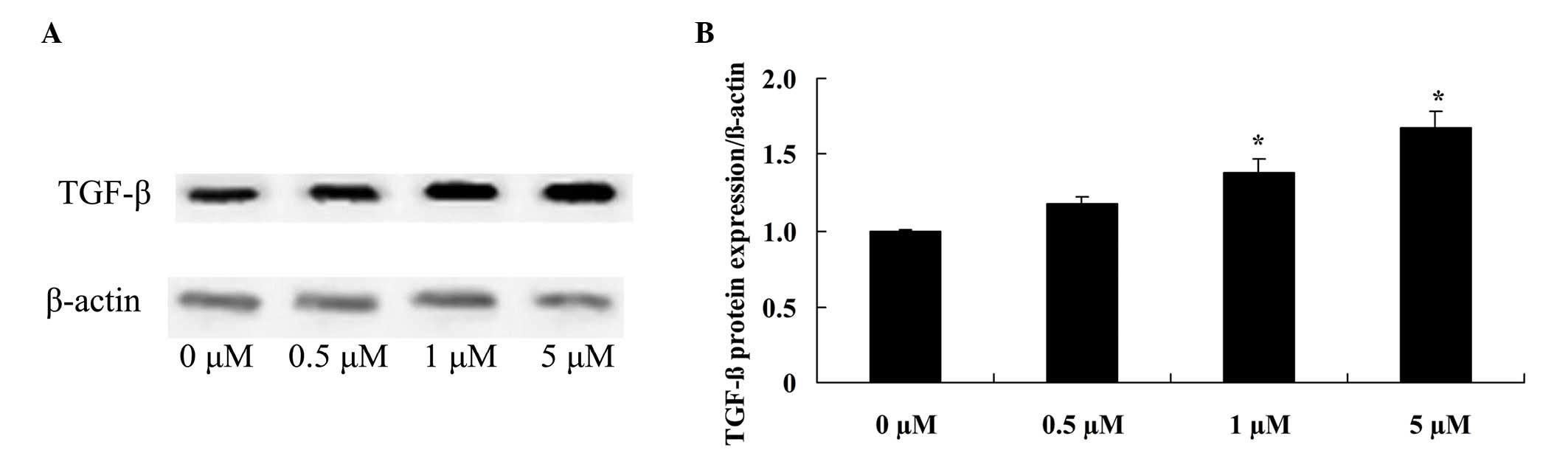
Glycitin increases TGF-β expression
levels in BMSCs
To confirm the effect of glycitin on TGF-β signaling
in BMSCs, TGF-β protein expression of BMSCs was detected. The
results indicated that pretreatment with 1 and 5 µM glycitin
significantly enhanced TGF-β protein expression of BMSCs (P=0.0063
and P=0.0021, respectively; Fig.
6).
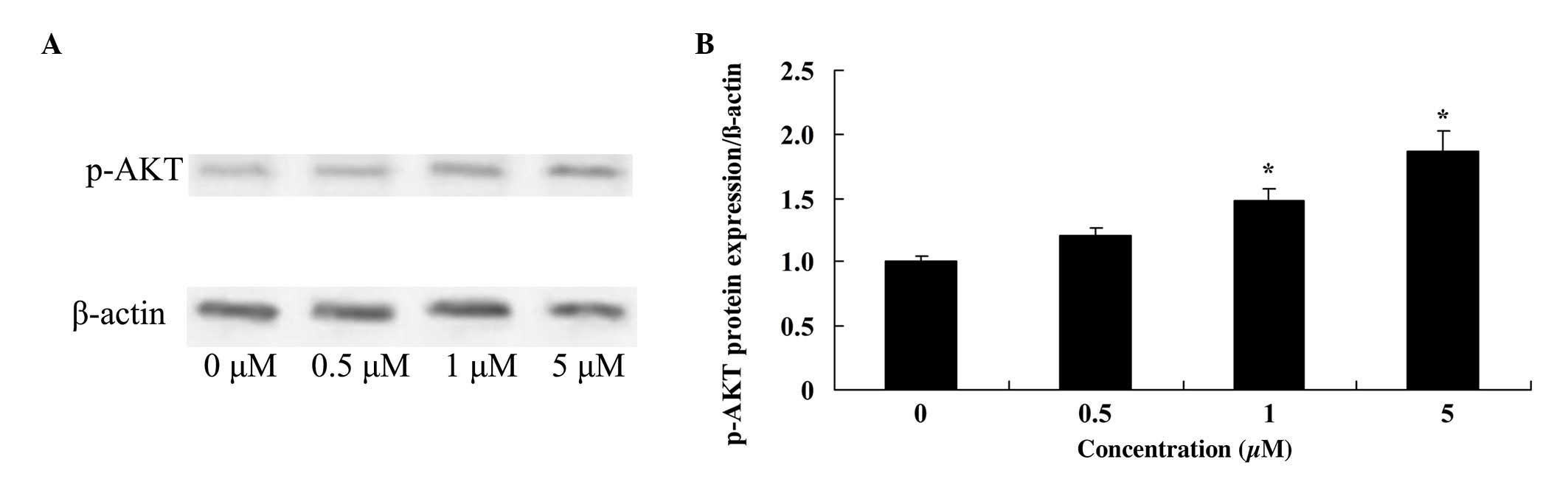
Glycitin increases p-AKT levels in
BMSCs
To investigate the effect of glycitin on p-AKT in
BMSCs, p-AKT was measured using western blotting. The results
demonstrated that 1 and 5 µM glycitin significantly increased the
presence of p-AKT in BMSCs (P=0.0071 and P=0.0033, respectively;
Fig. 7).
Discussion
Old bones and osteoblasts are absorbed by
osteoclasts and new bones are formed (17). During continuous reconstruction, the
equilibrium of the re-constructional process is regulated by a
complicated signal network consisting of hormones, growth factors,
cytokines, chemo-tactic factors and mechanical signals (18). BMSCs are capable of osteogenic
differentiation, which indicates the prospect of the clinical
application for biotherapy based on BMSCs (19). Osteogenic differentiation of BMSCs,
in vitro and in vivo, has been extensively studied
(20,21). The results of the present study
demonstrated that glycitin promotes cell proliferation, osteoblast
induction, and activates Col I mRNA expression and ALP activity of
BMSCs. Li et al (14)
reported that daidzin, genistin, and glycitin affects osteogenic
and adipogenic differentiation.
The TGF-β protein super family includes TGF,
activins, inhibin and bone morphogenetic proteins (22). These proteins have important roles in
cell proliferation, differentiation, formation of cell matrix, the
formation of tissues and organs, embryonic development and
immunoregulation. During bone formation, the combination of TGF-β
and its ligand may inhibit the formation of osteoclasts and bone
absorption, but may additionally facilitate the osteogenesis of
osteoblasts (23). Recent research
has shown that TGF-β1 may promote the osteogenic differentiation of
hMSCs and induce the expression of osteogenesis genes, ALP,
collagen type I and osteocalcins (24). Conversely, the addition of TGF-β3 may
inhibit the expression of ALP, suggesting that the TGF-β signaling
pathway has a different role in the osteogenic differentiation of
hMSCs (25). In addition, it was
demonstrated that glycitin significantly enhanced T GF-β protein
expression levels in BMSCs. Kim et al (26) indicated that glycitin promotes the
proliferation and migration of human dermal fibroblast cells
through TGF-β signaling.
Through the above data analysis, it was indicated
that oxygen deficit and Ang II may induce the phosphorylation of
Akt, a downstream modifier of PI3K, which can alter downstream
modifiers and lead to changes in cell proliferation (27). Glycitin significantly advanced p-AKT
formation in BMSCs. Kim et al (26) indicated that glycitin promotes
proliferation and migration of human dermal fibroblast cells
through TGF-β and p-AKT signaling.
In conclusion, the results of the present study
demonstrated that glycitin promotes cell proliferation and induces
osteoblast differentiation in BMSCs. A notable finding was that
molecules endowed with activating Col I mRNA expression, ALP
activity, and TGF-β and p-AKT signaling participated in the effect
of glycitin regulating osteoblasts in BMSCs.
Acknowledgements
This research was supported by the National Natural
Science Foundation of China (grant no. 81301,564) and the Army
Medical Science Youth Training Project (grant no. 13QNP184), the
‘Twelfth Five-year’ science and technology research project of
Jilin Department of Education (grant no. 2015-141) and the Jilin
province Department of Project (grant no. 20130624003JC).
References
|
1
|
Mathews S, Bhonde R, Gupta PK and Totey S:
Extracellular matrix protein mediated regulation of the osteoblast
differentiation of bone marrow derived human mesenchymal stem
cells. Differentiation. 84:185–192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamachika E, Tsujigiwa H, Matsubara M,
Hirata Y, Kita K, Takabatake K, Mizukawa N, Kaneda Y, Nagatsuka H
and Iida S: Basic fibroblast growth factor supports expansion of
mouse compact bone-derived mesenchymal stem cells (MSCs) and
regeneration of bone from MSC in vivo. J Mol Histol. 43:223–233.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Staudt ND, Aicher WK, Kalbacher H,
Stevanovic S, Carmona AK, Bogyo M and Klein G: Cathepsin X is
secreted by human osteoblasts, digests CXCL-12 and impairs adhesion
of hematopoietic stem and progenitor cells to osteoblasts.
Haematologica. 95:1452–1460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang C, Liu Y, Li C and Zhang B: Repair of
mandibular defects by bone marrow stromal cells expressing the
basic fibroblast growth factor transgene combined with multi-pore
mineralized Bio-Oss. Mol Med Rep. 7:99–104. 2013.PubMed/NCBI
|
|
5
|
Kim BS, Kang HJ, Park JY and Lee J:
Fucoidan promotes osteoblast differentiation via JNK- and
ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem
cells. Exp Mol Med. 47:e1282015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glynn ER, Londono AS, Zinn SA, Hoagland TA
and Govoni KE: Culture conditions for equine bone marrow
mesenchymal stem cells and expression of key transcription factors
during their differentiation into osteoblasts. J Anim Sci
Biotechnol. 4:402013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee HW, Kim SY, Kim AY, Lee EJ, Choi JY
and Kim JB: Adiponectin stimulates osteoblast differentiation
through induction of COX2 in mesenchymal progenitor cells. Stem
Cells. 27:2254–2262. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clabaut A, Delplace S, Chauveau C,
Hardouin P and Broux O: Human osteoblasts derived from mesenchymal
stem cells express adipogenic markers upon coculture with bone
marrow adipocytes. Differentiation. 80:40–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barbier V, Winkler IG, Wadley R and
Lévesque JP: Flow cytometry measurement of bone marrow perfusion in
the mouse and sorting of progenitors and stems cells according to
position relative to blood flow in vivo. Methods Mol Biol.
844:45–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang YB, Chen WH, Guo JJ, Fu ZH, Yi C,
Zhang M and Na XL: Soy isoflavone supplementation could reduce body
weight and improve glucose metabolism in non-Asian postmenopausal
women-a meta-analysis. Nutrition. 29:8–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu Z, Wu Q and Godber JS: Stabilities of
daidzin, glycitin, genistin, and generation of derivatives during
heating. J Agric Food Chem. 50:7402–7406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Robb EL and Stuart JA: Multiple
phytoestrogens inhibit cell growth and confer cytoprotection by
inducing manganese superoxide dismutase expression. Phytother Res.
28:120–131. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu A, Bray TM, Helferich WG, Doerge DR
and Ho E: Differential effects of whole soy extract and soy
isoflavones on apoptosis in prostate cancer cells. Exp Biol Med
(Maywood). 235:90–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li XH, Zhang JC, Sui SF and Yang MS:
Effect of daidzin, genistin, and glycitin on osteogenic and
adipogenic differentiation of bone marrow stromal cells and
adipocytic transdifferentiation of osteoblasts. Acta Pharmacol Sin.
26:1081–1086. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zang Y, Igarashi K and Yu C: Anti-obese
and anti-diabetic effects of a mixture of daidzin and glycitin on
C57BL/6J mice fed with a high-fat diet. Biosci Biotechnol Biochem.
79:117–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W, Ling W, Teng X, Quan C, Cai S and Hu
S: Effect of advanced glycation end products, extracellular matrix
metalloproteinase inducer and matrix metalloproteinases on type-I
collagen metabolism. Biomed Rep. 4:691–693. 2016.PubMed/NCBI
|
|
17
|
Peng KY, Horng LY, Sung HC, Huang HC and
Wu RT: Antiosteoporotic Activity of Dioscorea alata L. cv. Phyto
through driving mesenchymal stem cells differentiation for bone
formation. Evid Based Complement Alternat Med. 2011:7128922011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han DS, Chang HK, Kim KR and Woo SM:
Consideration of bone regeneration effect of stem cells: Comparison
of bone regeneration between bone marrow stem cells and
adipose-derived stem cells. J Craniofac Surg. 25:196–201. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mosig RA and Martignetti JA: Loss of MMP-2
in murine osteoblasts upregulates osteopontin and bone sialoprotein
expression in a circuit regulating bone homeostasis. Dis Model
Mech. 6:397–403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao M, Chen J, Lin G, Li S, Wang L, Qin A,
Zhao Z, Ren L, Wang Y and Tang BZ: Long-Term tracking of the
osteogenic differentiation of mouse BMSCs by aggregation-induced
emission nanoparticles. ACS Appl Mater Interfaces. 8:17878–17884.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang C, Liu D, Zhang C, Sun J, Feng W,
Liang XJ, Wang S and Zhang J: Defect-Related luminescent
hydroxyapatite-enhanced osteogenic differentiation of bone
mesenchymal stem cells via an ATP-induced cAMP/PKA pathway. ACS
Appl Mater Interfaces. 8:11262–11271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Batlle R, Alba-Castellón L,
Loubat-Casanovas J, Armenteros E, Francí C, Stanisavljevic J,
Banderas R, Martin-Caballero J, Bonilla F, Baulida J, et al: Snail1
controls TGF-β responsiveness and differentiation of mesenchymal
stem cells. Oncogene. 32:3381–3389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumar A, Ruan M, Clifton K, Syed F, Khosla
S and Oursler MJ: TGF-β mediates suppression of adipogenesis by
estradiol through connective tissue growth factor induction.
Endocrinology. 153:254–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Claros S, Rico-Llanos GA, Becerra J and
Andrades JA: A novel human TGF-β1 fusion protein in combination
with rhBMP-2 increases chondro-osteogenic differentiation of bone
marrow mesenchymal stem cells. Int J Mol Sci. 15:11255–11274. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin XX, Chen ZQ, Liu ZJ, Ma QJ and Dang
GT: Icariine stimulates proliferation and differentiation of human
osteoblasts by increasing production of bone morphogenetic protein
2. Chin Med J (Engl). 120:204–210. 2007.PubMed/NCBI
|
|
26
|
Kim YM, Huh JS, Lim Y and Cho M: Soy
isoflavone glycitin (4′-Hydroxy-6-Methoxyisoflavone-7-D-Glucoside)
promotes human dermal fibroblast cell proliferation and migration
via TGF-β signaling. Phytother Res. 29:757–769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C, Lin K, Chang J and Sun J:
Osteogenesis and angiogenesis induced by porous β-CaSiO (3)/PDLGA
composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt
pathways. Biomaterials. 34:64–77. 2013. View Article : Google Scholar : PubMed/NCBI
|